Dehydrogenation of Cycloalkanes over N-Doped Carbon-Supported Catalysts: The Effects of Active Component and Molecular Structure of the Substrate
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.1.1. Synthesis of the Nitrogen-Doped Carbon Support (CN)
2.1.2. Preparation of M/CN Catalysts
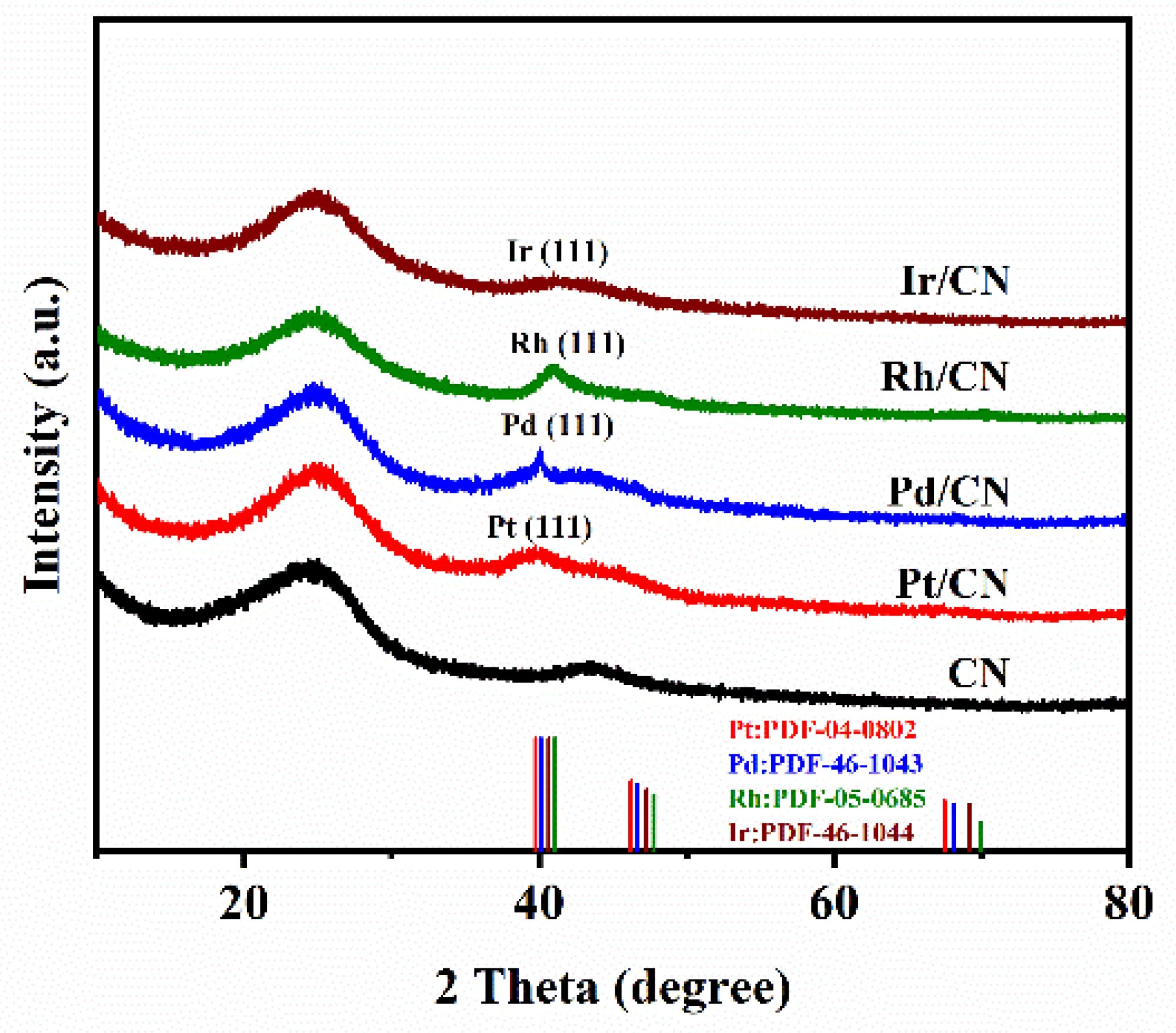
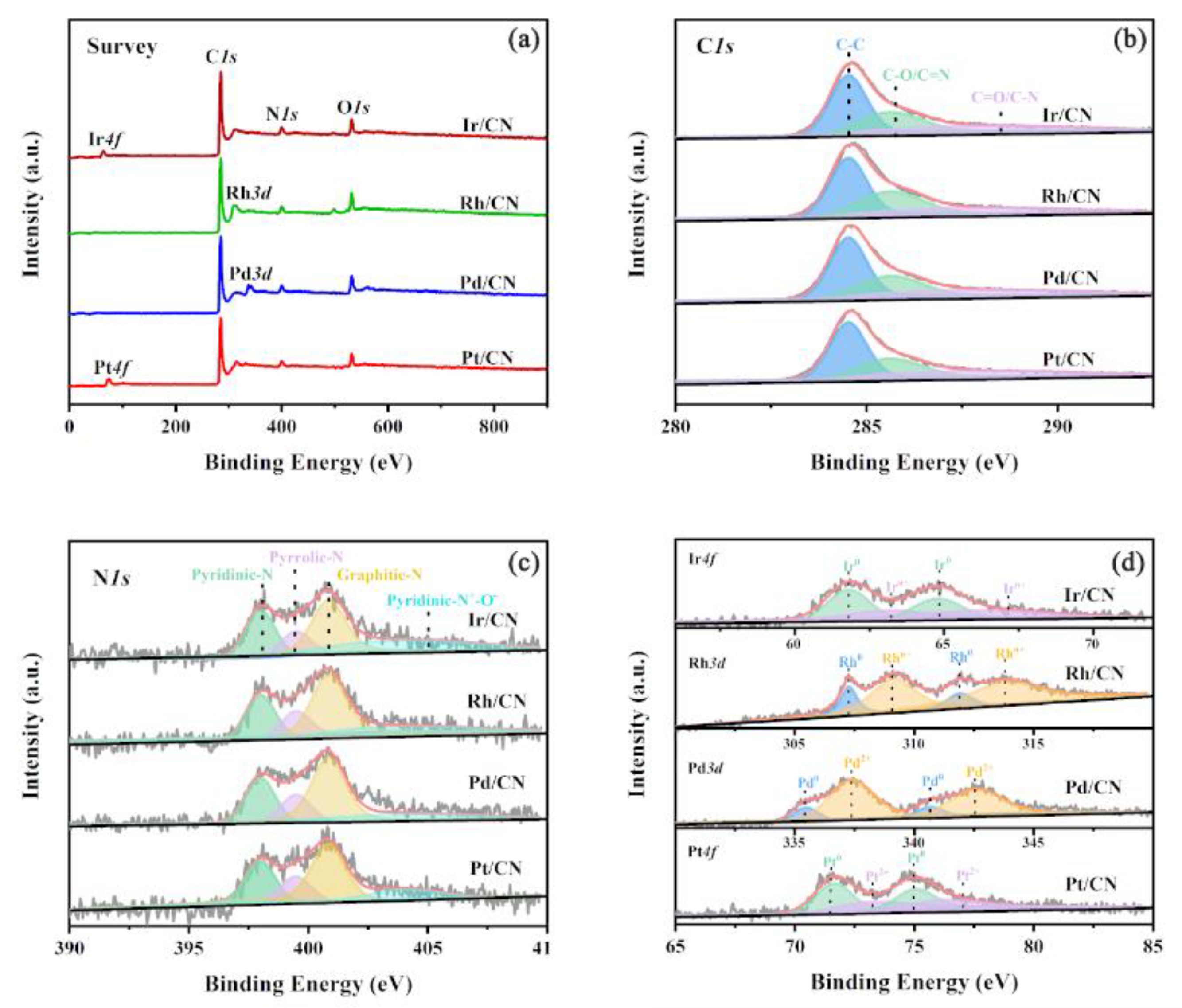
2.2. Characterization of Catalysts
2.3. Catalytic Testing
3. Results and Discussion
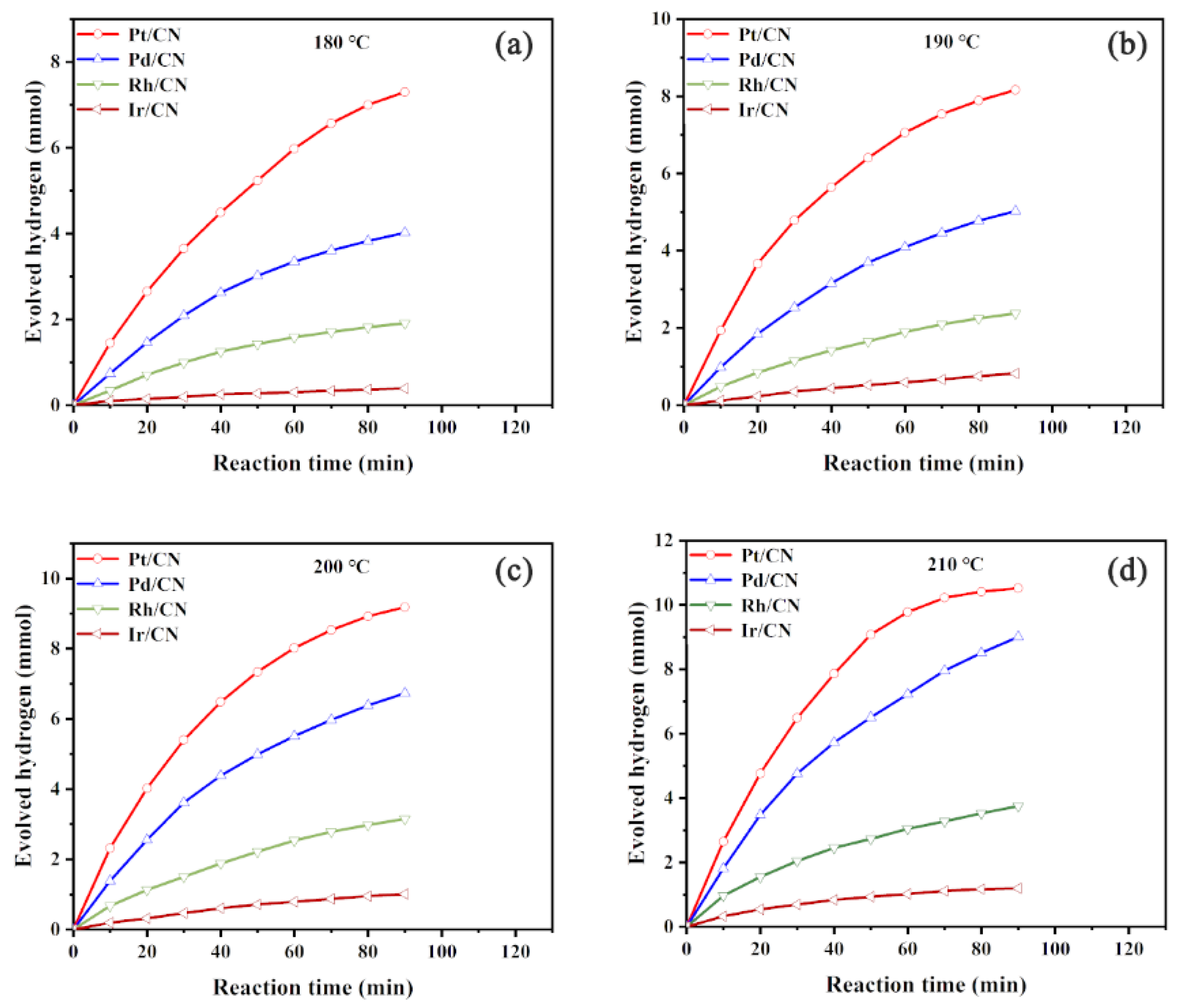
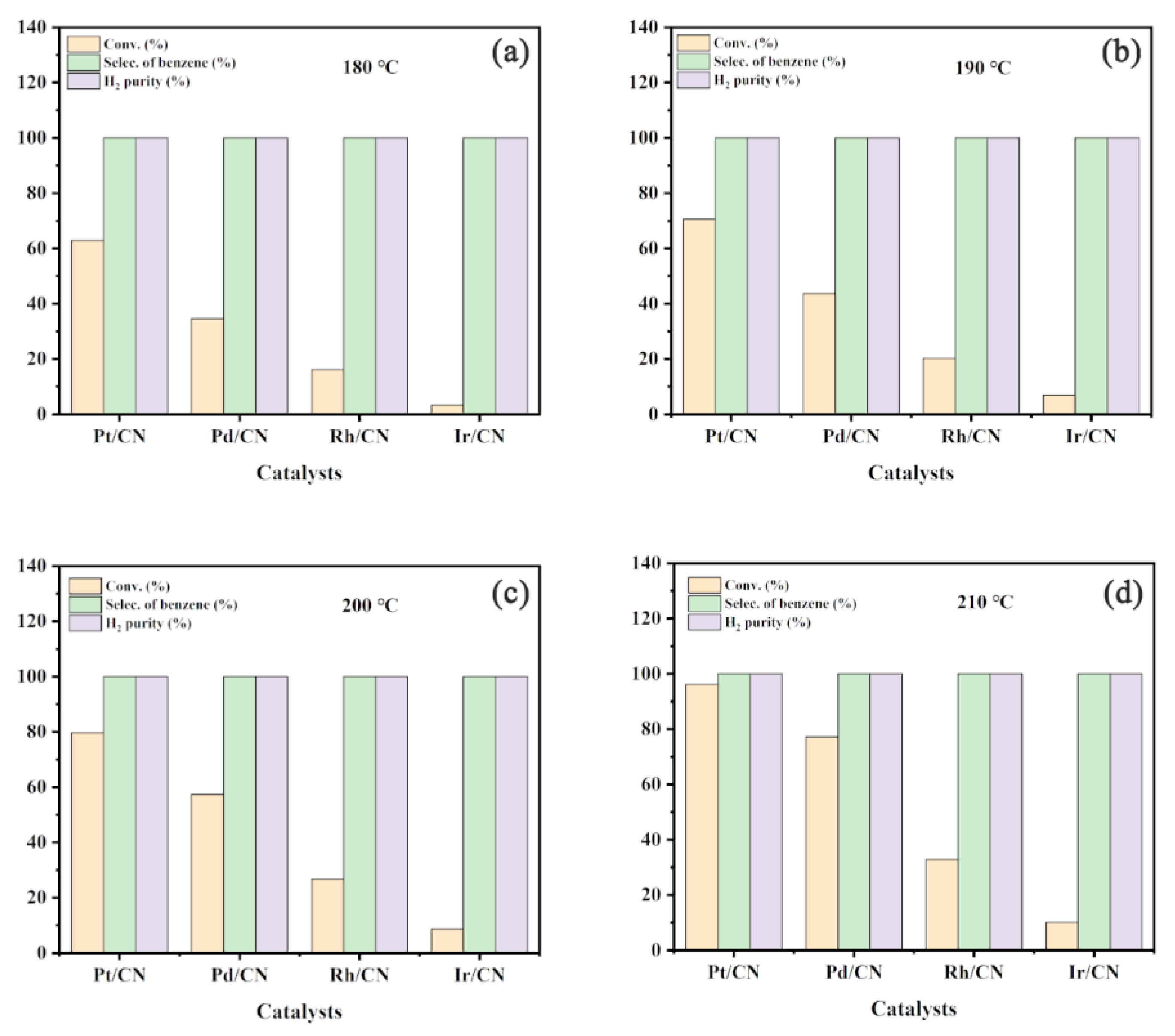
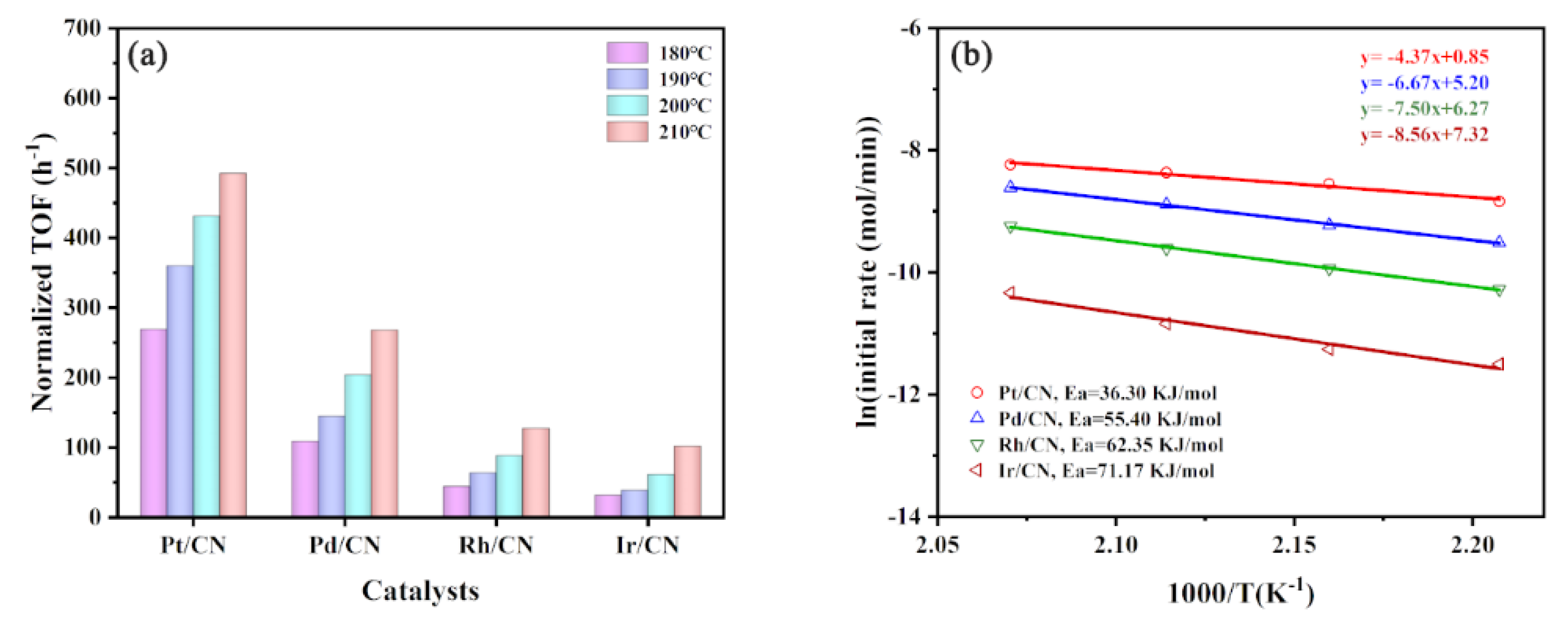
3.1. Effect of Metals
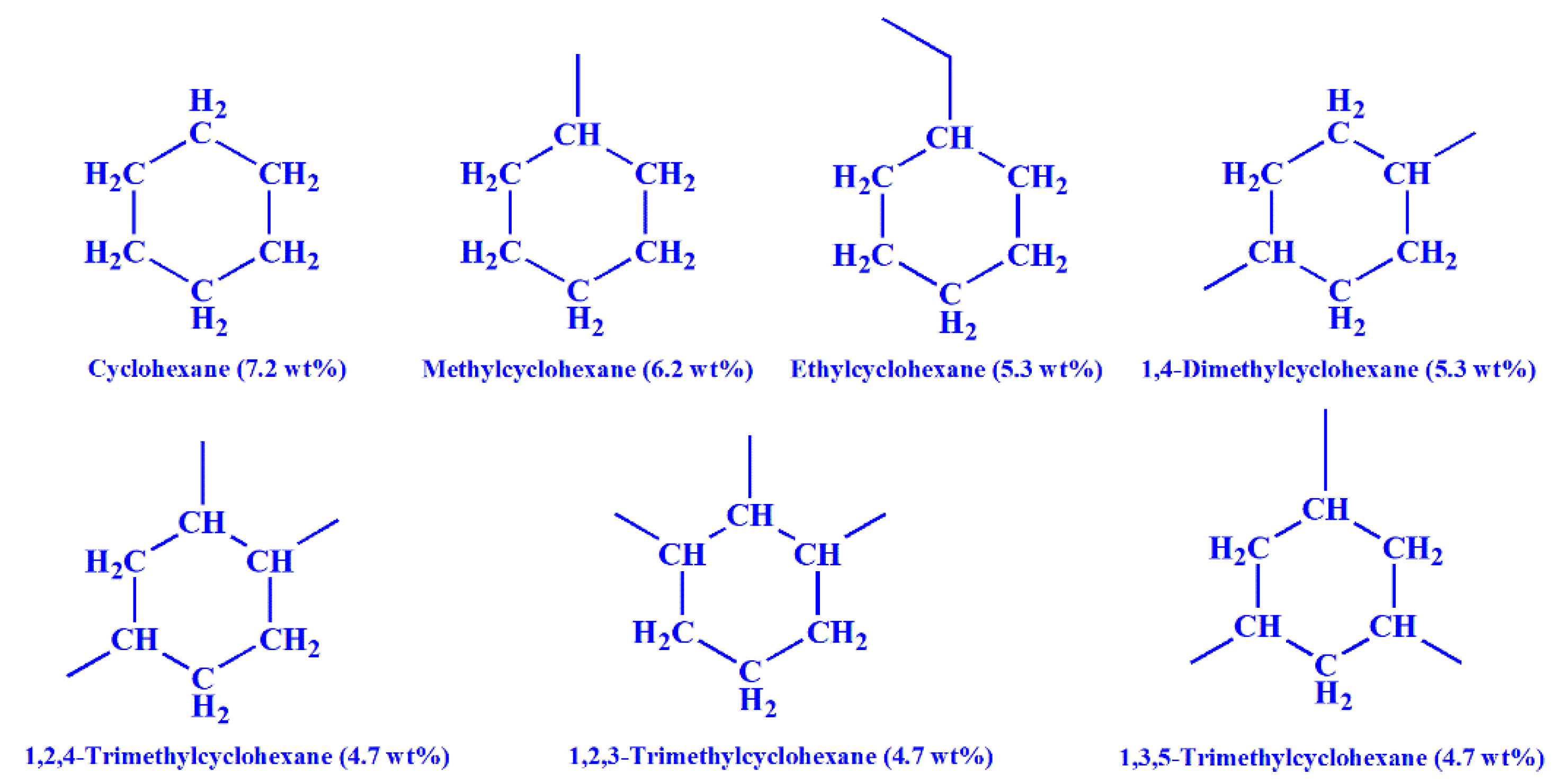
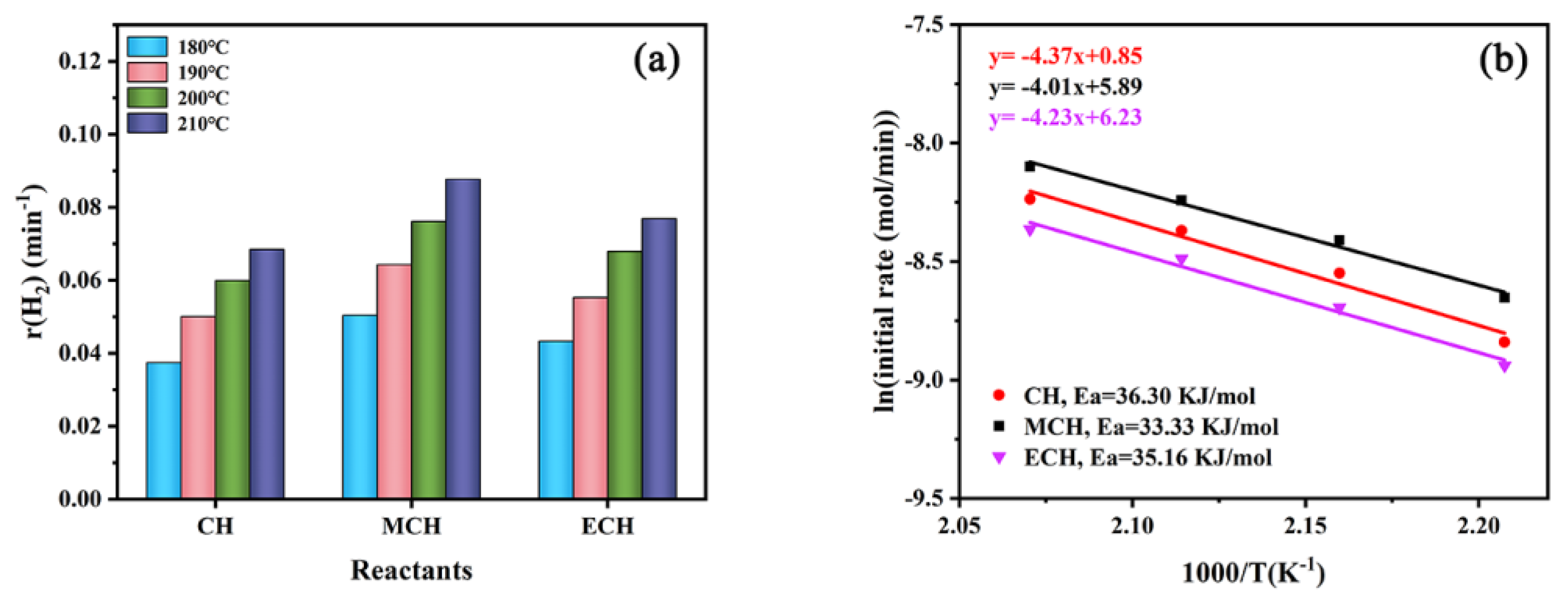
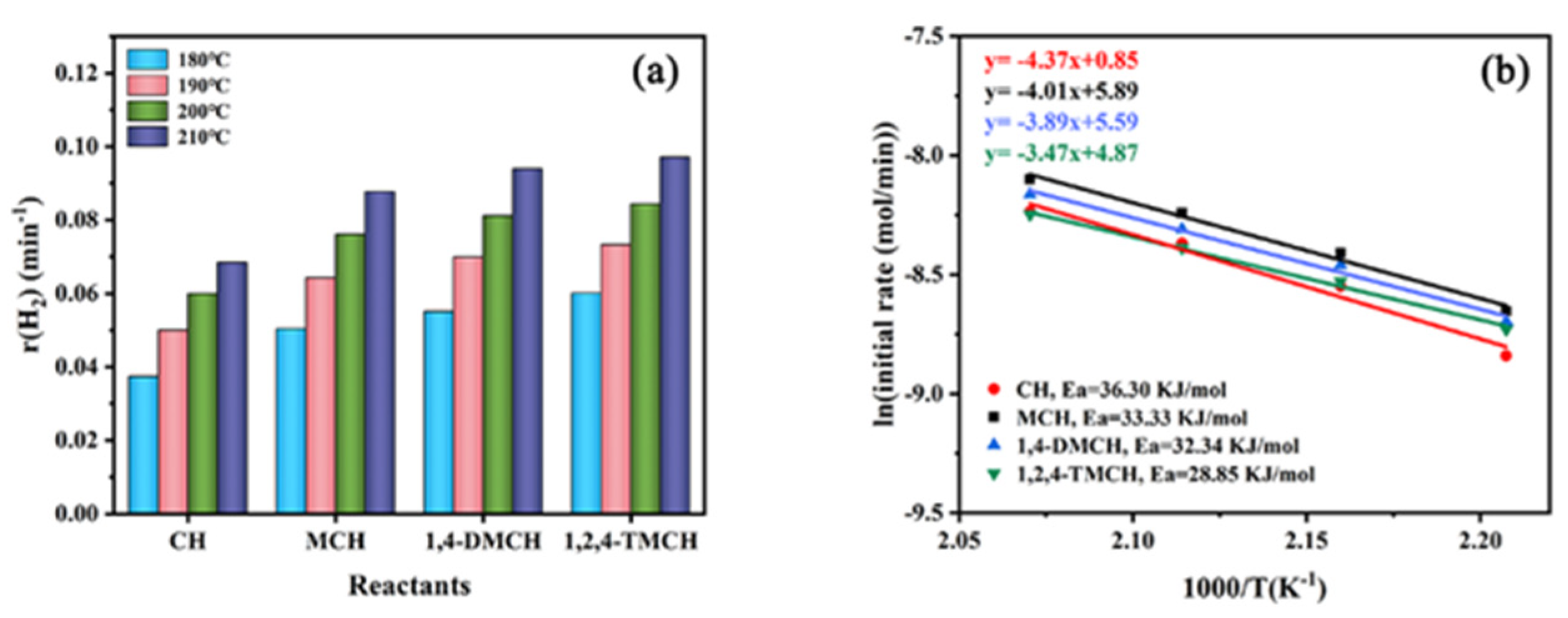
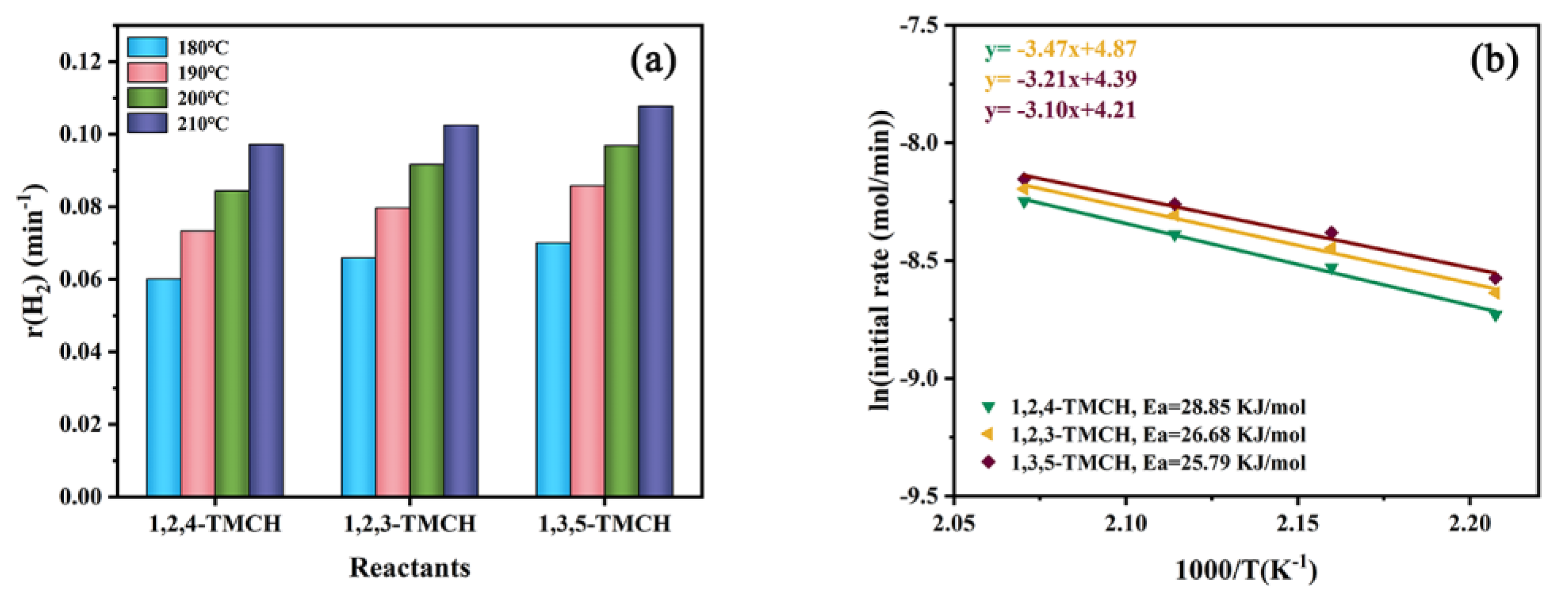
3.2. Effect of Molecular Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Patni, A.N.; Mantri, A.S.; Debashis, K. Ionic liquid promoted dehydrogenation of amine boranes: A review. Int. J. Hydrogen Energy 2021, 46, 11761–11781. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Nakayama, J.; Kasai, N.; Shibutani, T.; Miyake, A. Security risk analysis of a hydrogen fueling station with an on-site hydrogen production system involving methylcyclohexane. Int. J. Hydrogen Energy 2019, 44, 9110–9119. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, M.; Chen, X.; Dong, Y.; Zhang, Z.; Cheng, H. A highly active bifunctional Ru-Pd catalyst for hydrogenation and dehydrogenation of liquid organic hydrogen carriers. J. Catal. 2019, 378, 382–391. [Google Scholar] [CrossRef]
- Chen, H.; Shuang, H.; Lin, W.; Li, X.; Zhang, Z.; Li, J.; Fu, J. Tuning interfacial electronic properties of palladium oxide on vacancy-abundant carbon nitride for low-temperature dehydrogenation. ACS Catal. 2021, 11, 6193–6199. [Google Scholar] [CrossRef]
- Li, X.; Shen, P.; Han, X.; Wang, Y.; Zhu, Y.; Wu, Z. Dehydrogenation mechanisms of liquid organic hydrogen carriers over Pt, Pd, Rh, and Ni surfaces: Cyclohexane as a model compound. Appl. Surf. Sci. 2021, 543, 148769. [Google Scholar] [CrossRef]
- Marella, R.K.; Madduluri, V.R.; Yu, T.; Venkateswarlu, K.; Kumar, J.S.; Sreenivasan, M.; Lakkaboyana, S.K. Highly active biomorphic MgO/C supported Cu NPs direct catalytic coupling of 1,4-butanediol dehydrogenation and acetophenone hydrogenation using in-situ liberated H2. Mol. Catal. 2021, 507, 111561. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Goda, M.N.; Said, E. Selective dehydrogenation of isopropanol on carbonized metal-organic frameworks. Nano-Struct. Nano-Objects 2020, 24, 100605. [Google Scholar] [CrossRef]
- Biniwale, R.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high-capacity hydrogen storage and supply. Int. J. Hydrogen Energy 2008, 33, 360–365. [Google Scholar] [CrossRef]
- Skubic, L.; Sovdat, J.; Teran, N.; Huš, M.; Kopač, D.; Likozar, B. Ab initio multiscale process modeling of ethane, propane and butane dehydrogenation reactions: A review. Catalysts 2020, 10, 1405. [Google Scholar] [CrossRef]
- Gambo, Y.; Adamu, S.; Abdulrasheed, A.A.; Lucky, R.A.; Ba-Shammakh, M.S.; Hossain, M.M. Catalyst design and tuning for oxidative dehydrogenation of propane: A review. Appl. Catal. A-Gen. 2021, 609, 117914. [Google Scholar] [CrossRef]
- Rahman, S.T.; Choi, J.-R.; Lee, J.-H.; Park, S.-J. The role of CO2 as a mild oxidant in oxidation and dehydrogenation over catalysts: A review. Catalysts 2020, 10, 1075. [Google Scholar] [CrossRef]
- Al-ShaikhAli, A.H.; Jedidi, A.; Anjum, D.H.; Cavallo, L.; Takanabe, K. Kinetics on NiZn bimetallic catalysts for hydrogen evolution via selective dehydrogenation of methylcyclohexane to toluene. ACS Catal. 2017, 7, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Liu, H.; Lu, H.; Zhang, Z.; Chen, Y. Study on catalytic properties and carbon deposition of Ni-Cu/SBA-15 for cyclohexane dehydrogenation. Appl. Surf. Sci. 2017, 422, 905–912. [Google Scholar] [CrossRef]
- Hodoshima, S. Catalytic decalin dehydrogenation/naphthalene hydrogenation pair as a hydrogen source for fuel-cell vehicle. Int. J. Hydrogen Energy 2003, 28, 1255–1262. [Google Scholar] [CrossRef]
- Sebastián, D.; Bordejé, E.G.; Calvillo, L.; Lázaro, M.J.; Moliner, R. Hydrogen storage by decalin dehydrogenation/naphthalene hydrogenation pair over platinum catalysts supported on activated carbon. Int. J. Hydrogen Energy 2008, 33, 1329–1334. [Google Scholar] [CrossRef]
- Li, P.; Huang, Y.; Chen, D.; Zhu, J.; Zhao, T.; Zhou, X. CNFs-supported Pt catalyst for hydrogen evolution from decalin. Catal. Commun. 2009, 10, 815–818. [Google Scholar] [CrossRef]
- Li, X.; Tuo, Y.; Jiang, H.; Duan, X.; Yu, X.; Li, P. Engineering Pt/carbon-nanofibers/carbon-paper composite towards highly efficient catalyst for hydrogen evolution from liquid organic hydride. Int. J. Hydrogen Energy 2015, 40, 12217–12226. [Google Scholar] [CrossRef]
- Tuo, Y.; Shi, L.; Cheng, H.; Zhu, Y.; Yang, M.; Xu, J.; Han, Y.; Li, P.; Yuan, W. Insight into the support effect on the particle size effect of Pt/C catalysts in dehydrogenation. J. Catal. 2018, 360, 175–186. [Google Scholar] [CrossRef]
- Kariya, N.; Fukuoka, A.; Utagawa, T.; Sakuramoto, M.; Goto, Y.; Ichikawa, M. Efficient hydrogen production using cyclohexane and decalin by pulse-spray mode reactor with Pt catalysts. Appl. Catal. A-Gen. 2003, 247, 247–259. [Google Scholar] [CrossRef]
- Hodoshima, S.; Arai, H.; Saito, Y. Liquid-film-type catalytic decalin dehydrogeno-aromatization for long-term storage and long-distance transportation of hydrogen. Int. J. Hydrogen Energy 2003, 28, 197–204. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Fan, S.; Li, W.; Li, Z.; Yun, H.; Xu, X.; Guo, A.; Wang, Z. Size-dependent catalytic cyclohexane dehydrogenation with platinum nanoparticles on nitrogen-doped carbon. Energy Fuels 2020, 34, 16542–16551. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, F.; Ma, H.; Yuan, X.; Wang, Y.; Zhang, J. A review of catalysts for methylcyclohexane dehydrogenation. Top. Catal. 2021, 64, 509–520. [Google Scholar] [CrossRef]
- Pande, J.V.; Shukla, A.; Biniwale, R.B. Catalytic dehydrogenation of cyclohexane over Ag-M/ACC catalysts for hydrogen supply. Int. J. Hydrogen Energy 2012, 37, 6756–6763. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, H.; Lu, H.; Zhang, Z.; Chen, Y. High Selectivity of Cyclohexane Dehydrogenation for H2 Evolution Over Cu/SBA-15 Catalyst. Catal. Lett. 2017, 147, 1295–1302. [Google Scholar] [CrossRef]
- Pande, J.V.; Bindwal, A.B.; Pakade, Y.B.; Biniwale, R.B. Application of microwave synthesized Ag-Rh nanoparticles in cyclohexane dehydrogenation for enhanced H2 delivery. Int. J. Hydrogen Energy 2018, 43, 7411–7423. [Google Scholar] [CrossRef]
- Lu, R.; Boethius, G.; Wen, S.; Su, Y.; Deng, W. Improved organic hydrogen carriers with superior thermodynamic properties. Chem. Commun. 2009, 13, 1751–1753. [Google Scholar] [CrossRef]
- Clot, E.; Eisenstein, O.; Crabtree, R.H. Computational structure-activity relationships in H2 storage: How placement of N atoms affects release temperatures in organic liquid storage materials. Chem. Commun. 2007, 2231–2233. [Google Scholar] [CrossRef] [PubMed]
- Sotoodeh, F.; Smith, K.J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts. J. Catal. 2011, 279, 36–47. [Google Scholar] [CrossRef]
- Fan, H.; Cheng, M.; Wang, L.; Song, Y.; Cui, Y.; Wang, R. Extraordinary electrocatalytic performance for formic acid oxidation by the synergistic effect of Pt and Au on carbon black. Nano Energy 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.; Wang, B.; Wang, Y.; Wu, M.; Li, W.; Liu, B.; Lu, S. Carbon dots-confined CoP-CoO nanoheterostructure with strong interfacial synergy triggered the robust hydrogen evolution from ammonia borane. J. Energy Chem. 2021, 57, 198–205. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, C.; Zhang, Y.; Li, H.; Fu, D.; Du, X.; Zhang, X.M. N-doped mesoporous carbon embedded Co nanoparticles for highly efficient and stable H2 generation from hydrolysis of ammonia borane. J. Power Sources 2018, 399, 89–97. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Heras, F.; Alonso-Morales, N.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A. Enhancement of the activity of Pd/C catalysts in aqueous phase hydrodechlorination through doping of carbon supports. Catal. Sci. Technol. 2018, 8, 2598–2605. [Google Scholar] [CrossRef]
- Soni, Y.; Pradhan, S.; Bamnia, M.K.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Khan, T.S.; Haider, M.A.; Vinod, C.P. Spectroscopic evidences for the size dependent generation of Pd species responsible for the low temperature CO oxidation activity on Pd-SBA-15 nanocatalyst. Appl. Catal. B Environ. 2020, 272, 118934. [Google Scholar] [CrossRef]
- Liu, X.; Han, Q.; Zhang, Y.; Wang, X.; Cai, S.; Wang, C.; Yang, R. Green and facile synthesis of Rh/GO nanocomposites for high catalytic performance. Appl. Surf. Sci. 2019, 471, 929–934. [Google Scholar] [CrossRef]
- Xue, Q.; Gao, W.; Zhu, J.; Peng, R.; Xu, Q.; Chen, P.; Chen, Y. Carbon nanobowls supported ultrafine iridium nanocrystals: An active and stable electrocatalyst for the oxygen evolution reaction in acidic media. J. Colloid Interf. Sci. 2018, 529, 325–331. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.H. Understanding the effect of Pd size on formic acid dehydrogenation via size-controlled Pd/C catalysts prepared by NaBH4 treatment. Appl. Catal. B Environ. 2019, 244, 684–693. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Li, L.; Lin, J.; Yang, X.; Yu, C.; Liu, Z.; Fang, Y.; Huang, Y.; Tang, C. CuCo binary metal nanoparticles supported on boron nitride nanofibers as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. J. Power Sources 2019, 431, 135–143. [Google Scholar] [CrossRef]
- Sun, W.; Wu, S.; Lu, Y.; Wang, Y.; Cao, Q.; Fang, W. Effective control of particle size and electron density of Pd/C and Sn-Pd/C nanocatalysts for vanillin production via base-free oxidation. ACS Catal. 2020, 10, 7699–7709. [Google Scholar] [CrossRef]

| Sample | Metal (wt%) 1 | Mean Size (nm) 2 | Dispersion (%) 3 | SBET (m2/g) 4 | Vtotal (cm3/g) 4 | dBJH (nm) 4 |
|---|---|---|---|---|---|---|
| CN | - | - | - | 965.17 | 2.53 | 10.70 |
| Pt/CN | 3.22 | 2.07 ± 0.13 | 54.30 | 917.55 | 2.35 | 10.55 |
| Pd/CN | 2.64 | 2.46 ± 0.27 | 45.55 | 929.55 | 2.37 | 10.57 |
| Rh/CN | 2.32 | 2.29 ± 0.34 | 48.21 | 943.88 | 2.40 | 10.53 |
| Ir/CN | 2.69 | 2.50 ± 0.32 | 43.97 | 935.79 | 2.40 | 10.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, H.; Fan, S.; Wang, S.; Xu, G.; Guo, A.; Wang, Z. Dehydrogenation of Cycloalkanes over N-Doped Carbon-Supported Catalysts: The Effects of Active Component and Molecular Structure of the Substrate. Nanomaterials 2021, 11, 2846. https://doi.org/10.3390/nano11112846
Wang J, Liu H, Fan S, Wang S, Xu G, Guo A, Wang Z. Dehydrogenation of Cycloalkanes over N-Doped Carbon-Supported Catalysts: The Effects of Active Component and Molecular Structure of the Substrate. Nanomaterials. 2021; 11(11):2846. https://doi.org/10.3390/nano11112846
Chicago/Turabian StyleWang, Jian, He Liu, Shiguang Fan, Shuai Wang, Guanjun Xu, Aijun Guo, and Zongxian Wang. 2021. "Dehydrogenation of Cycloalkanes over N-Doped Carbon-Supported Catalysts: The Effects of Active Component and Molecular Structure of the Substrate" Nanomaterials 11, no. 11: 2846. https://doi.org/10.3390/nano11112846





