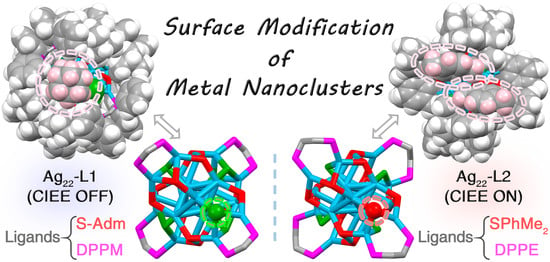
Ligand Effects on Intramolecular Configuration, Intermolecular Packing, and Optical Properties of Metal Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
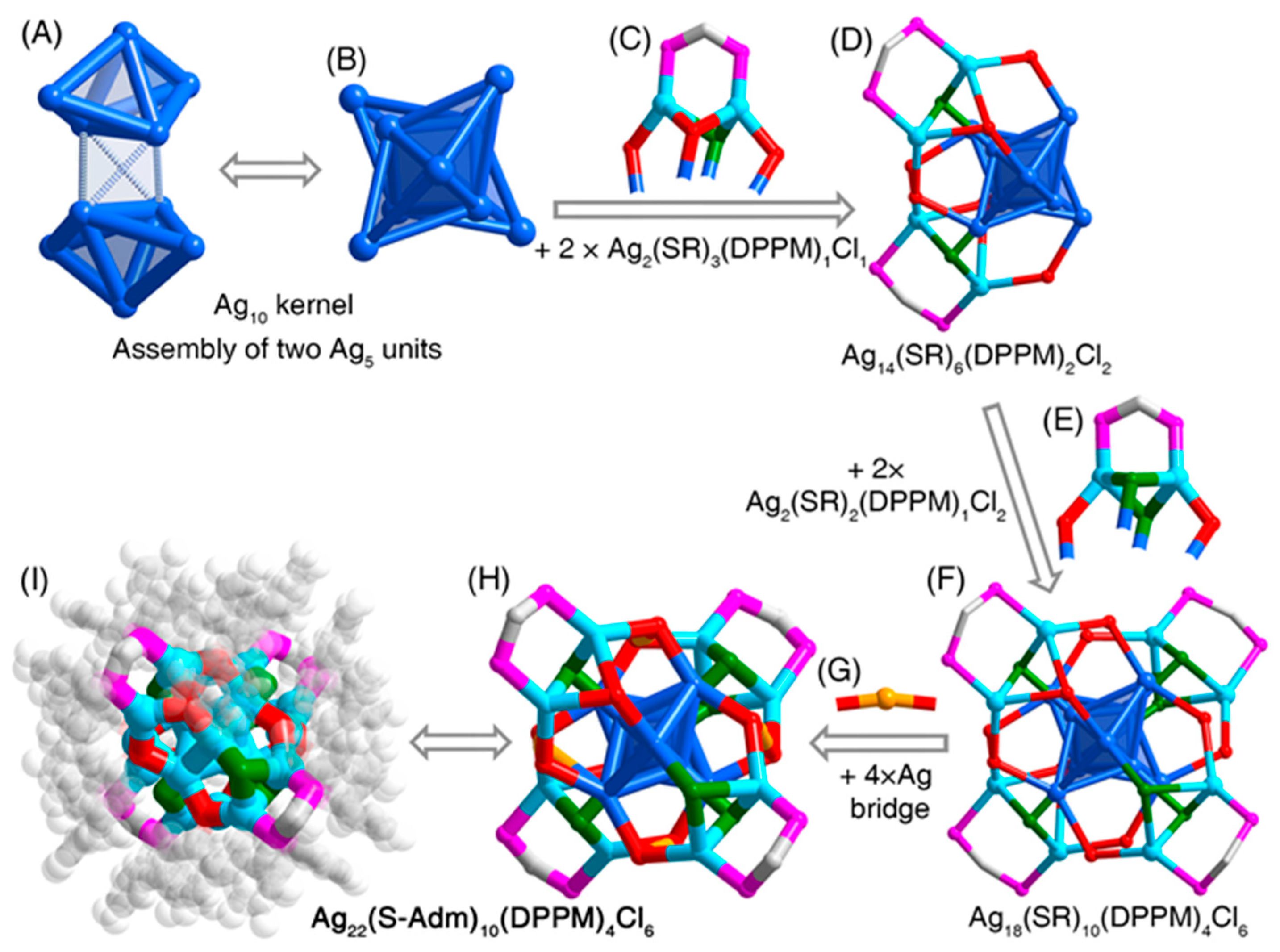
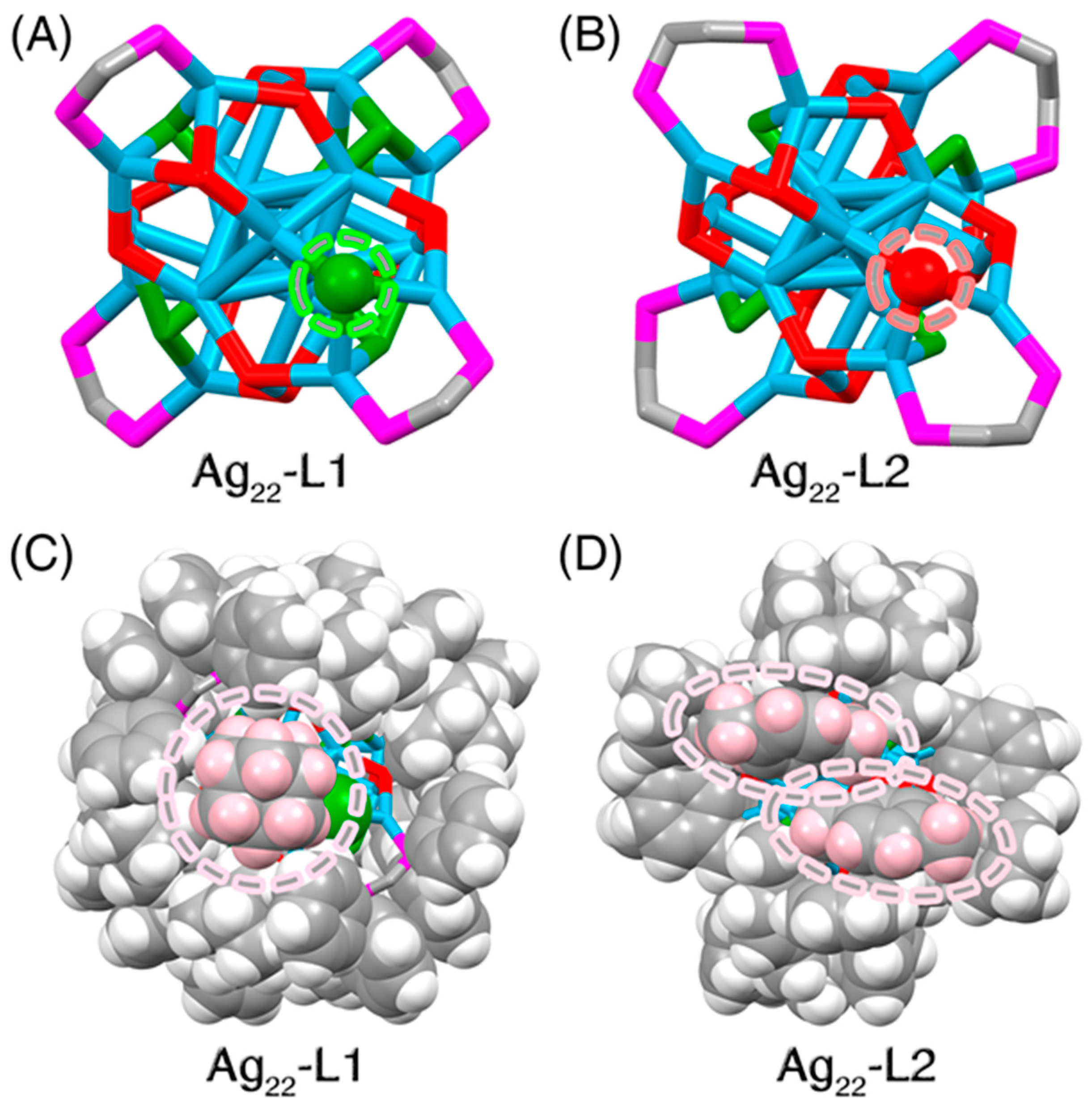
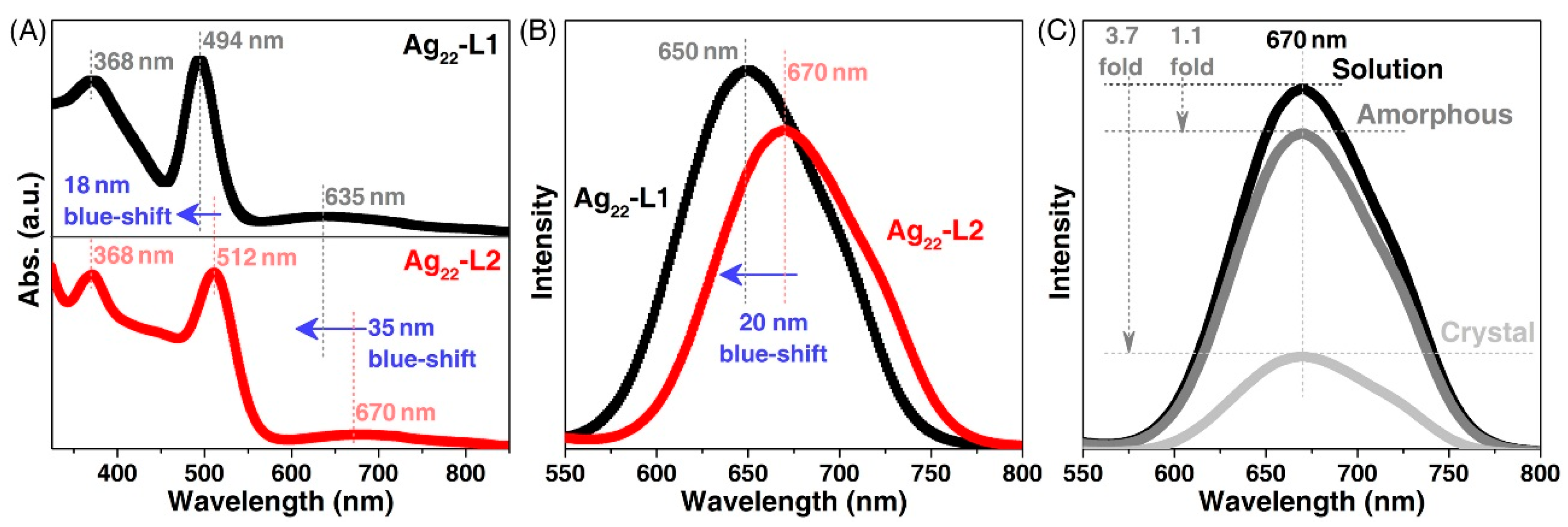
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, M.; Jin, R. Programmable Metal Nanoclusters with Atomic Precision. Adv. Mater. 2021. [Google Scholar] [CrossRef]
- Bhattarai, B.; Zaker, Y.; Atnagulov, A.; Yoon, B.; Landman, U.; Bigioni, T.P. Chemistry and Structure of Silver Molecular Nanoparticles. Acc. Chem. Res. 2018, 51, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Teo, B.K.; Zheng, N. Surface Chemistry of Atomically Precise Coinage-Metal Nanoclusters: From Structural Control to Surface Reactivity and Catalysis. Acc. Chem. Res. 2018, 51, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, W.; Niihori, Y.; Sharma, S.; Negishi, Y. Precise Synthesis, Functionalization and Application of Thiolate-Protected Gold Clusters. Coord. Chem. Rev. 2016, 320, 238–250. [Google Scholar] [CrossRef]
- Cook, A.W.; Hayton, T.W. Case Studies in Nanocluster Synthesis and Characterization: Challenges and Opportunities. Acc. Chem. Res. 2018, 51, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Hasegawa, S.; Suyama, M.; Tsukuda, T. Hydride Doping of Chemically Modified Gold-Based Superatoms. Acc. Chem. Res. 2018, 51, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hu, G.; Fung, V.; Jiang, D.-e. Insights into Interfaces, Stability, Electronic Properties, and Catalytic Activities of Atomically Precise Metal Nanoclusters from First Principles. Acc. Chem. Res. 2018, 51, 2793–2802. [Google Scholar] [CrossRef]
- Kang, X.; Chong, H.; Zhu, M. Au25(SR)18: The Captain of the Great Nanocluster Ship. Nanoscale 2018, 10, 10758–10834. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhu, M. Cocrystallization of Atomically Precise Nanoclusters. ACS Mater. Lett. 2020, 2, 1303–1314. [Google Scholar] [CrossRef]
- Sakthivel, N.A.; Dass, A. Aromatic Thiolate-Protected Series of Gold Nanomolecules and a Contrary Structural Trend in Size Evolution. Acc. Chem. Res. 2018, 51, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Hussain, A.I.; Chatha, S.A.S.; Mansha, A.; Ayub, K. Density Functional Theory Study of Geometric and Electronic Properties of Full Range of Bimetallic AgnYm (n + m = 10) Clusters. J. Alloys Compd. 2017, 705, 232–246. [Google Scholar] [CrossRef]
- Hussain, R.; Hussain, A.I.; Chatha, S.A.S.; Hussain, R.; Hanif, U.; Ayub, K. Density Functional Theory and Surface Reactivity Study of Bimetallic AgnYm (n + m = 10) Clusters. Solid State Sci. 2018, 80, 46–64. [Google Scholar] [CrossRef]
- Jadoon, T.; Carter-Fenk, K.; Siddique, M.B.A.; Herbert, J.M.; Hussain, R.; Iqbal, S.; Iqbal, J.; Ayub, K. Silver Clusters Tune Up Electronic Properties of Graphene Nanoflakes: A Comprehensive Theoretical Study. J. Mol. Liq. 2020, 297, 111902. [Google Scholar] [CrossRef]
- Jadoon, T.; Ahsin, A.; Ullah, F.; Mahmood, T.; Ayub, K. Adsorption Mechanism of p-Aminophenol over Silver-Graphene Composite: A First Principles Study. J. Mol. Liq. 2021, 341, 117415. [Google Scholar] [CrossRef]
- Agrachev, M.; Ruzzi, M.; Venzo, A.; Maran, F. Nuclear and Electron Magnetic Resonance Spectroscopies of Atomically Precise Gold Nanoclusters. Acc. Chem. Res. 2019, 52, 44–52. [Google Scholar] [CrossRef]
- Kwak, K.; Lee, D. Electrochemistry of Atomically Precise Metal Nanoclusters. Acc. Chem. Res. 2019, 52, 12–22. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Tailoring the Photoluminescence of Atomically Precise Nanoclusters. Chem. Soc. Rev. 2019, 48, 2422–2457. [Google Scholar] [CrossRef]
- Gan, Z.; Xia, N.; Wu, Z. Discovery, Mechanism, and Application of Antigalvanic Reaction. Acc. Chem. Res. 2018, 51, 2774–2783. [Google Scholar] [CrossRef]
- Lei, Z.; Wan, X.-K.; Yuan, S.-F.; Guan, Z.-J.; Wang, Q.-M. Alkynyl Approach toward the Protection of Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Konishi, K.; Iwasaki, M.; Shichibu, Y. Phosphine-Ligated Gold Clusters with Core+exo Geometries: Unique Properties and Interactions at the Ligand-Cluster Interface. Acc. Chem. Res. 2018, 51, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Nag, A.; Chakraborty, A.; Pradeep, T. Approaching Materials with Atomic Precision Using Supramolecular Cluster Assemblies. Acc. Chem. Res. 2019, 52, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jin, R.; Jin, R. Opportunities and Challenges in CO2 Reduction by Gold- and Silver-Based Electrocatalysts: From Bulk Metals to Nanoparticles and Atomically Precise Nanoclusters. ACS Energy Lett. 2018, 3, 452–462. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.; Yan, N.; Xie, J. Roles of Thiolate Ligands in the Synthesis, Properties and Catalytic Application of Gold Nanoclusters. Coord. Chem. Rev. 2018, 368, 60–79. [Google Scholar] [CrossRef]
- Liu, Y.; Chai, X.; Cai, X.; Chen, M.; Jin, R.; Ding, W.; Zhu, Y. Central Doping of a Foreign Atom into the Silver Cluster for Catalytic Conversion of CO2 toward C-C Bond Formation. Angew. Chem. Int. Ed. 2018, 57, 9775–9779. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, Y.; Zhou, J.; Fang, D.; Jiang, H.; Wang, X. Silver-Assisted Thiolate Ligand Exchange Induced Photoluminescent Boost of Gold Nanoclusters for Selective Imaging of Intracellular Glutathione. Chem. Mater. 2018, 30, 1947–1955. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent Noble Metal Nanoclusters as an Emerging Optical Probe for Sensor Development. Chem. Asian. J. 2013, 8, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Astruc, D. Atomically Precise Copper Nanoclusters and Their Applications. Coord. Chem. Rev. 2018, 359, 112–126. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Intra-Cluster Growth Meets Inter-Cluster Assembly: The Molecular and Supramolecular Chemistry of Atomically Precise Nanoclusters. Coord. Chem. Rev. 2019, 394, 1–38. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Transformation of Atomically Precise Nanoclusters by Ligand-Exchange. Chem. Mater. 2019, 31, 9939–9969. [Google Scholar] [CrossRef]
- Gan, Z.; Chen, J.; Liao, L.; Zhang, H.; Wu, Z. Surface Single-Atom Tailoring of a Gold Nanoparticle. J. Phys. Chem. Lett. 2018, 9, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Huang, L.; Liu, W.; Xiong, L.; Pei, Y.; Sun, Z.; Wang, S.; Wei, S.; Zhu, M. Reversible Nanocluster Structure Transformation between Face-Centered Cubic and Icosahedral Isomers. Chem. Sci. 2019, 10, 8685–8693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sels, A.; Salassa, G.; Cousin, F.; Lee, L.-T.; Bürgi, T. Covalently Bonded Multimers of Au25(SBut)18 as a Conjugated System. Nanoscale 2018, 10, 12754–12762. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Mohammed, O.F.; Bakr, O.M. Atomic-Level Doping of Metal Clusters. Acc. Chem. Res. 2018, 51, 3094–3103. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Li, Y.; Zhu, M.; Jin, R. Atomically Precise Alloy Nanoclusters: Syntheses, Structures, and Properties. Chem. Soc. Rev. 2020, 49, 6443–6514. [Google Scholar] [CrossRef]
- Kang, X.; Wei, X.; Jin, S.; Yuan, Q.; Luan, X.; Pei, Y.; Wang, S.; Zhu, M.; Jin, R. Rational Construction of a Library of M29 Nanoclusters from Monometallic to Tetrametallic. Proc. Natl. Acad. Sci. USA 2019, 116, 18834–18840. [Google Scholar] [CrossRef] [Green Version]
- Fei, W.; Antonello, S.; Dainese, T.; Dolmella, A.; Lahtinen, M.; Rissanen, K.; Venzo, A.; Maran, F. Metal Doping of Au25(SR)18− Clusters: Insights and Hindsights. J. Am. Chem. Soc. 2019, 141, 16033–16045. [Google Scholar] [CrossRef]
- Lee, S.; Bootharaju, M.S.; Deng, G.; Malola, S.; Häkkinen, H.; Zheng, N.; Hyeon, T. [Pt2Cu34(PET)22Cl4]2−: An Atomically Precise, 10-Electron PtCu Bimetal Nanocluster with a Direct Pt-Pt Bond. J. Am. Chem. Soc. 2021, 143, 12100–12107. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hendrich, M.P.; Gupta, R.; Qian, H.; Schatz, G.C.; Jin, R. Reversible Switching of Magnetism in Thiolate-Protected Au25 Superatoms. J. Am. Chem. Soc. 2009, 131, 2490–2492. [Google Scholar] [CrossRef]
- Kang, X.; Xu, F.; Wei, X.; Wang, S.; Zhu, M. Valence Self-Regulation of Sulfur in Nanoclusters. Sci. Adv. 2019, 5, eaax7863. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Weitz, A.; Withers, G.; Higaki, T.; Zhao, S.; Chen, Y.; Gil, R.R.; Hendrich, M.; Jin, R. Controlling Magnetism of Au133(TBBT)52 Nanoclusters at Single Electron Level and Implication for Nonmetal to Metal Transition. Chem. Sci. 2019, 10, 9684–9691. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Zhang, C.; Dong, X.-Y.; Zang, S.-Q.; Mak, T.C.W. Shell Engineering to Achieve Modification and Assembly of Atomically-Precise Silver Clusters. Chem. Soc. Rev. 2021, 50, 2297–2319. [Google Scholar] [CrossRef]
- Huang, R.-W.; Wei, Y.-S.; Dong, X.-Y.; Wu, X.-H.; Du, C.-X.; Zang, S.-Q.; Mak, T.C.W. Hypersensitive Dual-Function Luminescence Switching of a Silver-Chalcogenolate Cluster-Based Metal-Organic Framework. Nat. Chem. 2017, 9, 689–697. [Google Scholar] [CrossRef]
- Lei, Z.; Pei, X.-L.; Jiang, Z.-G.; Wang, Q.-M. Cluster Linker Approach: Preparation of a Luminescent Porous Framework with NbO Topology by Linking Silver Ions with Gold(I) Clusters. Angew. Chem. Int. Ed. 2014, 53, 12771–12775. [Google Scholar] [CrossRef]
- Wei, X.; Kang, X.; Zuo, Z.; Song, F.; Wang, S.; Zhu, M. Hierarchical Structural Complexity in Atomically Precise Nanocluster Frameworks. Natl. Sci. Rev. 2021, 8, nwaa077. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, S.; Zhu, M. Observation of a New Type of Aggregation-induced Emission in Nanoclusters. Chem. Sci. 2018, 9, 3062–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Liu, H.; Li, T.; Liu, J.; Yin, J.; Mohammed, O.F.; Bakr, O.M.; Liu, Y.; Yang, B.; Zhang, H. Contribution of Metal Defects in the Assembly Induced Emission of Cu Nanoclusters. J. Am. Chem. Soc. 2017, 139, 4318–4321. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, R.; Selenius, E.; Ruan, P.; Yao, Y.; Zhou, Y.; Malola, S.; Häkkinen, H.; Teo, B.K.; Cao, Y.; et al. Solvent-Mediated Assembly of Atom-Precise Gold-Silver Nanoclusters to Semiconducting One-Dimensional Materials. Nat. Commun. 2020, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Du, Y.; Liu, J.; Yao, Q.; Chen, T.; Cao, Y.; Zhang, H.; Xie, J. Aurophilic Interactions in the Self-Assembly of Gold Nanoclusters into Nanoribbons with Enhanced Luminescence. Angew. Chem. Int. Ed. 2019, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Si, Y.; Dong, X.-Y.; Wang, Z.-Y.; Liu, L.-Y.; Zang, S.-Q.; Mak, T.C.W. Symmetry Breaking of Atomically Precise Fullerene-like Metal Nanoclusters. J. Am. Chem. Soc. 2021, 143, 12439–12444. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Chen, R. Pd-Mediated Synthesis of Ag33 Chiral Nanocluster with Core–Shell Structure in T Point Group. J. Am. Chem. Soc. 2019, 141, 7107–7114. [Google Scholar] [CrossRef] [PubMed]
- Khatun, E.; Bodiuzzaman, M.; Sugi, K.S.; Chakraborty, P.; Paramasivam, G.; Dar, W.A.; Ahuja, T.; Antharjanam, S.; Pradeep, T. Confining an Ag10 Core in an Ag12 Shell: A Four-Electron Superatom with Enhanced Photoluminescence upon Crystallization. ACS Nano 2019, 13, 5753–5759. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Jin, S.; Xiong, L.; Wei, X.; Zhou, M.; Qin, C.; Pei, Y.; Wang, S.; Zhu, M. Nanocluster Growth via “Graft-Onto”: Effects on Geometric Structures and Optical Properties. Chem. Sci. 2020, 11, 1691–1697. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.; Akola, J.; Lopez-Acevedo, O.; Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Whetten, R.L.; Grönbeck, H.; Häkkinen, H. A Unified View of Ligand-Protected Gold Clusters as Superatom Complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 9157–9162. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Li, J.-J.; Guan, Z.-J.; Yuan, S.-F.; Wang, Q.-M. Formation of an Alkynyl-Protected Ag112 Silver Nanocluster as Promoted by Chloride Released in Situ from CH2Cl2. Angew. Chem. Int. Ed. 2020, 59, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-L.; Guan, Z.-J.; Du, Y.; Nan, Z.-A.; Lin, Y.-M.; Wang, Q.-M. Chloride-Promoted Formation of a Bimetallic Nanocluster Au80Ag30 and the Total Structure Determination. J. Am. Chem. Soc. 2016, 138, 7848–7851. [Google Scholar] [CrossRef]
- Zou, X.; Jin, S.; Wei, X.; Li, X.; Zhou, M.; Wang, S.; Zhu, M. Overall Structures of Two Metal Nanoclusters: Chloride as a Bridge Fills the Space between the Metal Core and the Metal Shell. Inorg. Chem. 2020, 59, 11905–11909. [Google Scholar] [CrossRef]
- Wei, X.; Shen, H.; Xu, C.; Li, H.; Jin, S.; Kang, X.; Zhu, M. Ag48 and Ag50 Nanoclusters: Toward Active-Site Tailoring of Nanocluster Surface Structures. Inorg. Chem. 2021, 60, 5931–5936. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wei, X.; Li, H.; Shen, H.; Han, J.; Kang, X.; Zhu, M. Ligand Effects on Intramolecular Configuration, Intermolecular Packing, and Optical Properties of Metal Nanoclusters. Nanomaterials 2021, 11, 2655. https://doi.org/10.3390/nano11102655
Wu S, Wei X, Li H, Shen H, Han J, Kang X, Zhu M. Ligand Effects on Intramolecular Configuration, Intermolecular Packing, and Optical Properties of Metal Nanoclusters. Nanomaterials. 2021; 11(10):2655. https://doi.org/10.3390/nano11102655
Chicago/Turabian StyleWu, Sainan, Xiao Wei, Hao Li, Honglei Shen, Jiaojiao Han, Xi Kang, and Manzhou Zhu. 2021. "Ligand Effects on Intramolecular Configuration, Intermolecular Packing, and Optical Properties of Metal Nanoclusters" Nanomaterials 11, no. 10: 2655. https://doi.org/10.3390/nano11102655