Selective Thermal Transformation of Automotive Shredder Residues into High-Value Nano Silicon Carbide
Abstract
:1. Introduction
2. Material and Methods
2.1. Raw Materials
2.2. Experimental
3. Results and Discussion
3.1. Characterisation of Raw Materials
3.2. Thermal Transformation of the Waste Glass and Mixed Plastics of ASR
3.3. Formation Mechanism of the SiC Nanospheres
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Report, N.W. National Waste Report 2018–2019; Department of the Environment and Energy: Victoria, Australia, 2018.
- Jody, B.; Daniels, E.; Duranceau, C.; Pomykala, J.; Spangenberger, J. End-of-Life Vehicle Recycling: State of the Art of Resource Recovery from Shredder Residue; Argonne National Lab. (ANL): Argonne, IL, USA, 2011.
- GHK; BIOIS. A Study to Examine the Benefits of the End of Life Vehicles Directive and the Costs and Benefits of a Revision of the 2015 Targets for Recycling, Re-Use and Recovery under the ELV Directive; GHK in association with Bio Intelligence Service: Birmingham, AL, USA, 2006. [Google Scholar]
- Cossu, R.; Lai, T. Automotive shredder residue (ASR) management: An overview. Waste Manag. 2015, 45, 143–151. [Google Scholar] [CrossRef]
- Nourreddine, M. Recycling of auto shredder residue. J. Hazard. Mater. 2007, 139, 481–490. [Google Scholar] [CrossRef]
- Taylor, R.; Ray, R.; Chapman, C. Advanced thermal treatment of auto shredder residue and refuse derived fuel. Fuel 2013, 106, 401–409. [Google Scholar] [CrossRef]
- Hossain, R.; Al Mahmood, A.; Sahajwalla, V. Recovering renewable carbon materials from automotive shredder residue (ASR) for micro-supercapacitor electrodes. J. Clean. Prod. 2021, 304, 127131. [Google Scholar] [CrossRef]
- Committee, L.A.E. Where There’s Muck There’s Brass–Waste to Energy Schemes in London; Greater London Authority: London, UK, 2009. [Google Scholar]
- Heermann, C.; Schwager, F.; Whiting, K. Pyrolysis & Gasification of Waste. A Worldwide Technology & Business Review, 2nd ed.; Juniper Consultancy Services Ltd.: Dursley, UK, 2001. [Google Scholar]
- Biswal, B.; Kumar, S.; Singh, R.K. Production of Hydrocarbon Liquid by Thermal Pyrolysis of Paper Cup Waste. J. Waste Manag. 2013, 2013, 731858. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sarkar, A. Reaction Kinetics and Product Distribution of Slow Pyrolysis of Indian Textile Wastes. Int. J. Chem. React. Eng. 2012, 10. [Google Scholar] [CrossRef]
- Carrott, P.; Nabais, J.M.V.; Carrott, M.R.; Pajares, J. Preparation of activated carbon fibres from acrylic textile fibres. Carbon 2001, 39, 1543–1555. [Google Scholar] [CrossRef]
- Hossain, A.; Davies, P. Pyrolysis liquids and gases as alternative fuels in internal combustion engines—A review. Renew. Sustain. Energy Rev. 2013, 21, 165–189. [Google Scholar] [CrossRef]
- Nabais, J.V.; Carrott, P.; Carrott, M.R. From commercial textile fibres to activated carbon fibres: Chemical transformations. Mater. Chem. Phys. 2005, 93, 100–108. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Activated carbons from acrylic textile waste. J. Anal. Appl. Pyrolysis 2010, 89, 51–59. [Google Scholar] [CrossRef]
- Quek, A.; Balasubramanian, R. Liquefaction of waste tires by pyrolysis for oil and chemicals—A review. J. Anal. Appl. Pyrolysis 2013, 101, 1–16. [Google Scholar] [CrossRef]
- Williams, P. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, R.; Sahajwalla, V. Molecular recycling: A key approach to tailor the waste recycling for high-value nano silicon carbide. J. Clean. Prod. 2021, 316, 128344. [Google Scholar] [CrossRef]
- Braslaw, J.; Melotik, D.; Gealer, R.; Wingfield, R., Jr. Hydrocarbon generation during the inert gas pyrolysis of automobile shredder waste. Thermochim. Acta 1991, 186, 1–18. [Google Scholar] [CrossRef]
- Day, M.; Cooney, J.; Shen, Z. Pyrolysis of automobile shredder residue: An analysis of the products of a commercial screw kiln process. J. Anal. Appl. Pyrolysis 1996, 37, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Day, M.; Shen, Z.; Cooney, J. Pyrolysis of auto shredder residue: Experiments with a laboratory screw kiln reactor. J. Anal. Appl. Pyrolysis 1999, 51, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Galvagno, S.; Fortuna, F.; Cornacchia, G.; Casu, S.; Coppola, T.; Sharma, V. Pyrolysis process for treatment of automobile shredder residue: Preliminary experimental results. Energy Convers. Manag. 2001, 42, 573–586. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A. Vacuum pyrolysis of automobile shredder residues. Resour. Conserv. Recycl. 2001, 32, 1–27. [Google Scholar] [CrossRef]
- Shen, Z.; Day, M.; Cooney, J.; Lu, G.; Briens, C.; Bergougnou, M. Ultrapyrolysis of automobile shredder residue. Can. J. Chem. Eng. 1995, 73, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Pasel, C.; Wanzl, W. Experimental investigations on reactor scale-up and optimisation of product quality in pyrolysis of shredder waste. Fuel Process. Technol. 2003, 80, 47–67. [Google Scholar] [CrossRef] [Green Version]
- Zolezzi, M.; Nicolella, C.; Ferrara, S.; Iacobucci, C.; Rovatti, M. Conventional and fast pyrolysis of automobile shredder residues (ASR). Waste Manag. 2004, 24, 691–699. [Google Scholar] [CrossRef]
- Marco, I.d.; Caballero, B.; Torres, A.; Laresgoiti, M.F.; Chomon, M.J.; Cabrero, M.A. Recycling polymeric wastes by means of pyrolysis. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2002, 77, 817–824. [Google Scholar] [CrossRef]
- Balakrishnan, V.; Phan, H.-P.; Dinh, T.; Dao, D.V.; Nguyen, N.-T. Thermal Flow Sensors for Harsh Environments. Sensors 2017, 17, 2061. [Google Scholar] [CrossRef] [Green Version]
- Ramrakhiani, M. Nanostructures and their applications. Recent Res. Sci. Technol. 2012, 4, 14–19. [Google Scholar]
- Castelletto, S.; Johnson, B.C.; Boretti, A. Quantum Effects in Silicon Carbide Hold Promise for Novel Integrated Devices and Sensors. Adv. Opt. Mater. 2013, 1, 609–625. [Google Scholar] [CrossRef]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.-P.; Dao, D.V.; Nakamura, K.; Dimitrijev, S.; Nguyen, N.-T. The Piezoresistive Effect of SiC for MEMS Sensors at High Temperatures: A Review. J. Microelectromech. Syst. 2015, 24, 1663–1677. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, G.; Lanig, P.; Theis, D.; Weyrich, C. Single crystal growth of SiC substrate material for blue light emitting diodes. IEEE Trans. Electron Devices 1983, 30, 277–281. [Google Scholar] [CrossRef]
- Wright, N.G.; Horsfall, A.B.; Vassilevski, K. Prospects for SiC electronics and sensors. Mater. Today 2008, 11, 16–21. [Google Scholar] [CrossRef]
- Oliveros, A.; Guiseppi-Elie, A.; Saddow, S.E. Silicon carbide: A versatile material for biosensor applications. Biomed. Microdevices 2013, 15, 353–368. [Google Scholar] [CrossRef]
- Hatakeyama, F.; Kanzaki, S. Synthesis of Monodispersed Spherical β-Silicon Carbide Powder by a Sol-Gel Process. J. Am. Ceram. Soc. 1990, 73, 2107–2110. [Google Scholar] [CrossRef]
- Seog, I.-S.; Kim, C.H. Preparation of monodispersed spherical silicon carbide by the sol-gel method. J. Mater. Sci. 1993, 28, 3277–3282. [Google Scholar] [CrossRef]
- Qian, J.-M.; Wang, J.-P.; Qiao, G.-J.; Jin, Z.-H. Preparation of porous SiC ceramic with a woodlike microstructure by sol-gel and carbothermal reduction processing. J. Eur. Ceram. Soc. 2004, 24, 3251–3259. [Google Scholar] [CrossRef]
- Guo, J.; Zuo, Y.; Li, Z.; Gao, W.; Zhang, J. Preparation of SiC nanowires with fins by chemical vapor deposition. Phys. E Low-Dimens. Syst. Nanostruct. 2007, 39, 262–266. [Google Scholar] [CrossRef]
- Henderson, E.J.; Veinot, J.G. From phenylsiloxane polymer composition to size-controlled silicon carbide nanocrystals. J. Am. Chem. Soc. 2009, 131, 809–815. [Google Scholar] [CrossRef]
- Zhu, C.W.; Zhao, G.Y.; Revankar, V.; Hlavacek, V. Synthesis of ultra-fine SiC powders in a dc plasma reactor. J. Mater. Sci. 1993, 28, 659–668. [Google Scholar] [CrossRef]
- Guo, J.Y.; Gitzhofer, F.; Boulos, M.I. Induction plasma synthesis of ultrafine SiC powders from silicon and CH4. J. Mater. Sci. 1995, 30, 5589–5599. [Google Scholar] [CrossRef]
- Ramesh, P.; Vaidhyanathan, B.; Ganguli, M.; Rao, K.J.J.o.M.R. Synthesis of β-SiC powder by use of microwave radiation. J. Mater. Res. 1994, 9, 3025–3027. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Braunegg, G.; Hermann, C.; Horvat, P.; Kroutil, M.; Martinz, J.; Neto, J.; Pereira, A.L.; Varila, P. Production of Polyhydroxyalkanoates from Agricultural Waste and Surplus Materials. Biomacromolecules 2005, 6, 561–565. [Google Scholar] [CrossRef]
- Bazargan, A.; McKay, G. A review–synthesis of carbon nanotubes from plastic wastes. Chem. Eng. J. 2012, 195, 377–391. [Google Scholar] [CrossRef]
- Jie, X.; Li, W.; Slocombe, D.; Gao, Y.; Banerjee, I.; Gonzalez-Cortes, S.; Yao, B.; AlMegren, H.; Alshihri, S.; Dilworth, J.; et al. Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons. Nat. Catal. 2020, 3, 902–912. [Google Scholar] [CrossRef]
- Zhang, B.; Piao, G.; Zhang, J.; Bu, C.; Xie, H.; Wu, B.; Kobayashi, N. Synthesis of carbon nanotubes from conventional biomass-based gasification gas. Fuel Process. Technol. 2018, 180, 105–113. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Sahajwalla, V.J.W.M. Characteristics of waste automotive glasses as silica resource in ferrosilicon synthesis. Waste Manag. Res. 2016, 34, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, S.; Mayyas, M.; Sahajwalla, V. Novel Synthesis of Silicon Carbide Nanowires from e-Waste. ACS Sustain. Chem. Eng. 2017, 5, 4171–4178. [Google Scholar] [CrossRef]
- Maroufi, S.; Mayyas, M.; Sahajwalla, V. Waste materials conversion into mesoporous silicon carbide nanocermics: Nanofibre/particle mixture. J. Clean. Prod. 2017, 157, 213–221. [Google Scholar] [CrossRef]
- Rajarao, R.; Farzana, R.; Khanna, R.; Sahajwalla, V. Synthesis of SiC/Si3N4 nanocomposite by using automotive waste tyres as resource. J. Ind. Eng. Chem. 2015, 29, 35–38. [Google Scholar] [CrossRef]
- Mayyas, M.; Pahlevani, F.; Handoko, W.; Sahajwalla, V. Preliminary investigation on the thermal conversion of automotive shredder residue into value-added products: Graphitic carbon and nano-ceramics. Waste Manag. 2016, 50, 173–183. [Google Scholar] [CrossRef]
- Kim, K.-H.; Joung, H.-T.; Nam, H.; Seo, Y.-C.; Hong, J.H.; Yoo, T.-W.; Lim, B.-S.; Park, J.-H. Management status of end-of-life vehicles and characteristics of automobile shredder residues in Korea. Waste Manag. 2004, 24, 533–540. [Google Scholar] [CrossRef]
- Ferrão, P.; Nazareth, P.; Amaral, J. Strategies for Meeting EU End-of-Life Vehicle Reuse/Recovery Targets. J. Ind. Ecol. 2008, 10, 77–93. [Google Scholar] [CrossRef]
- Edo, M.; Aracil, I.; Font, R.; Anzano, M.; Fullana, A.; Collina, E. Viability study of automobile shredder residue as fuel. J. Hazard. Mater. 2013, 260, 819–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, I.; Caballero, B.; Cabrero, M.; Laresgoiti, M.; Torres, A.; Chomón, M. Recycling of automobile shredder residues by means of pyrolysis. J. Anal. Appl. Pyrolysis 2007, 79, 403–408. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M. Py–FTIR–GC/MS Analysis of Volatile Products of Automobile Shredder Residue Pyrolysis. Polymers 2020, 12, 2734. [Google Scholar] [CrossRef] [PubMed]
- Öhman, M.; Persson, D.; Leygraf, C. In situ ATR-FTIR studies of the aluminium/polymer interface upon exposure to water and electrolyte. Prog. Org. Coatings 2006, 57, 78–88. [Google Scholar] [CrossRef]
- Yang, S.; Zhong, F.; Wang, M.; Bai, S.; Wang, Q. Recycling of automotive shredder residue by solid state shear milling technology. J. Ind. Eng. Chem. 2018, 57, 143–153. [Google Scholar] [CrossRef]
- Mayyas, M.; Mayyas, M.; Pahlevani, P.; Liu, Z.; Rajarao, R.; Sahajwalla, V. From automotive shredder residue to nano-ceramics and graphitic carbon—Thermal degradation kinetics. J. Anal. Appl. Pyrolysis 2016, 120, 60–74. [Google Scholar] [CrossRef]
- Choi, M.; Kim, J.-C.; Kim, D.-W. Waste Windshield-Derived Silicon/Carbon Nanocomposites as High-Performance Lithium-Ion Battery Anodes. Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Crystalline Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Dwivedi, N.; Yeo, R.J.; Satyanarayana, N.; Kundu, S.; Tripathy, S.; Bhatia, C.S. Understanding the Role of Nitrogen in Plasma-Assisted Surface Modification of Magnetic Recording Media with and without Ultrathin Carbon Overcoats. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Goohpattader, P.S.; Dwivedi, N.; Rismani-Yazdi, E.; Satyanarayana, N.; Yeo, R.J.; Kundu, S.; Bhatia, C.S. Probing the role of C+ ion energy, thickness and graded structure on the functional and microstructural characteristics of ultrathin carbon films (<2 nm). Tribol. Int. 2015, 81, 73–88. [Google Scholar]
- Liste, S.; González, P.; Serra, J.; Borrajo, J.; Chiussi, S.; León, B.; Pérez-Amor, M.; López, J.G.; Ferrer, F.; Morilla, Y.; et al. Study of the stoichiometry transfer in pulsed laser deposition of bioactive silica-based glasses. Thin Solid Films 2004, 453–454, 219–223. [Google Scholar] [CrossRef]
- An, Z.; Wang, H.; Zhu, C.; Cao, H.; Xue, J. Synthesis and formation mechanism of porous silicon carbide stacked by nanoparticles from precipitated silica/glucose composites. J. Mater. Sci. 2018, 54, 2787–2795. [Google Scholar] [CrossRef]
- Nallathambi, G.; Ramachandran, T.; Rajendran, V.; Palanivelu, R. Effect of silica nanoparticles and BTCA on physical properties of cotton fabrics. Mater. Res. 2011, 14, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Finster, J.; Klinkenberg, E.-D.; Heeg, J.; Braun, W. ESCA and SEXAFS investigations of insulating materials for ULSI microelectronics’. Vacuum 1990, 41, 1586. [Google Scholar] [CrossRef]
- Kaur, A.; Chahal, P.; Hogan, T. Selective Fabrication of SiC/Si Diodes by Excimer Laser Under Ambient Conditions. IEEE Electron Device Lett. 2015, 37, 142–145. [Google Scholar] [CrossRef]
- Galuska, A.A.; Uht, J.C.; Marquez, N. Reactive and nonreactive ion mixing of Ti films on carbon substrates. J. Vac. Sci. Technol. A Vac. Surf. Films 1988, 6, 110–122. [Google Scholar] [CrossRef]
- McKenna, J.; Patel, J.; Mitra, S.; Soin, N.; Svrcek, V.; Maguire, P.; Mariotti, D. Synthesis and surface engineering of nanomaterials by atmospheric-pressure microplasmas. Eur. Phys. J. Appl. Phys. 2011, 56, 24020. [Google Scholar] [CrossRef]
- Lee, K.-H.; Lee, S.-K.; Jeon, K.-S. Photoluminescent properties of silicon carbide and porous silicon carbide after annealing. Appl. Surf. Sci. 2009, 255, 4414–4420. [Google Scholar] [CrossRef]
- Tamayo, A.; Rubio, F.; Mazo, M.A.; Rubio, J. Further characterization of the surface properties of the SiC particles through complementarity of XPS and IGC-ID techniques. Boletín Soc. Española Cerámica Vidr. 2018, 57, 231–239. [Google Scholar] [CrossRef]
- Rajarao, R.; Ferreira, R.; Sadi, S.H.F.; Khanna, R.; Sahajwalla, V. Synthesis of silicon carbide nanoparticles by using electronic waste as a carbon source. Mater. Lett. 2014, 120, 65–68. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, W.; Zhang, J.; Li, Y.; Zhou, G.; Jia, G.J.D. Uniform mesoporous CaSiO3: Eu3+ nanospheres: Template-directed synthesis, luminescence and sustained drug release properties. Dye Pigment. 2017, 136, 427–433. [Google Scholar] [CrossRef]
- Mizokawa, Y.; Geib, K.; Wilmsen, C. Characterization of β-SiC surfaces and the Au/SiC interface. J. Vac. Sci. Technol. A Vac. Surf. Film. 1986, 4, 1696–1700. [Google Scholar] [CrossRef]
- Bachman, B.J.; Vasile, M.J. Ion bombardment of polyimide films. J. Vac. Sci. Technol. A Vac. Surf. Film. 1989, 7, 2709–2716. [Google Scholar] [CrossRef]
- Hijikata, Y.; Yaguchi, H.; Yoshikawa, M.; Yoshida, S.J.A.S.S. Composition analysis of SiO2/SiC interfaces by electron spectroscopic measurements using slope-shaped oxide films. Appl. Surf. Sci. 2001, 184, 161–166. [Google Scholar] [CrossRef]
- Shimoda, K.; Park, J.-S.; Hinoki, T.; Kohyama, A. Influence of surface structure of SiC nano-sized powder analyzed by X-ray photoelectron spectroscopy on basic powder characteristics. Appl. Surf. Sci. 2007, 253, 9450–9456. [Google Scholar] [CrossRef]
- Dua, A.K.; George, V.C.; Agarwala, R.P. aluminum(III) oxide. Thin Solid Film. 1988, 165, 163–172. [Google Scholar] [CrossRef]
- Wagner, C.D.; Zatko, D.A.; Raymond, R.H. magnesium oxide. Anal. Chem. 1980, 52, 1445–1451. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, H.-H.; Chun, J.S.; Lee, W.-J. Compositional and structural analysis of aluminum oxide films prepared by plasma-enhanced chemical vapor deposition. Thin Solid Film. 1994, 237, 57–65. [Google Scholar] [CrossRef]
- Pahlevani, F.; Dippenaar, R.; Gorjizadeh, N.; Cholake, S.T.; Hossain, R.; Kumar, R.; Sahajwalla, V. Surface Modification of Steel Using Automotive Waste as Raw Materials. Procedia Manuf. 2017, 7, 387–394. [Google Scholar] [CrossRef]
- Hagio, T.; Takase, A.; Umebayashi, S. X-ray photoelectron spectroscopic studies of β-sialons. J. Mater. Sci. Lett. 1992, 11, 878–880. [Google Scholar] [CrossRef]
- Huang, H.-H.; Shih, W.-C.; Lai, C.-H. Nonpolar resistive switching in the Pt/MgO/Pt nonvolatile memory device. Appl. Phys. Lett. 2010, 96, 193505. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Kishi, K.; Miyoshi, H. XPS studies of NaCl deposited on Cu (100) and Ni/Cu (100) surfaces. J. Electron Spectrosc. Relat. Phenom. 1991, 53, 237–249. [Google Scholar] [CrossRef]
- Wagner, C.D. Chapter 7. In Handbook of X-ray and Ultraviolet Photoelectron Spectroscopy; Briggs, D., Ed.; Heyden & Son Ltd.: London, UK, 1977. [Google Scholar]
- Jayaramulu, K.; Geyer, F.; Petr, M.; Zboril, R.; Vollmer, D.; Fischer, R.A. Shape controlled hierarchical porous hydrophobic/oleophilic metal-organic nanofibrous gel composites for oil adsorption. Adv. Mater. 2017, 29, 1605307. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.-N.; Zheng, Y.; Zhan, Y.-Y.; Cai, G.-H.; Xiao, Y.-H. Synthesis of porous SiC and application in the CO oxidation reaction. Mater. Lett. 2007, 61, 4766–4768. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Wang, L.; Cheng, Q.; Qin, H.; Li, Z.; Lou, Z.; Lu, J.; Zhang, J.; Zhou, Q. Synthesis of silicon carbide nanocrystals from waste polytetrafluoroethylene. Dalton Trans. 2017, 46, 2756–2759. [Google Scholar] [CrossRef]
- Shcherban, N.D. Review on synthesis, structure, physical and chemical properties and functional characteristics of porous silicon carbide. J. Ind. Eng. Chem. 2017, 50, 15–28. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, S.H.; Song, I.H.; Kim, H.D.; Park, C.B. Fabrication of open-cell, microcellular silicon carbide ceramics by carbothermal reduction. J. Am. Ceram. Soc. 2005, 88, 2949–2951. [Google Scholar] [CrossRef]
- Kim, Y.W.; Eom, J.H.; Wang, C.; Park, C.B. Processing of porous silicon carbide ceramics from carbon-filled polysiloxane by extrusion and carbothermal reduction. J. Am. Ceram. Soc. 2008, 91, 1361–1364. [Google Scholar] [CrossRef]
- Petzow, G.; Herrmann, M. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2002; Volume 102. [Google Scholar]
- Zhang, Y.; Shi, E.-W.; Chen, Z.-Z.; Li, X.-B.; Xiao, B. Large-scale fabrication of silicon carbide hollow spheres. J. Mater. Chem. 2006, 16, 4141–4145. [Google Scholar] [CrossRef]
- Krstic, V.D. Production of fine, high-purity beta silicon carbide powders. J. Am. Ceram. Soc. 1992, 75, 170–174. [Google Scholar] [CrossRef]
- Wei, G.C.T. Beta SiC Powders Produced by Carbothermic Reduction of Silica in a High-Temperature Rotary Furnace. J. Am. Ceram. Soc. 1983, 66, c111–c113. [Google Scholar] [CrossRef]
- Filsinger, D.H.; Bourrie, D.B. Silica to silicon: Key carbothermic reactions and kinetics. J. Am. Ceram. Soc. 1990, 73, 1726–1732. [Google Scholar] [CrossRef]
- Yang, J.-F.; Zhang, G.-J.; Kondo, N.; She, J.-H.; Jin, Z.-H.; Ohji, T.; Kanzaki, S. Porous 2H-Silicon Carbide Ceramics Fabricated by Carbothermal Reaction between Silicon Nitride and Carbon. J. Am. Ceram. Soc. 2003, 86, 910–914. [Google Scholar] [CrossRef]
- Carroll, D.F.; Weimer, A.W.; Dunmead, S.D.; Eisman, G.A.; Hwang, J.H.; Cochran, G.A.; Susnitzky, D.W.; Beaman, D.R.; Conner, C.L. Carbothermally prepared nanophase SiC/ Si3N4 composite powders and densified parts. AIChE J. 1997, 43, 2624–2635. [Google Scholar] [CrossRef]

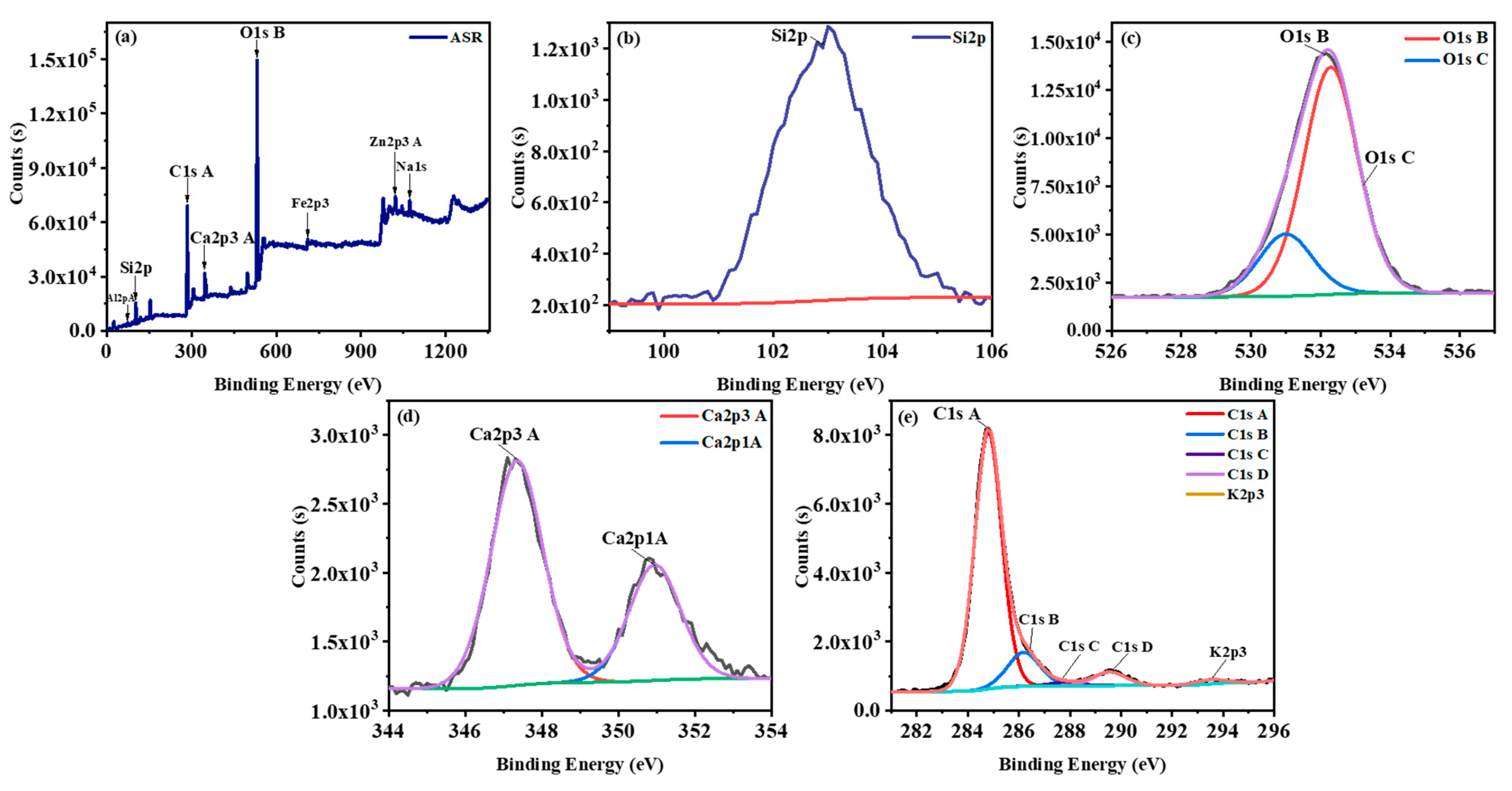
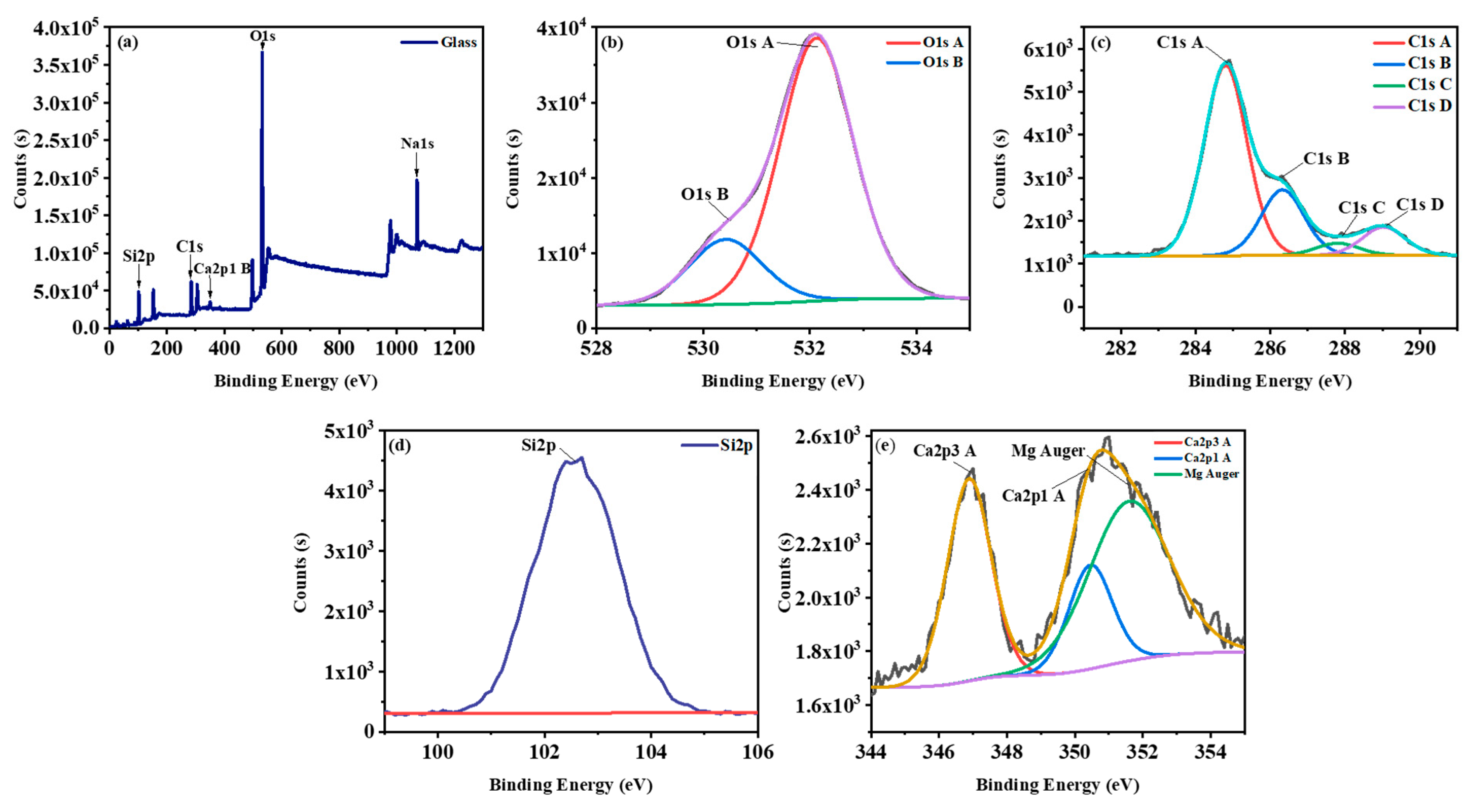

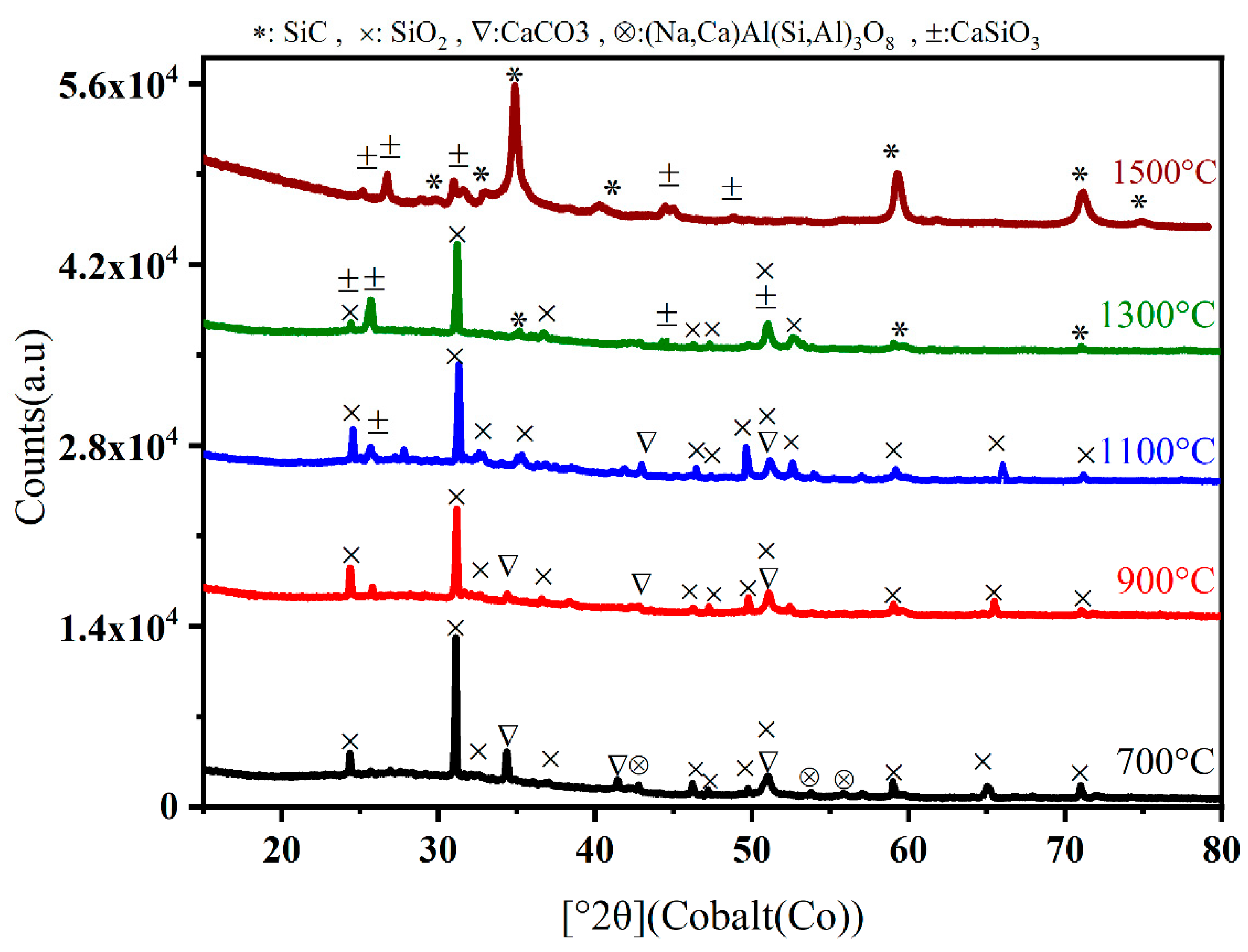
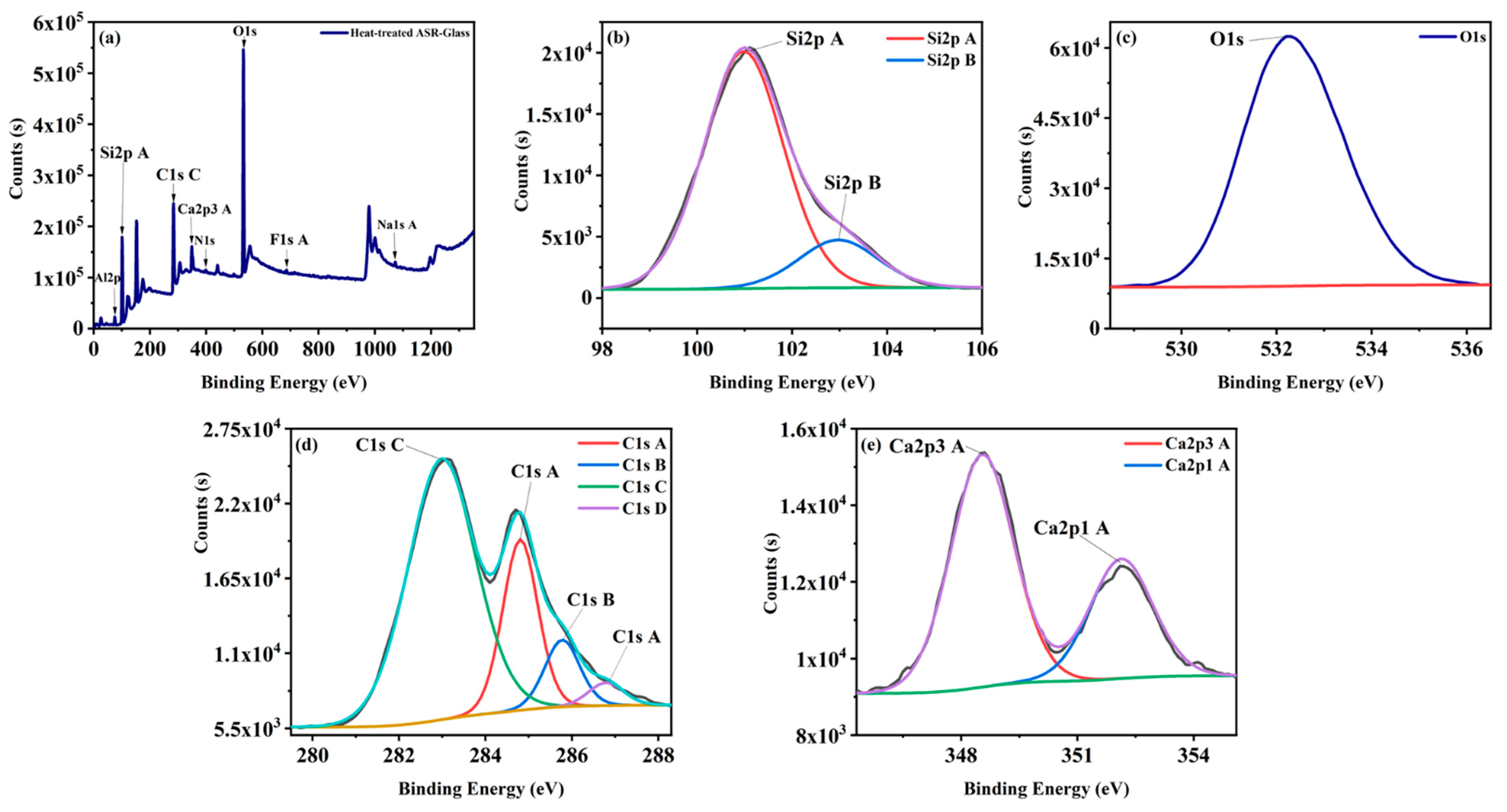
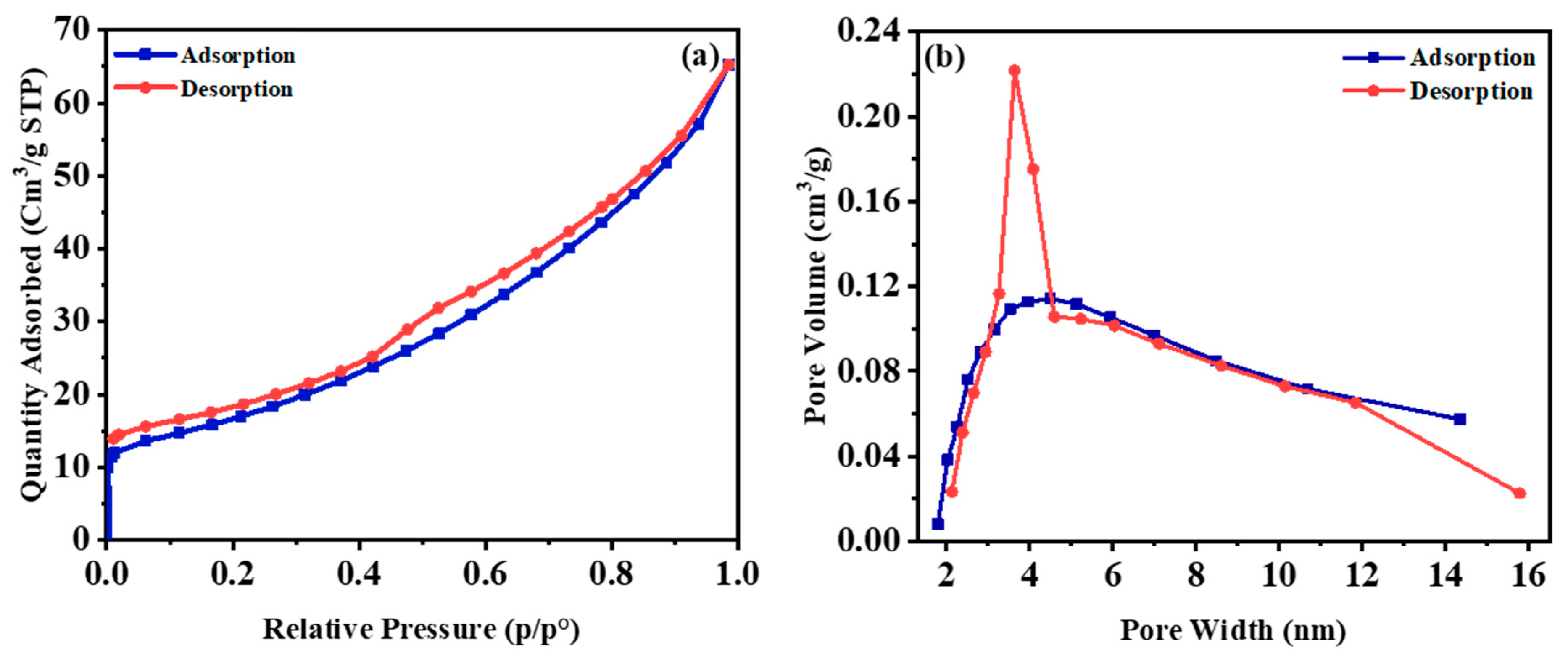
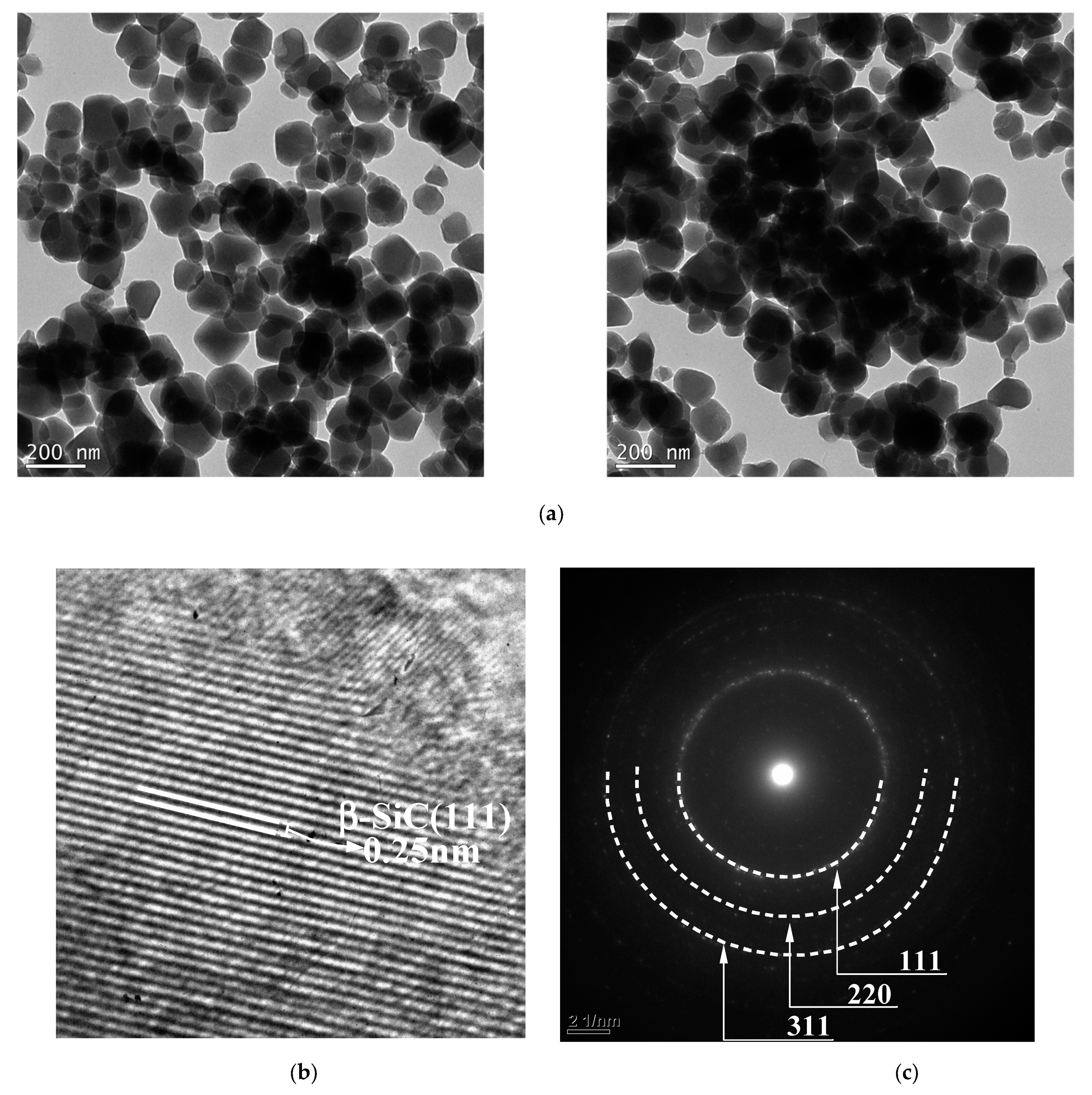
| Oxide | (wt%) |
|---|---|
| SiO2 | 71.25 |
| Na2O | 15.50 |
| CaO | 8.30 |
| MgO | 3.73 |
| Al2O3 | 0.47 |
| Fe2O3 | 0.46 |
| K2O | 0.13 |
| TOTAL | 99.84 |
| N (%) | C (%) | H (%) | S (%) | O (%) |
|---|---|---|---|---|
| 0.72 | 25.74 | 3.002 | 0.258 | 11.66 |
| Name | Peak BE | Atomic% | Possible Compounds | Ref. |
|---|---|---|---|---|
| Si2p A | 101 | 29.96 | SiC | [70,71] |
| Si2P B | 102.5 | 5.34 | SiOC/CaSiO3 | [69,76] |
| C1s C | 283 | 22 | SiC | [71,73,77] |
| C1s A | 284.8 | 7.46 | C | [72,78] |
| O1s | 532 | 28.86 | SiOC/MgO/Al2O3/CaSiO3 | [75,76,79,80,81,82] |
| Ca2p3 A | 347.54 | 1.84 | CaSiO3 | [76] |
| Al2p | 75 | 3.88 | Al2O3 | [83,84,85] |
| Mg2p | 51 | 0.10 | MgO | [86] |
| N1s | 399 | 0.13 | pyridinic N | [87] |
| Na1s A | 1073 | 0.23 | NaCl/Cu | [88] |
| F1s A | 686 | 0.2 | Na2SiF6 | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemati, S.; Hossain, R.; Sahajwalla, V. Selective Thermal Transformation of Automotive Shredder Residues into High-Value Nano Silicon Carbide. Nanomaterials 2021, 11, 2781. https://doi.org/10.3390/nano11112781
Hemati S, Hossain R, Sahajwalla V. Selective Thermal Transformation of Automotive Shredder Residues into High-Value Nano Silicon Carbide. Nanomaterials. 2021; 11(11):2781. https://doi.org/10.3390/nano11112781
Chicago/Turabian StyleHemati, Sepideh, Rumana Hossain, and Veena Sahajwalla. 2021. "Selective Thermal Transformation of Automotive Shredder Residues into High-Value Nano Silicon Carbide" Nanomaterials 11, no. 11: 2781. https://doi.org/10.3390/nano11112781
APA StyleHemati, S., Hossain, R., & Sahajwalla, V. (2021). Selective Thermal Transformation of Automotive Shredder Residues into High-Value Nano Silicon Carbide. Nanomaterials, 11(11), 2781. https://doi.org/10.3390/nano11112781









