3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Composite Carbon Nanofiber Electrodes
2.3. Characterization of Electrode Material
2.4. Electro-Oxidation of AO7
3. Results and Discussion
3.1. Material Composition and Morphology
3.2. Application of Materials to the Electrochemical Degradation of the AO7 Dye
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Nnaji, C.O.; Jeevanandam, J.; Chan, Y.S.; Danquah, M.K.; Pan, S.; Barhoum, A. Engineered nanomaterials for wastewater treatment: Current and future trends. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–168. [Google Scholar]
- Wang, A.; Qu, J.; Ru, J.; Liu, H.; Ge, J. Mineralization of an azo dye Acid Red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dye Pigment. 2005, 65, 227–233. [Google Scholar] [CrossRef]
- Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Advanced reduction processes: A new class of treatment processes. Environ. Eng. Sci. 2013, 30, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.; Fidal, V.T.; Bowman, K.; Breheny, M.; Chandra, T.S.; Keshavarz, T.; Kyazze, G. Degradation of azo dye (acid orange 7) in a microbial fuel cell: Comparison between anodic microbial-mediated reduction and cathodic laccase-mediated oxidation. Front. Energy Res. 2019, 7, 101. [Google Scholar] [CrossRef]
- Ali, G.; Barhoum, A.; Gupta, V.K.; Nada, A.; El-Maghrabi, H.H.; Kanthasamy, R.; Shaaban, E.R.; Algarni, H.; Chong, K.F. High surface area mesoporous silica for hydrogen sulfide effective removal. Curr. Nanosci. 2020, 16, 226–234. [Google Scholar] [CrossRef]
- Wang, C.-T.; Chou, W.-L.; Chung, M.-H.; Kuo, Y.-M. COD removal from real dyeing wastewater by electro-Fenton technology using an activated carbon fiber cathode. Desalination 2010, 253, 129–134. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Le, T.X.H.; Bechelany, M.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A hierarchical CoFe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Le, T.X.H.; Bechelany, M.; Oturan, N.; Papirio, S.; Esposito, G.; van Hullebusch, E.D.; Cretin, M.; Oturan, M.A. Electrochemical mineralization of sulfamethoxazole over wide pH range using FeIIFeIII LDH modified carbon felt cathode: Degradation pathway, toxicity and reusability of the modified cathode. Chem. Eng. J. 2018, 350, 844–855. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: A critical review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Lacour, S.; Oturan, N.; Oturan, M.A.; Cretin, M. High removal efficiency of dye pollutants by electron-Fenton process using a graphene based cathode. Carbon 2015, 94, 1003–1011. [Google Scholar] [CrossRef]
- Le, T.X.H.; Dumée, L.F.; Lacour, S.; Rivallin, M.; Yi, Z.; Kong, L.; Bechelany, M.; Cretin, M. Hybrid graphene-decorated metal hollow fibre membrane reactors for efficient electro-Fenton—Filtration co-processes. J. Membr. Sci. 2019, 587, 117182. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar] [CrossRef]
- Le, T.X.H.; Charmette, C.; Bechelany, M.; Cretin, M. Facile preparation of porous carbon cathode to eliminate paracetamol in aqueous medium using electro-fenton system. Electrochim. Acta 2016, 188, 378–384. [Google Scholar] [CrossRef]
- Zhao, H.; Qian, L.; Guan, X.; Wu, D.; Zhao, G. Continuous bulk FeCuC aerogel with ultradispersed metal nanoparticles: An efficient 3D heterogeneous electro-fenton cathode over a wide range of pH 3–9. Environ. Sci. Technol. 2016, 50, 5225–5233. [Google Scholar] [CrossRef]
- Félix, R.; Beltrán-Gastélum, M.; Salazar-Gastélum, M.I.; Silva-Carrillo, C.; Reynoso-Soto, E.A.; Pérez-Sicairos, S.; Lin, S.W.; Paraguay-Delgado, F.; Alonso-Núñez, G. Pt–Pd bimetallic nanoparticles on MWCNTs: Catalyst for hydrogen peroxide electrosynthesis. J. Nanopart. Res. 2013, 15, 1–11. [Google Scholar] [CrossRef]
- Le, T.X.H.; Esmilaire, R.; Drobek, M.; Bechelany, M.; Vallicari, C.; Nguyen, D.L.; Julbe, A.; Tingry, S.; Cretin, M. Design of a novel fuel cell-Fenton system: A smart approach to zero energy depollution. J. Mater. Chem. A 2016, 4, 17686–17693. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yang, X.; Huang, J.; Zhu, Y.; Zhou, Y.; Yao, Y.; Li, C. Iron oxide containing graphene/carbon nanotube based carbon aerogel as an efficient E-Fenton cathode for the degradation of methyl blue. Electrochim. Acta 2016, 200, 75–83. [Google Scholar] [CrossRef]
- Khataee, A.; Marandizadeh, H.; Vahid, B.; Zarei, M.; Joo, S.W. Combination of photocatalytic and photoelectro-Fenton/citrate processes for dye degradation using immobilized N-doped TiO2 nanoparticles and a cathode with carbon nanotubes: Central composite design optimization. Chem. Eng. Process. Process. Intensif. 2013, 73, 103–110. [Google Scholar] [CrossRef]
- Barros, W.; Steter, J.R.; Lanza, M.; Tavares, A.C. Catalytic activity of Fe3-xCuxO4 (0 ≤ x ≤ 0.25) nanoparticles for the degradation of Amaranth food dye by heterogeneous electro-Fenton process. Appl. Catal. B Environ. 2016, 180, 434–441. [Google Scholar] [CrossRef]
- Xiaochao, G.; Xuebin, L.; Jin, T.; Xiaoyun, L.; Bin, Z.; Xujing, Z.; Jin, X. Degradation of folic acid wastewater by electro-Fenton with three-dimensional electrode and its kinetic study. R. Soc. Open Sci. 2018, 5, 170926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.-L.; Xia, X.; Han, J.-L.; Ding, Y.-C.; Haider, M.R.; Wang, A.-J. Graphene modified electro-fenton catalytic membrane for in situ degradation of antibiotic florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Tu, C.-H.; Lin, Y.-S. Application of graphene and carbon nanotubes on carbon felt electrodes for the electro-fenton system. Materials 2019, 12, 1698. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.R.; Jiang, W.-L.; Han, J.-L.; Sharif, H.M.A.; Ding, Y.-C.; Cheng, H.-Y.; Wang, A.-J. In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants. Appl. Catal. B Environ. 2019, 256, 117774. [Google Scholar] [CrossRef]
- Özcan, A.; Şahin, Y.; Koparal, A.S.; Oturan, M.A. Carbon sponge as a new cathode material for the electro-Fenton process: Comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J. Electroanal. Chem. 2008, 616, 71–78. [Google Scholar] [CrossRef]
- Le, T.X.H.; Drobek, M.; Bechelany, M.; Motuzas, J.; Julbe, A.; Cretin, M. Application of Fe-MFI zeolite catalyst in heterogeneous electro-Fenton process for water pollutants abatement. Microporous Mesoporous Mater. 2018, 278, 64–69. [Google Scholar] [CrossRef]
- Le, T.X.H.; Cowan, M.G.; Drobek, M.; Bechelany, M.; Julbe, A.; Cretin, M. Le Fe-nanoporous carbon derived from MIL-53 (Fe): A heterogeneous catalyst for mineralization of organic pollutants. Nanomaterials 2019, 9, 641. [Google Scholar] [CrossRef] [Green Version]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Barhoum, A.; Shalan, A.E.; El-Hout, S.I.; Ali, G.A.M.; Abdelbasir, S.M.; Serea, E.S.A.; Ibrahim, A.H.; Pal, K. A broad family of carbon nanomaterials: Classification, properties, synthesis, and emerging applications. In Handbook of Nanofibers; Springer Nature: Cham, Switzerland, 2019; pp. 1–40. [Google Scholar]
- Barhoum, A.; Rasouli, R.; Yousefzadeh, M.; Rahier, H.; Bechelany, M. Nanofiber technology: History and developments. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–42. [Google Scholar]
- Gopalakrishnan, R.; Li, Y.; Smekens, J.; Barhoum, A.; Van Assche, G.; Omar, N.; Van Mierlo, J. Electrochemical impedance spectroscopy characterization and parameterization of lithium nickel manganese cobalt oxide pouch cells: Dependency analysis of temperature and state of charge. Ionics 2019, 25, 111–123. [Google Scholar] [CrossRef]
- Haichao, L.; Haoyi, L.; Bubakir, M.M.; Weimin, Y.; Barhoum, A. Engineering nanofibers as electrode and membrane materials for batteries, supercapacitors, and fuel cells. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 1105–1130. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Engel, A.B.; Cretin, M.; Tingry, S. Gold particles growth on carbon felt for efficient micropower generation in a hybrid biofuel cell. Electrochimica Acta 2016, 219, 121–129. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Saad, M.; Barhoum, A.; Bechelany, M.; Rizk, M.S. PVC membrane, coated-wire, and carbon-paste ion-selective electrodes for potentiometric determination of galantamine hydrobromide in physiological fluids. Mater. Sci. Eng. C 2018, 89, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Salah, A.; Rizk, M.S.; Moustafa, H.; Bechelany, M.; Barhoum, A. Carbon-based Nanosensors for Salicylate Determination in Pharmaceutical Preparations. Electroanalysis 2019, 31, 778–789. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.; Barhoum, A. t-Butyl calixarene/Fe2O3@MWCNTs composite-based potentiometric sensor for determination of ivabradine hydrochloride in pharmaceutical formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for biomedical and healthcare applications. Macromol. Biosci. 2018, 19, e1800256. [Google Scholar] [CrossRef]
- Barhoum, A.; El-Maghrabi, H.H.; Iatsunskyi, I.; Coy, E.; Renard, A.; Salameh, C.; Weber, M.; Sayegh, S.; Nada, A.; Roualdes, S.; et al. Atomic layer deposition of Pd nanoparticles on self-supported carbon-Ni/NiO-Pd nanofiber electrodes for electrochemical hydrogen and oxygen evolution reactions. J. Colloid Interface Sci. 2020, 569, 286–297. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.; Li, H.; Barhoum, A.; Zhang, Y.; He, X.; Yang, W.; Bubakir, M.M.; Chen, H. Magnetic nanofibers: Unique properties, fabrication techniques, and emerging applications. ChemistrySelect 2018, 3, 9127–9143. [Google Scholar] [CrossRef]
- Barhoum, A.; Samyn, P.; Öhlund, T.; Dufresne, A. Review of recent research on flexible multifunctional nanopapers. Nanoscale 2017, 9, 15181–15205. [Google Scholar] [CrossRef]
- Turky, A.O.; Barhoum, A.; Rashad, M.; Bechelany, M. Enhanced the structure and optical properties for ZnO/PVP nanofibers fabricated via electrospinning technique. J. Mater. Sci. Mater. Electron. 2017, 28, 17526–17532. [Google Scholar] [CrossRef]
- Prasad, S.; Kumar, V.; Kirubanandam, S.; Barhoum, A. Engineered nanomaterials: Nanofabrication and surface functionalization. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 305–340. [Google Scholar]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Theories of nanoparticle and nanostructure formation in liquid phase. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 597–619. [Google Scholar]
- Mantzaris, N.V. Liquid-phase synthesis of nanoparticles: Particle size distribution dynamics and control. Chem. Eng. Sci. 2005, 60, 4749–4770. [Google Scholar] [CrossRef]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Liquid-phase synthesis of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar]
- Samyn, P.; Barhoum, A. Engineered nanomaterials for papermaking industry. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–277. [Google Scholar]
- Ngom, B.; Guo, Y.; Wang, X.; Bi, D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: A review. Anal. Bioanal. Chem. 2010, 397, 1113–1135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ruan, J.; Du, T. Recent advances on photocatalytic and electrochemical oxidation for ammonia treatment from water/wastewater. ACS EST Eng. 2020, 1, 310–325. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Argyriou, R.; Economou, C.N.; Charalampous, N.; Dailianis, S.; Tatoulis, T.I.; Tekerlekopoulou, A.; Vayenas, D.V. Treatment of printing ink wastewater using electrocoagulation. J. Environ. Manag. 2019, 237, 442–448. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Khattab, T.A.; Gätjen, L.; Wilken, R. Colored, photocatalytic, antimicrobial and UV-protected viscose fibers decorated with Ag/Ag2CO3 and Ag/Ag3PO4 nanoparticles. Cellulose 2019, 26, 5437–5453. [Google Scholar] [CrossRef]
- Wakelyn, P.J. Health and safety issues in cotton production and processing. In Cotton: Science and Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2006; pp. 460–483. [Google Scholar]
- Said, M.M.; Rehan, M.; El-Sheikh, S.M.; Zahran, M.K.; Abdel-Aziz, M.S.; Bechelany, M.; Barhoum, A. Multifunctional hydroxyapatite/silver nanoparticles/cotton gauze for antimicrobial and biomedical applications. Nanomaterials 2021, 11, 429. [Google Scholar] [CrossRef]
- Le, T.X.H.; Esmilaire, R.; Drobek, M.; Bechelany, M.; Vallicari, C.; Cerneaux, S.; Julbe, A.; Cretin, M. Nitrogen-doped graphitized carbon electrodes for biorefractory pollutant removal. J. Phys. Chem. C 2017, 121, 15188–15197. [Google Scholar] [CrossRef]
- Wang, T.; Wu, J.; Liu, Y.; Cui, X.; Ding, P.; Deng, J.; Zha, C.; Coy, E.; Li, Y. Scalable preparation and stabilization of atomic-thick CoNi layered double hydroxide nanosheets for bifunctional oxygen electrocatalysis and rechargeable zinc-air batteries. Energy Storage Mater. 2019, 16, 24–30. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Xie, A.; Chen, P.; Li, S.; Shen, Y. Practical, cost-effective and large-scale production of nitrogen-doped porous carbon particles and their use as metal-free electrocatalysts for oxygen reduction. Electrochim. Acta 2015, 165, 29–35. [Google Scholar] [CrossRef]
- Zhong, H.; Deng, C.; Qiu, Y.; Yao, L.; Zhang, H. Nitrogen-doped hierarchically porous carbon as efficient oxygen reduction electrocatalysts in acid electrolyte. J. Mater. Chem. A 2014, 2, 17047–17057. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L.; Rahier, H.; Van Assche, G. Physicochemical characterization of nanomaterials: Polymorph, composition, wettability, and thermal stability. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 255–278. [Google Scholar]
- Barhoum, A.; García-Betancourt, M.L. Physicochemical characterization of nanomaterials: Size, morphology, optical, magnetic, and electrical properties. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 279–304. [Google Scholar]
- Yang, D.-S.; Chaudhari, S.; Rajesh, K.P.; Yu, J.-S. Preparation of nitrogen-doped porous carbon nanofibers and the effect of porosity, electrical conductivity, and nitrogen content on their oxygen reduction performance. ChemCatChem 2014, 6, 1236–1244. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, X.-X.; Ding, X.-L.; Kong, H.-C.; Sha, H.-D.; Lin, H.; Wen, W.; Shen, G.; Guo, Z.; Ma, Z.-F.; et al. Effects of cobalt precursor on pyrolyzed carbon-supported cobalt-polypyrrole as electrocatalyst toward oxygen reduction reaction. Nanoscale Res. Lett. 2013, 8, 478. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-J.; Yuan, X.; Wen, W.; Zhang, D.-Y.; Sun, L.; Jiang, Q.-Z.; Ma, Z.-F. Electrochemical performance of a novel CoTETA/C catalyst for the oxygen reduction reaction. Electrochem. Commun. 2009, 11, 206–208. [Google Scholar] [CrossRef]
- Faubert, G.; Côté, R.; Guay, D.; Dodelet, J.; Dénès, G.; Poleunis, C.; Bertrand, P. Activation and characterization of Fe-based catalysts for the reduction of oxygen in polymer electrolyte fuel cells. Electrochim. Acta 1998, 43, 1969–1984. [Google Scholar] [CrossRef]
- Yang, R.; Dahn, J.R.; Bonakdarpour, A.; Easton, E.B. Co-C-N oxygen reduction catalysts prepared by combinatorial magnetron sputter deposition. ECS Trans. 2006, 3, 221–230. [Google Scholar] [CrossRef]
- Niwa, H.; Kobayashi, M.; Horiba, K.; Harada, Y.; Oshima, M.; Terakura, K.; Ikeda, T.; Koshigoe, Y.; Ozaki, J.-I.; Miyata, S.; et al. X-ray photoemission spectroscopy analysis of N-containing carbon-based cathode catalysts for polymer electrolyte fuel cells. J. Power Sources 2011, 196, 1006–1011. [Google Scholar] [CrossRef]
- Hayes, W.I.; Joseph, P.; Mughal, M.Z.; Papakonstantinou, P. Production of reduced graphene oxide via hydrothermal reduction in an aqueous sulphuric acid suspension and its electrochemical behaviour. J. Solid State Electrochem. 2014, 19, 361–380. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, J.; Wu, T.; Su, X.; Su, H.; Qu, S.; Xie, Y.; Chen, M.; Diao, G. Graphitized porous carbon nanofibers prepared by electrospinning as anode materials for lithium ion batteries. RSC Adv. 2016, 6, 83185–83195. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Toprakci, O.; Yao, Y.; Xu, G.; Li, S.; Zhang, X. Electrospun carbon nanofibers decorated with various amounts of electrochemically-inert nickel nanoparticles for use as high-performance energy storage materials. RSC Adv. 2012, 2, 192–198. [Google Scholar] [CrossRef] [Green Version]
- George, G.; Anandhan, S. Synthesis and characterisation of nickel oxide nanofibre webs with alcohol sensing characteristics. RSC Adv. 2014, 4, 62009–62020. [Google Scholar] [CrossRef]
- Bao, G.; Bai, J.; Li, C. Synergistic effect of the Pd–Ni bimetal/carbon nanofiber composite catalyst in Suzuki coupling reaction. Org. Chem. Front. 2019, 6, 352–361. [Google Scholar] [CrossRef]
- Guo, S.-X.; Liu, Y.; Bond, A.M.; Zhang, J.; Karthik, P.E.; Maheshwaran, I.; Kumar, S.S.; Phani, K.L.N. Facile electrochemical co-deposition of a graphene–cobalt nanocomposite for highly efficient water oxidation in alkaline media: Direct detection of underlying electron transfer reactions under catalytic turnover conditions. Phys. Chem. Chem. Phys. 2014, 16, 19035–19045. [Google Scholar] [CrossRef] [PubMed]
- Di Blasi, A.; Busacca, C.; Di Blasi, O.; Briguglio, N.; Antonucci, V. Synthesis and Characterization of Electrospun Nickel-Carbon Nanofibers as Electrodes for Vanadium Redox Flow Battery. J. Electrochem. Soc. 2018, 165, A1478–A1485. [Google Scholar] [CrossRef]
- Luo, Q.-X.; Guo, L.-P.; Yao, S.-Y.; Bao, J.; Liu, Z.-T.; Liu, Z.-W. Cobalt nanoparticles confined in carbon matrix for probing the size dependence in Fischer-Tropsch synthesis. J. Catal. 2019, 369, 143–156. [Google Scholar] [CrossRef]
- Kim, M.; Nam, D.-H.; Park, H.-Y.; Kwon, C.; Eom, K.; Yoo, S.; Jang, J.; Kim, H.-J.; Cho, E.; Kwon, H. Cobalt-carbon nanofibers as an efficient support-free catalyst for oxygen reduction reaction with a systematic study of active site formation. J. Mater. Chem. A 2015, 3, 14284–14290. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Mahmoud, S.; Abdel-Ghani, N.E.T.; El Nashar, R.M.; Bechelany, M.; Barhoum, A. Polyvinyl chloride modified carbon paste electrodes for sensitive determination of levofloxacin drug in serum, urine, and pharmaceutical formulations. Sensors 2021, 21, 3150. [Google Scholar] [CrossRef]
- Le, T.X.H.; Van Nguyen, T.; Yacouba, Z.A.; Zoungrana, L.; Avril, F.; Petit, E.; Mendret, J.; Bonniol, V.; Bechelany, M.; Lacour, S.; et al. Toxicity removal assessments related to degradation pathways of azo dyes: Toward an optimization of Electro-Fenton treatment. Chemosphere 2016, 161, 308–318. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, H.; Hou, L. Degradation of C. I. Acid Orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process. J. Hazard. Mater. 2014, 276, 182–191. [Google Scholar] [CrossRef]
- Fernandes, A.; Morao, A.; Magrinho, M.; Lopes, A.; Gonçalves, I. Electrochemical degradation of C. I. Acid Orange 7. Dye. Pigment. 2004, 61, 287–296. [Google Scholar] [CrossRef]
- Özcan, A.; Oturan, M.A.; Oturan, N.; Sahin, Y. Removal of Acid Orange 7 from water by electrochemically generated Fenton’s reagent. J. Hazard. Mater. 2009, 163, 1213–1220. [Google Scholar] [CrossRef]
- Daneshvar, N.; Aber, S.; Vatanpour, V.; Rasoulifard, M.H. Electro-Fenton treatment of dye solution containing Orange II: Influence of operational parameters. J. Electroanal. Chem. 2008, 615, 165–174. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, C.; Li, W.; Cui, J. Indirect electrochemical oxidation of dye wastewater containing Acid Orange 7 using Ti/RuO 2-Pt Electrode. Int. J. Electrochem. Sci. 2014, 9, 943–954. [Google Scholar]
- Han, Y.; Quan, X.; Ruan, X.; Zhang, W. Integrated electrochemically enhanced adsorption with electrochemical regeneration for removal of acid orange 7 using activated carbon fibers. Sep. Purif. Technol. 2008, 59, 43–49. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, G.; Guo, L.; Dai, Q.; Ma, X. Electrochemical oxidation of Acid Orange 7 azo dye using a PbO2 electrode: Parameter optimization, reaction mechanism and toxicity evaluation. Chemosphere 2020, 241, 125010. [Google Scholar] [CrossRef]
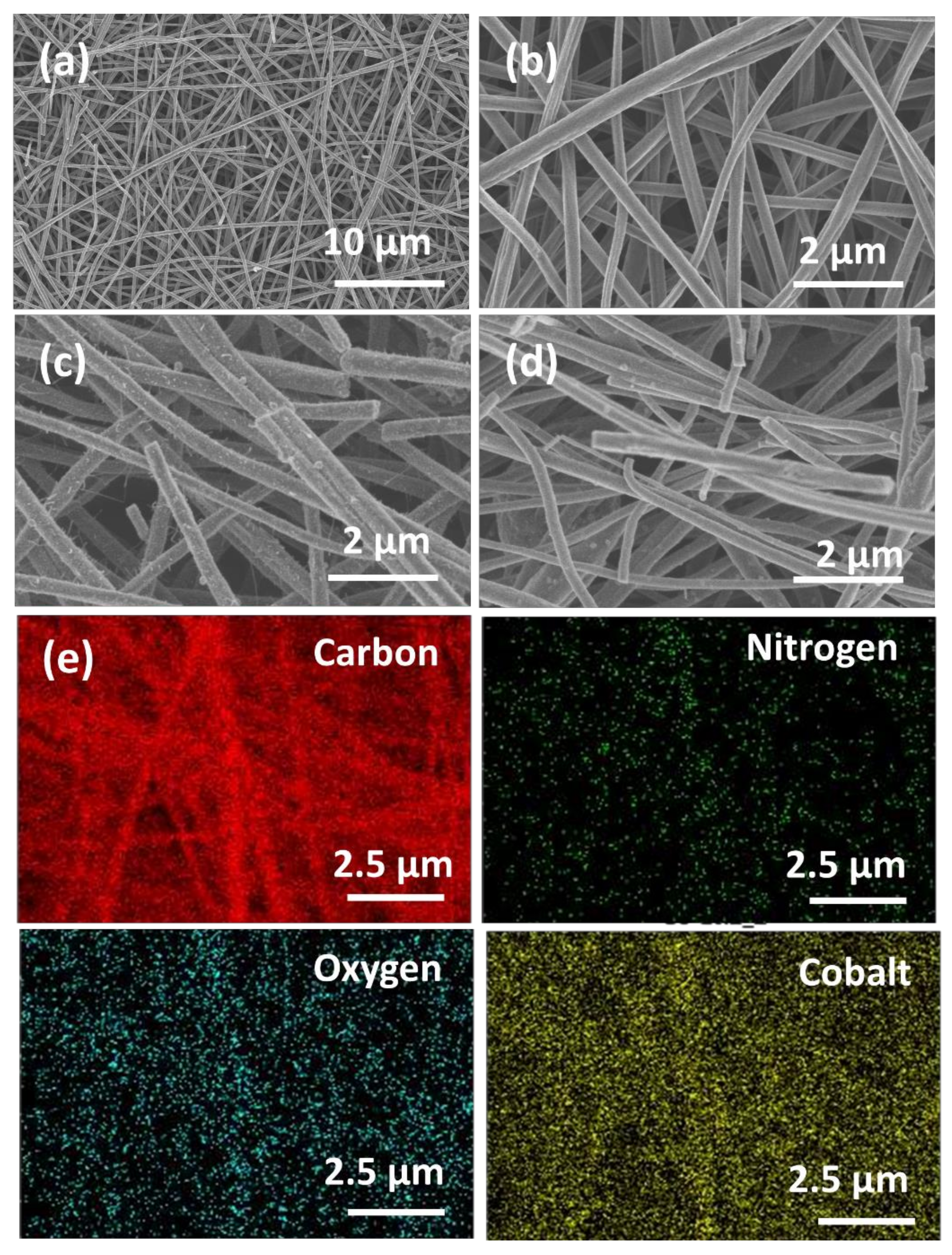
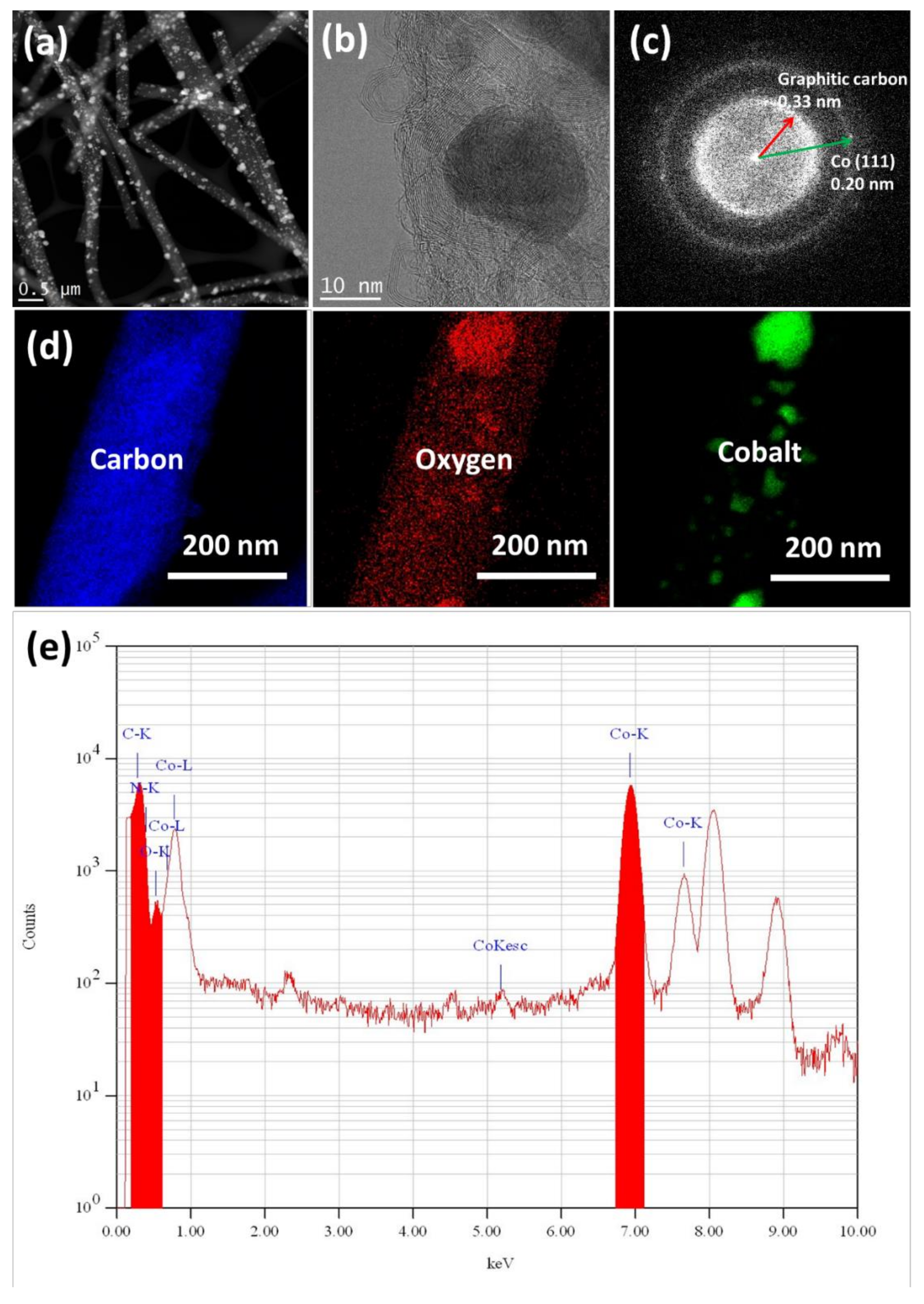
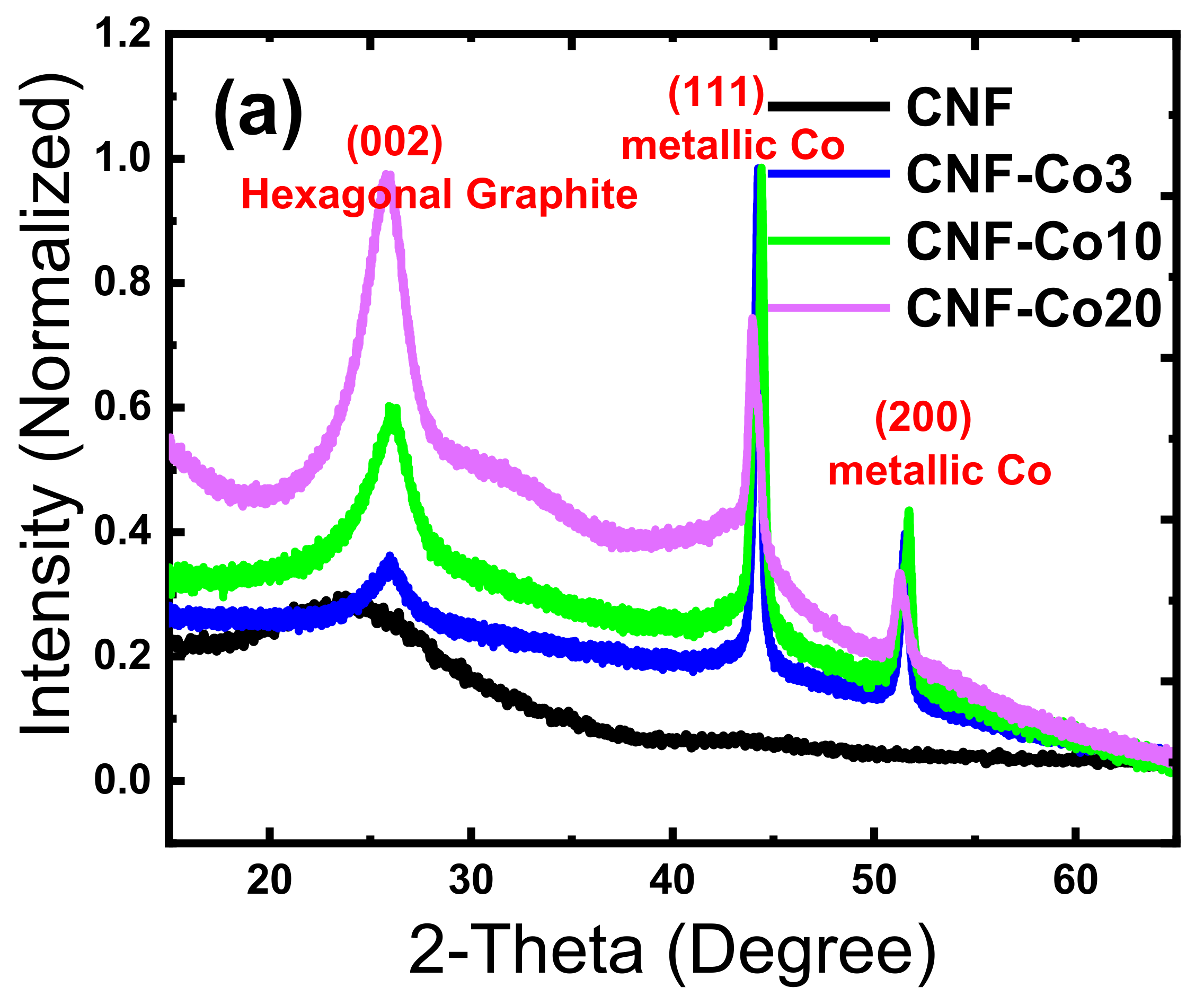
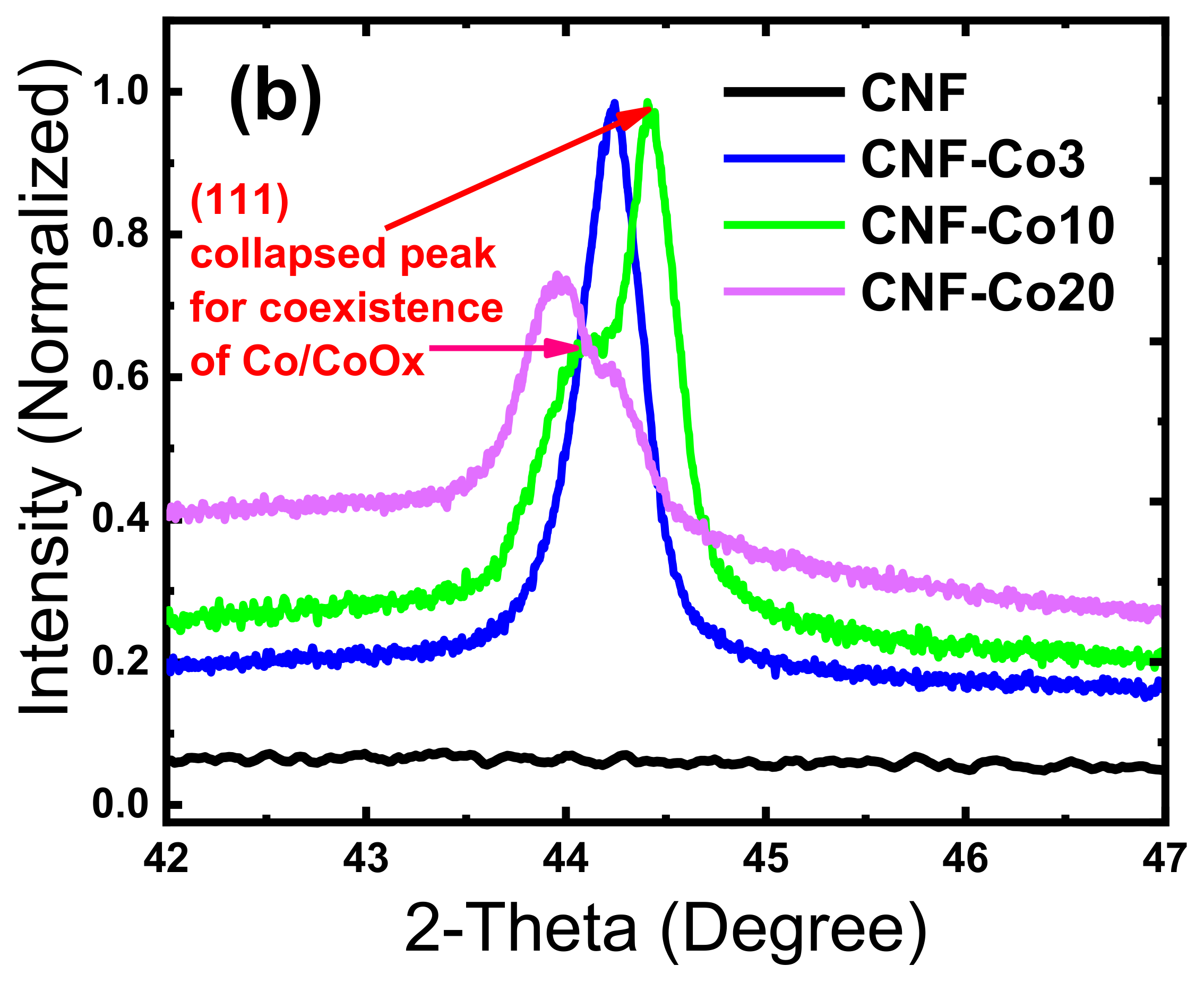

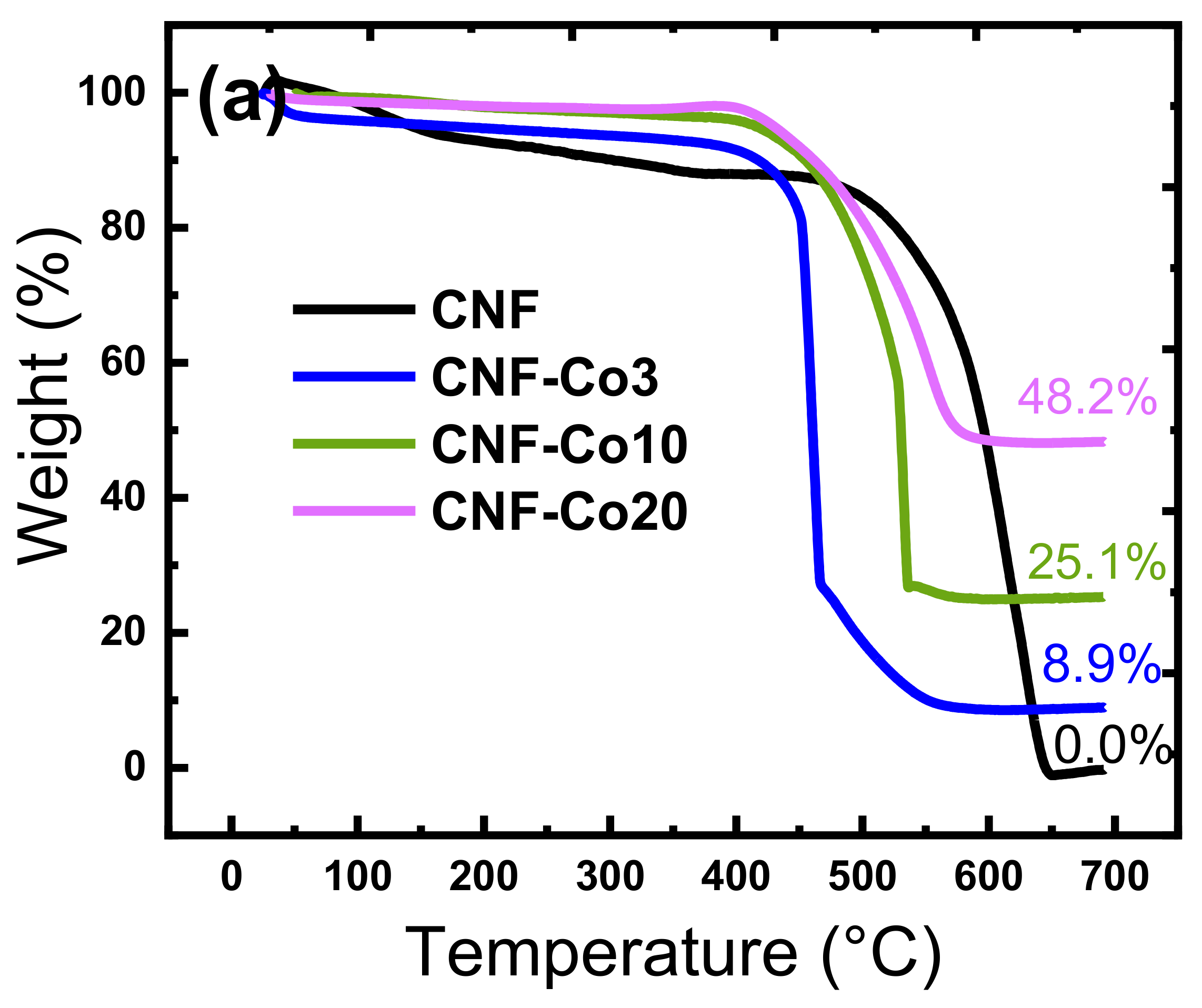
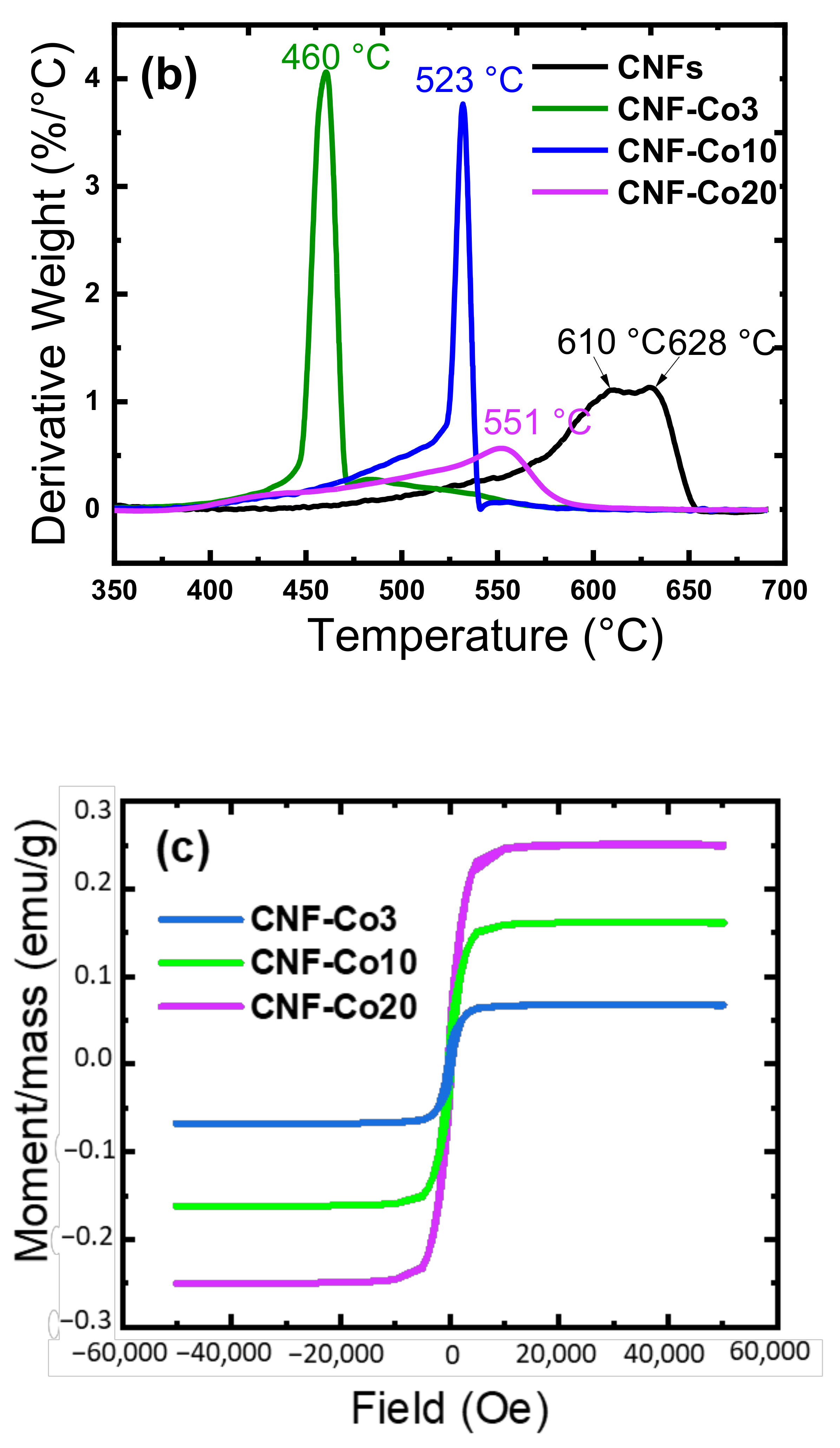
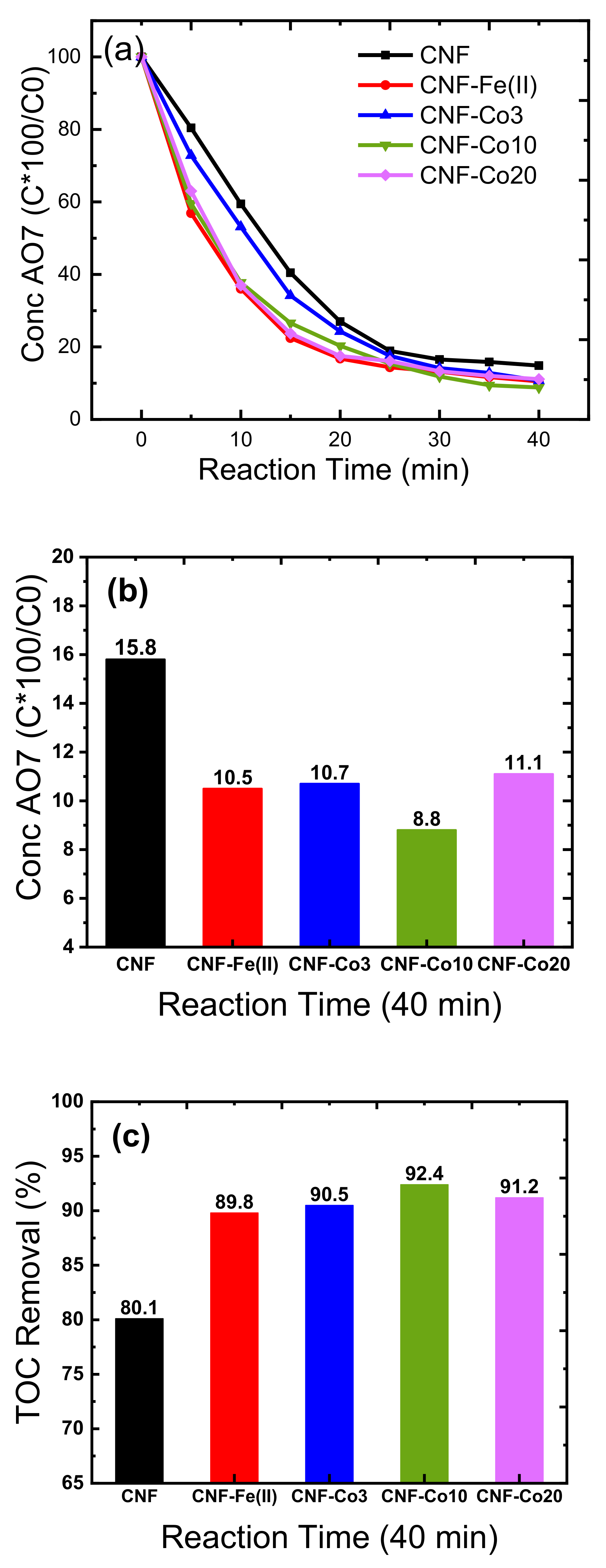


| Electrodes | Co Crystallite Size a | R = (ID/IG) b | BET Surface Area | Average Pore Diameter | Co Content c | AO7 Residue d | Rate Constant e | TOC Removal at pH 3 f | TOC Removal at pH 6 g |
|---|---|---|---|---|---|---|---|---|---|
| nm | -- | cm2/g | nm | wt/wt% | % | min−1 | % | % | |
| CNF | -- | 1.13 | 7.1 | 13.9 | No | 15.8 ± 0.3 | 0.068 | 80.1 ± 0.6 | 81.2 ± 0.5 |
| CNF-Fe(II) h | -- | 1.13 | 7.1 | 13.9 | No | 10.5 ± 0.2 | 0.090 | 89.8 ± 0.4 | -- |
| CNF-Co3 | 37.0 ± 0.6 | 1.1 | 50.5 | 8.6 | 8.9 ± 0.5 | 10.7 ± 0.3 | 0.071 | 90.5 ± 0.3 | 90.5 ± 0.5 |
| CNF-Co10 | 35.0 ± 0.4 | 1.01 | 115.3 | 3.8 | 25.1 ± 0.4 | 8.8 ± 0.3 | 0.080 | 92.4 ± 0.6 | 93.3 ± 0.5 |
| CNF-Co20 | 32.1 ± 0.3 | 0.92 | 240.2 | 5.6 | 48.2 ± 0.7 | 11.1 ± 0.2 | 0.089 | 91.2 ± 0.6 | 91.0 ± 0.7 |
| Cathode | Electrolyte | Initial AO7 Conc. | Current Density | Time | TOC Removal | pH | Ref. |
|---|---|---|---|---|---|---|---|
| mM | mA·cm−2 | min | % | -- | -- | ||
| rGO/carbon felt | Fe (II) + Na2SO4 | 0.1 | 30 | 120 | 73 | 3 | [14] |
| Carbon felt | Fe (II) + Na2SO4 | 0.1 | 8.3 | 120 | 90 | 3 | [78] |
| Stainless steel | Fe3O4 + peroxydisulfate (PDS) | 0.07 | 8.4 | 90 | 30 | 3 | [79] |
| Boron-doped diamond electrode | KCl + Na2SO4 | 1.7 | 10 | 600 | 90 | 3.5 | [80] |
| CNFs-Co10 | Na2SO4 | 0.1 | 10 | 40 | 92.5 | 3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barhoum, A.; Favre, T.; Sayegh, S.; Tanos, F.; Coy, E.; Iatsunskyi, I.; Razzouk, A.; Cretin, M.; Bechelany, M. 3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process. Nanomaterials 2021, 11, 2686. https://doi.org/10.3390/nano11102686
Barhoum A, Favre T, Sayegh S, Tanos F, Coy E, Iatsunskyi I, Razzouk A, Cretin M, Bechelany M. 3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process. Nanomaterials. 2021; 11(10):2686. https://doi.org/10.3390/nano11102686
Chicago/Turabian StyleBarhoum, Ahmed, Therese Favre, Syreina Sayegh, Fida Tanos, Emerson Coy, Igor Iatsunskyi, Antonio Razzouk, Marc Cretin, and Mikhael Bechelany. 2021. "3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process" Nanomaterials 11, no. 10: 2686. https://doi.org/10.3390/nano11102686
APA StyleBarhoum, A., Favre, T., Sayegh, S., Tanos, F., Coy, E., Iatsunskyi, I., Razzouk, A., Cretin, M., & Bechelany, M. (2021). 3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process. Nanomaterials, 11(10), 2686. https://doi.org/10.3390/nano11102686











