MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the d-MXene Flake Solution
2.3. Fabrication of MXene Coatings on Pipe Steel
2.4. Electrochemical Hydrogen Permeation Evaluation
2.5. Slow Strain Rate Measurement
2.6. Electrochemical Characterization
2.7. Materials Characterization
3. Results and Discussion
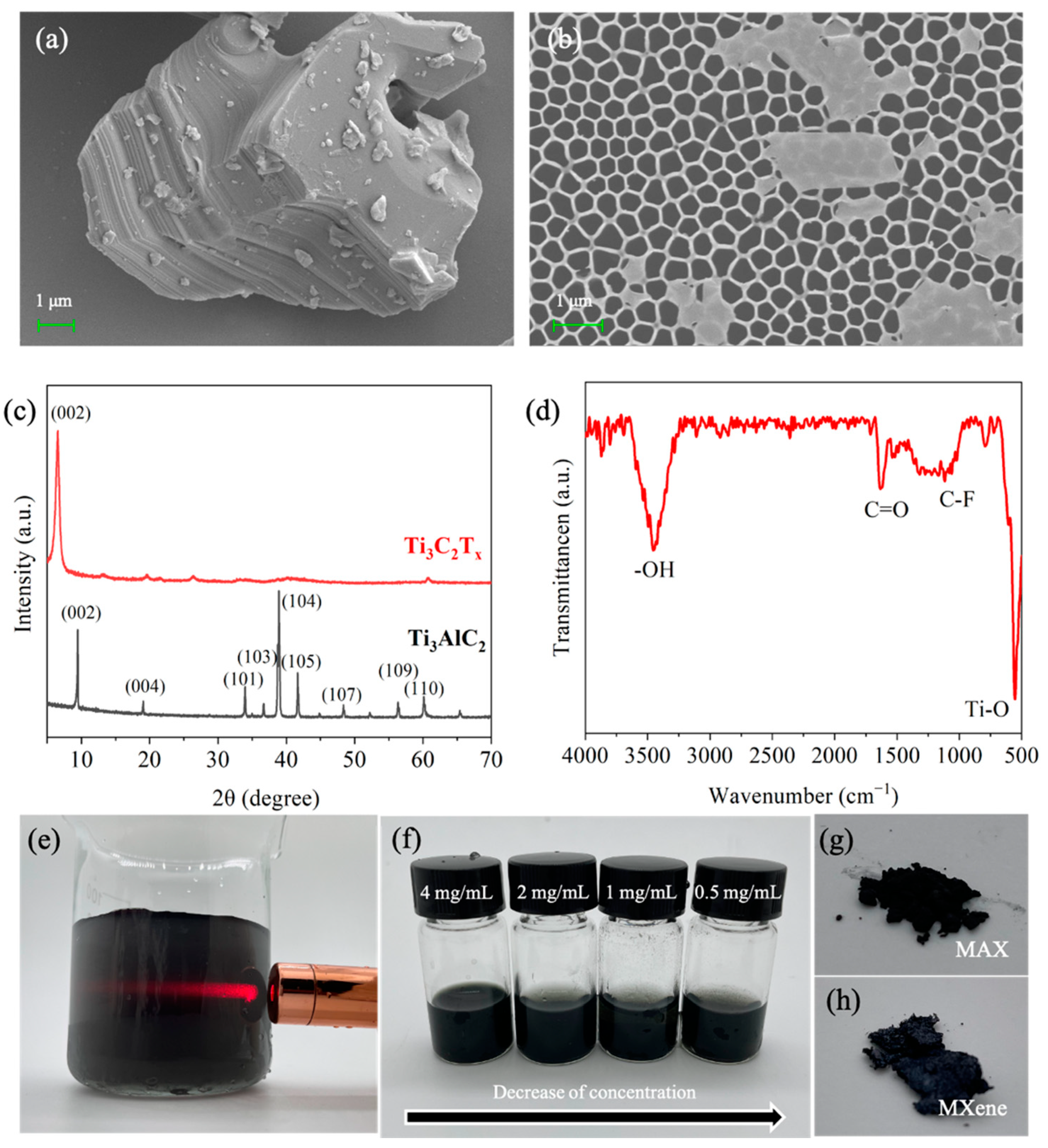
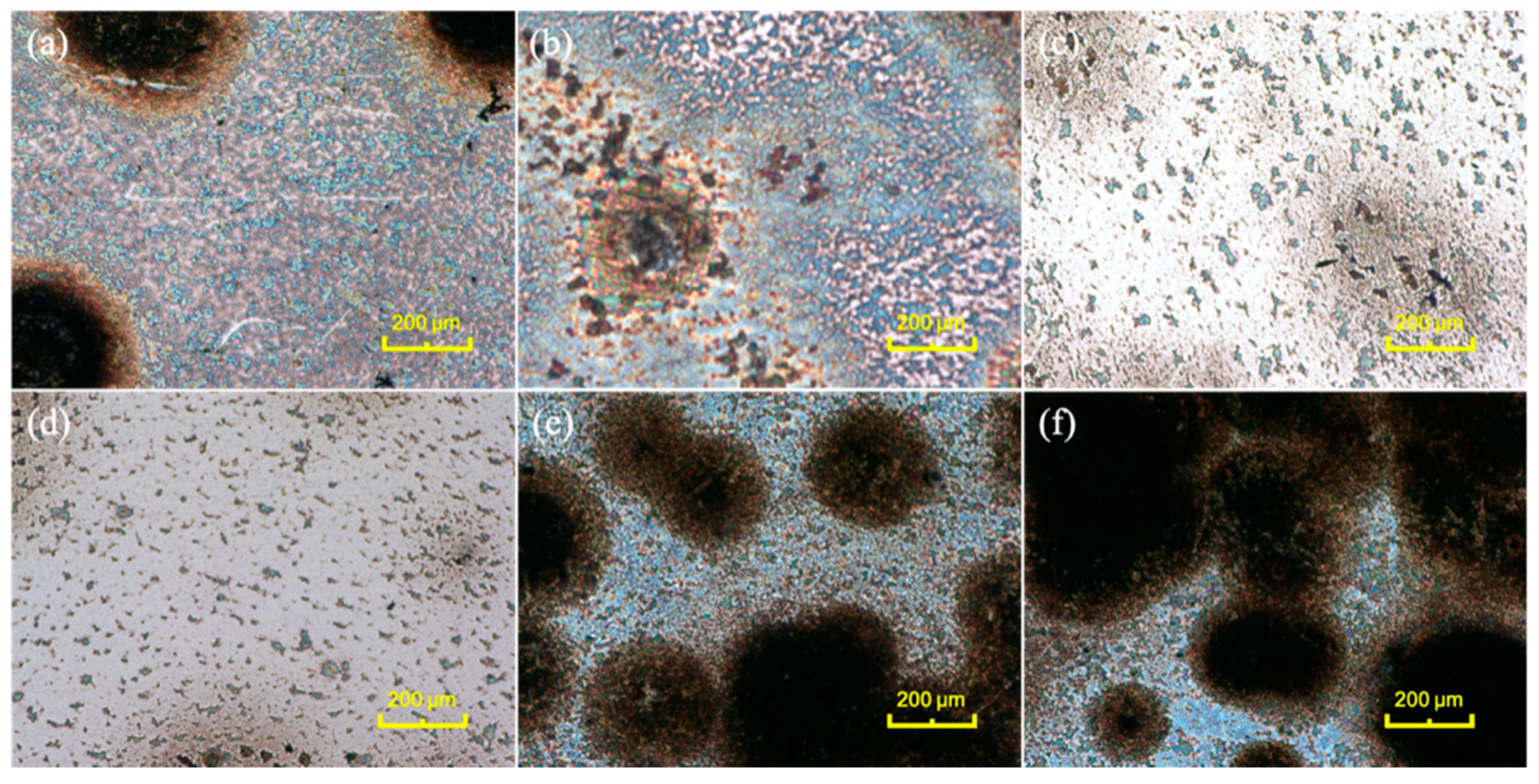
3.1. Fabrication and Characterization
3.2. Hydrogen Permeation Behavior of the MXene Coatings
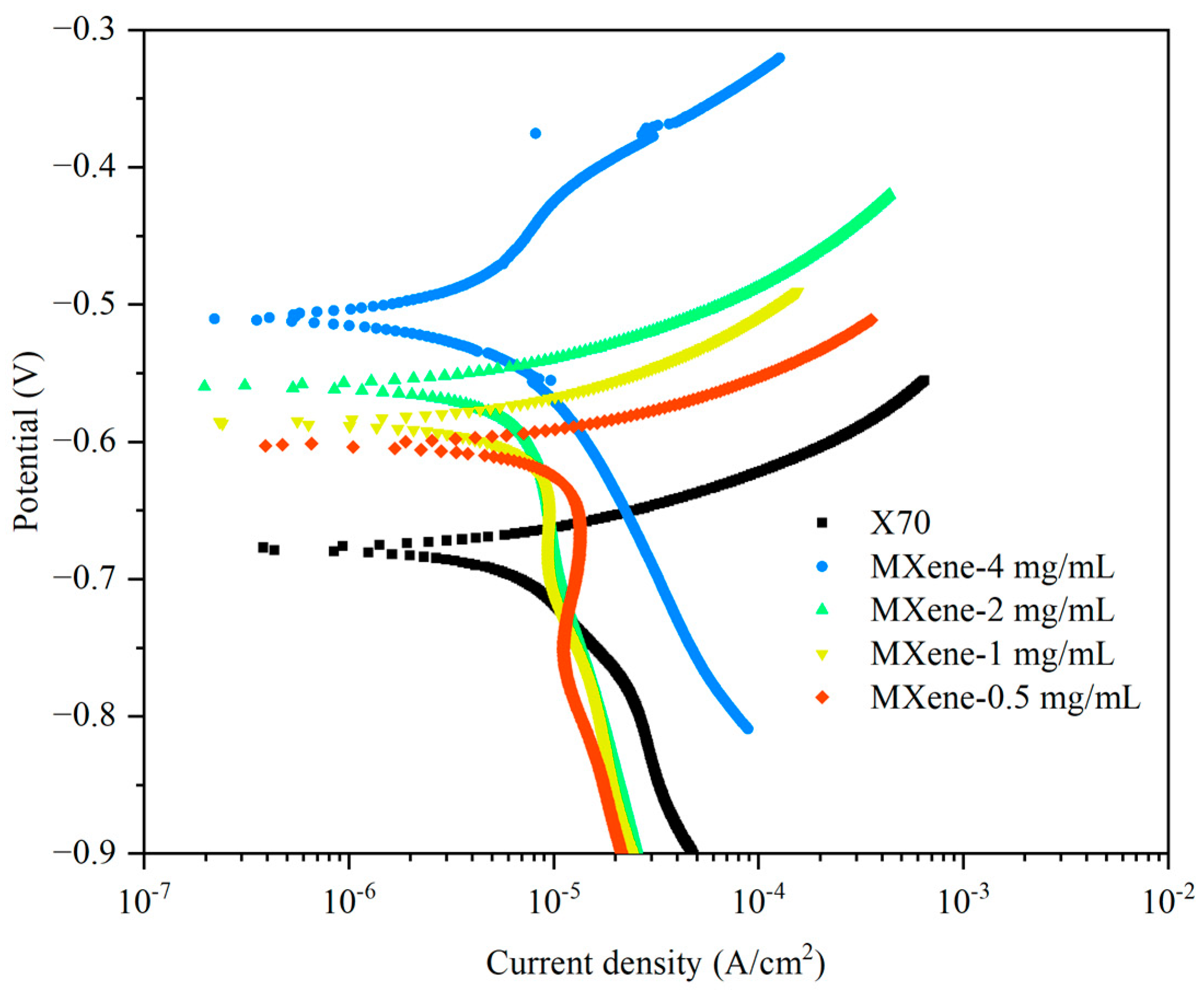
3.3. Corrosion Resistance
3.4. Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, S.J.; Kim, K.Y. An Overview on Hydrogen Uptake, Diffusion and Transport Behavior of Ferritic Steel, and Its Susceptibility to Hydrogen Degradation. Corros. Sci. Technol. 2017, 16, 209–225. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Lee, Y.-K.; Raabe, D.; Tsuzaki, K. Overview of Hydrogen Embrittlement in High-Mn Steels. Int. J. Hydrog. Energy 2017, 42, 12706–12723. [Google Scholar] [CrossRef]
- Lakdhar, I.; Alhussein, A.; Capelle, J.; Creus, J. Al-Ti-W alloys deposited by magnetron sputtering: Effective barrier to prevent steel hydrogen embrittlement. Appl. Surf. Sci. 2021, 567, 150786. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Xiang, Q.-Y.; Yan, K.; Yao, W.-Q.; Cao, J.-L. Study on influence factors of permeation reduction factor of Al2O3 -hydrogen isotopes permeation barriers. Int. J. Hydrogen Energy 2016, 41, 4326–4331. [Google Scholar] [CrossRef]
- Wu, Y.; He, D.; Li, S.; Yang, Y.; Liu, X.; Wang, S.; Jiang, L. Effect of microstructure change on the deuterium permeation properties of erbium oxide coatings prepared via MOCVD. Int. J. Hydrogen Energy 2016, 41, 431–438. [Google Scholar] [CrossRef]
- Wang, J.; Ling, Y.; Lu, Z.; Zhang, M.; Zhou, Q.; Wang, R.; Li, Y.; Zhang, Z. Hydrogen interaction characteristics of a Cr2O3-Y2O3 coating formed on stainless steel in an ultra-low oxygen environment. Int. J. Hydrogen Energy 2019, 44, 8669–8679. [Google Scholar] [CrossRef]
- Yao, Z.; Suzuki, A.; Levchuk, D.; Terai, T. SiC Coating by RF Sputtering as Tritium Permeation Barrier for Fusion Blanket. Fusion Sci. Technol. 2017, 52, 865–869. [Google Scholar] [CrossRef]
- Kalin, B.; Yakushin, V.; Fomina, E. Tritium barrier development for austenitic stainless steel by its aluminizing in a lithium melt. Fusion Eng. Des. 1998, 41, 119–127. [Google Scholar] [CrossRef]
- Seel, M.; Pandey, R. Proton and hydrogen transport through two-dimensional monolayers. 2D Mater. 2016, 3, 025004. [Google Scholar] [CrossRef]
- Young, K.T.; Smith, C.; Krentz, T.M.; Hitchcock, D.A.; Vogel, E.M. Graphene synthesized by chemical vapor deposition as a hydrogen isotope permeation barrier. Carbon 2021, 176, 106–117. [Google Scholar] [CrossRef]
- Bull, S.K.; Champ, T.A.; Raj, S.V.; O’Brien, R.C.; Musgrave, C.B.; Weimer, A.W. Atomic layer deposited boron nitride nanoscale films act as high temperature hydrogen barriers. Appl. Surf. Sci. 2021, 565, 150428. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Kumar, J.P.; Natarajan, R.; Vasseghian, Y.; Saravanan, P.; Manivasagan, R. Methods of synthesis, characteristics, and environmental applications of Mxene: A comprehensive review. Chemosphere 2021, 286, 131607. [Google Scholar] [CrossRef]
- Montazeri, K.; Currie, M.; Verger, L.; Dianat, P.; Barsoum, M.W.; Nabet, B. Beyond Gold: Spin-Coated Ti3C2-Based MXene Photodetectors. Adv. Mater. 2019, 31, 1903271. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.-X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, G.; Ji, Y.; Liu, Q.; Cheng, L.; Guan, K.; Zhang, M.; Liu, G.; Xiong, J.; Yang, J. 2D MXene nanofilms with tunable gas transport channels. Adv. Funct. Mater. 2018, 28, 1801511. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Wang, S.; Meng, X.; Zhang, W.; Jin, Y.; Yang, N.; Tan, X.; Li, J.; Liu, S. Voltage-enhanced ion sieving and rejection of Pb2+ through a thermally cross-linked two-dimensional MXene membrane. Chem. Eng. J. 2020, 401, 126073. [Google Scholar] [CrossRef]
- Ren, D.; Liu, Y.; Huang, N. Chemical bonding states of TiC films before and after hydrogen ion irradiation. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 630–633. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, S.; Liang, R.; Sun, P.; Hai, Y.; Zhang, L. Thermal-triggered insulating fireproof layers: A novel fire-extinguishing MXene composites coating. Chem. Eng. J. 2020, 391, 123621. [Google Scholar] [CrossRef]
- Zhou, P.; Li, W.; Zhu, X.; Li, Y.; Jin, X.; Chen, J. Graphene Containing Composite Coatings as a Protective Coatings against Hydrogen Embrittlement in Quenching & Partitioning High Strength Steel. J. Electrochem. Soc. 2016, 163, D160–D166. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.Z. Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, X.; Lyu, F.; Zhong, Q.; Hu, H.; Pan, Q.; Zhang, Q. Integrating MXene nanosheets with cobalt-tipped carbon nanotubes for an efficient oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 1281–1286. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Li, H.; Fan, X.; Zhu, M. Ti3C2 MXene nanosheets toward high-performance corrosion inhibitor for epoxy coating. Prog. Org. Coat. 2019, 135, 156–167. [Google Scholar] [CrossRef]
- Matsuyama, M.; Takeuchi, T. Trap and Release of Tritium Formed in Silica and Alumina. J. Nucl. Sci. Technol. 1981, 18, 15–20. [Google Scholar] [CrossRef][Green Version]
- Belonoshko, A.B.; Rosengren, A.; Dong, Q.; Hultquist, G.; Leygraf, C. First-principles study of hydrogen diffusion in α−Al2O3 and liquid alumina. Phys. Rev. B 2004, 69. [Google Scholar] [CrossRef]
- Lee, R.W.; Frank, R.C.; Swets, D.E. Diffusion of Hydrogen and Deuterium in Fused Quartz. J. Chem. Phys. 1962, 36, 1062–1071. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Yang, F.; Shi, Y.; Song, J.; Lai, X. Energetics and diffusion of hydrogen in hydrogen permeation barrier of α-Al2O3/FeAl with two different interfaces. Int. J. Hydrogen Energy 2013, 38, 7550–7560. [Google Scholar] [CrossRef]
- Shan, C.; Wu, A.; Li, Y.; Zhao, Z.; Chen, O.; Huang, Q.; Shi, S. The behaviour of diffusion and permeation of tritium through 316L stainless steel with coating of TiC and TiN + TiC. J. Nucl. Mater. 1992, 191, 221–225. [Google Scholar] [CrossRef]
- Checchetto, R.; Bonelli, M.; Gratton, L.M.; Miotello, A.; Sabbioni, A.; Guzman, L.; Benamati, G. Analysis of the hydrogen permeation properties of TiN-TiC bilayers deposited on martensitic stainless steel. Surf. Coat. Technol. 1996, 83, 40–44. [Google Scholar] [CrossRef]
- Yao, Z.; Hao, J.; Zhou, C.; Shan, C.; Yu, J. The permeation of tritium through 316L stainless steel with multiple coatings. J. Nucl. Mater. 2000, 283, 1287–1291. [Google Scholar] [CrossRef]




| Samples | Breakthrough Time tb/s | Diffusion Coefficient D/cm2·s−1 | Permeation Current It=40,000s/μA·cm−1 | Permeability J/mol·cm−1·s−1 |
|---|---|---|---|---|
| X70 | 110 | 5.94 × 10−6 | 27.5 | 2.85 × 10−5 |
| MXene-4 mg/mL-1 L | 1330 | 4.91 × 10−7 | 9.1 | 9.43 × 10−6 |
| MXene-2 mg/mL-1 L | 1200 | 5.45 × 10−7 | 13.1 | 1.36 × 10−5 |
| MXene-1 mg/mL-1 L | 850 | 7.69 × 10−7 | 12.9 | 1.34 × 10−5 |
| MXene-0.5 mg/mL-1 L | 820 | 7.97 × 10−7 | 23.0 | 2.38 × 10−5 |
| MXene-4 mg/mL-2 L | 1290 | 5.07 × 10−7 | 11.3 | 1.17 × 10−5 |
| MXene-4 mg/mL-3 L | 1120 | 5.84 × 10−7 | 13.5 | 1.40 × 10−5 |
| Samples | Ecorr (V vs. SCE) | Icorr (μA·cm−2) |
|---|---|---|
| X70 | −0.681 | 5.6 |
| MXene-4 mg/mL | −0.511 | 1.5 |
| MXene-2 mg/mL | −0.561 | 3.3 |
| MXene-1 mg/mL | −0.588 | 4.2 |
| MXene-0.5 mg/mL | −0.605 | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Meng, X.; Xiao, S.; Chen, G.; Wu, H.; Zhou, C.; Jiang, S.; Chu, P.K. MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels. Nanomaterials 2021, 11, 2737. https://doi.org/10.3390/nano11102737
Shi K, Meng X, Xiao S, Chen G, Wu H, Zhou C, Jiang S, Chu PK. MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels. Nanomaterials. 2021; 11(10):2737. https://doi.org/10.3390/nano11102737
Chicago/Turabian StyleShi, Kejun, Xinyu Meng, Shu Xiao, Guohua Chen, Hao Wu, Chilou Zhou, Saihua Jiang, and Paul K. Chu. 2021. "MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels" Nanomaterials 11, no. 10: 2737. https://doi.org/10.3390/nano11102737
APA StyleShi, K., Meng, X., Xiao, S., Chen, G., Wu, H., Zhou, C., Jiang, S., & Chu, P. K. (2021). MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels. Nanomaterials, 11(10), 2737. https://doi.org/10.3390/nano11102737










