Europium(III) Complex-Functionalized SiO2@mTiO2 Nanospheres for Al3+-Modulated Multicolor Emission
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
2.2. Preparation of H2bpdc Ligand
2.3. Preparation of Eu(tta)3(H2O)2
2.4. Preparation of SiO2@mTiO2 Core–Shell Nanospheres
2.5. Preparation of SiO2@mTiO2-bpdc
2.6. Preparation of Hybrid Material Eu(tta)3bpdc-SiO2@mTiO2
2.7. Luminescent Sensing Experiment
2.8. Characterization
3. Results and Discussion
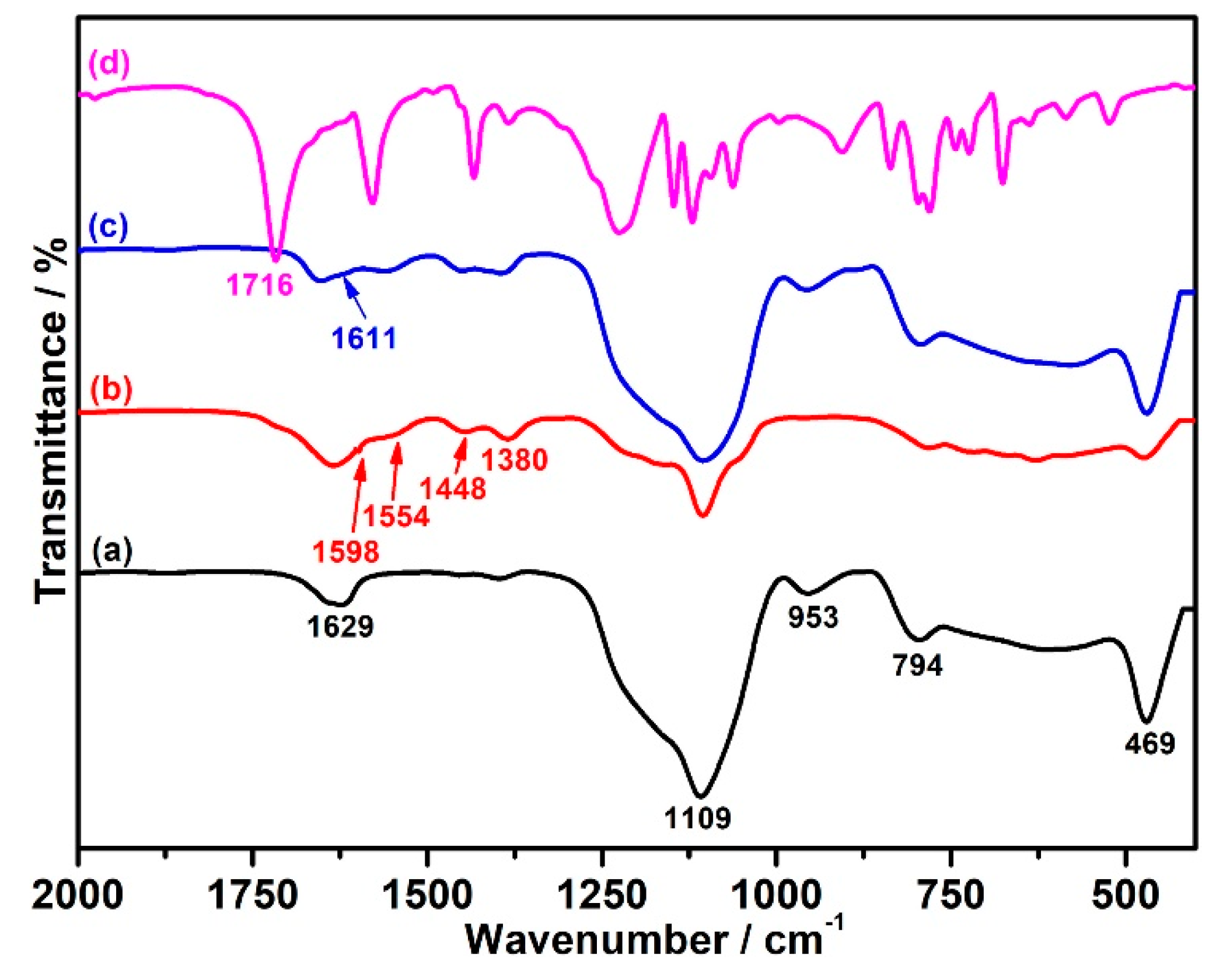
3.1. FT-IR Spectra
3.2. PXRD
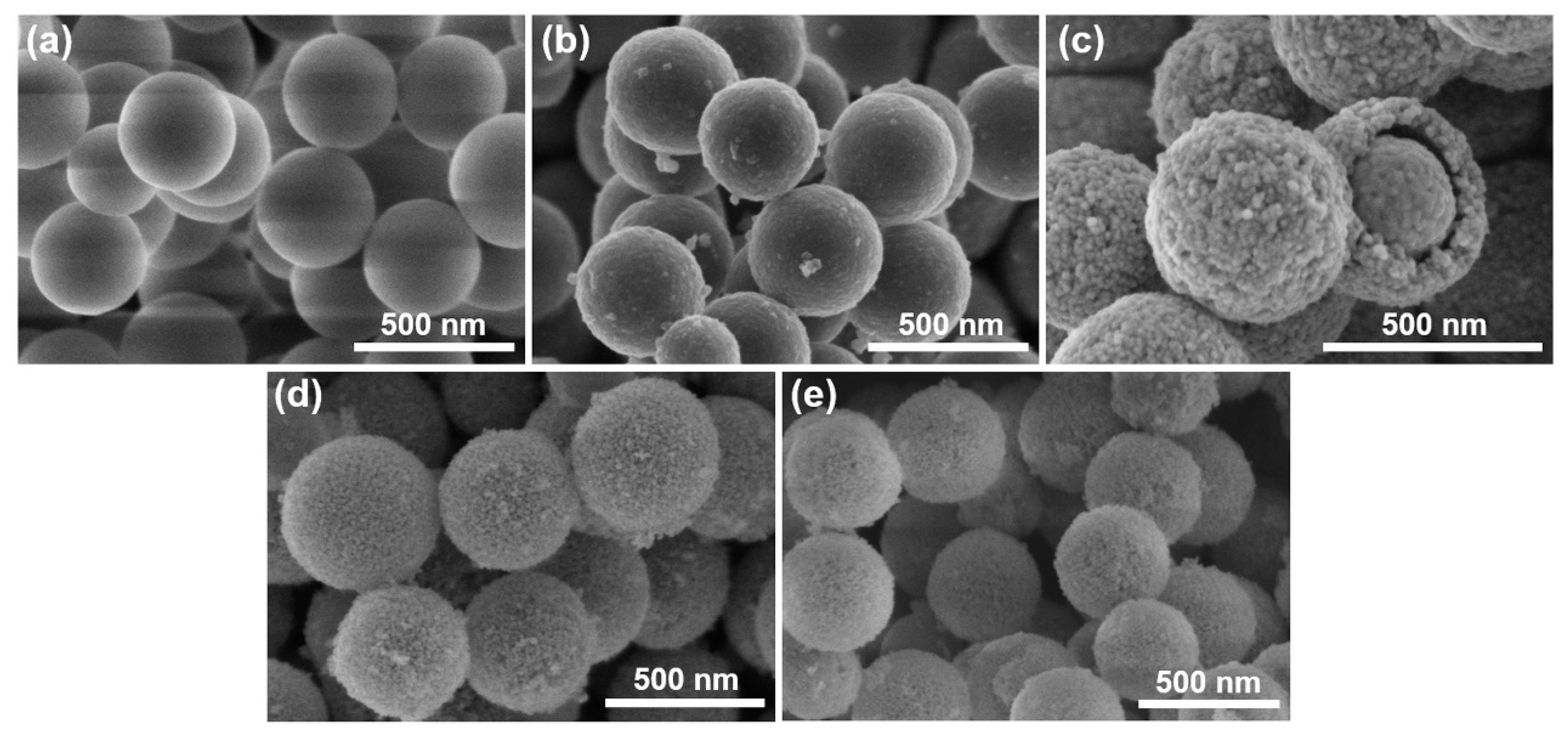
3.3. SEM Images
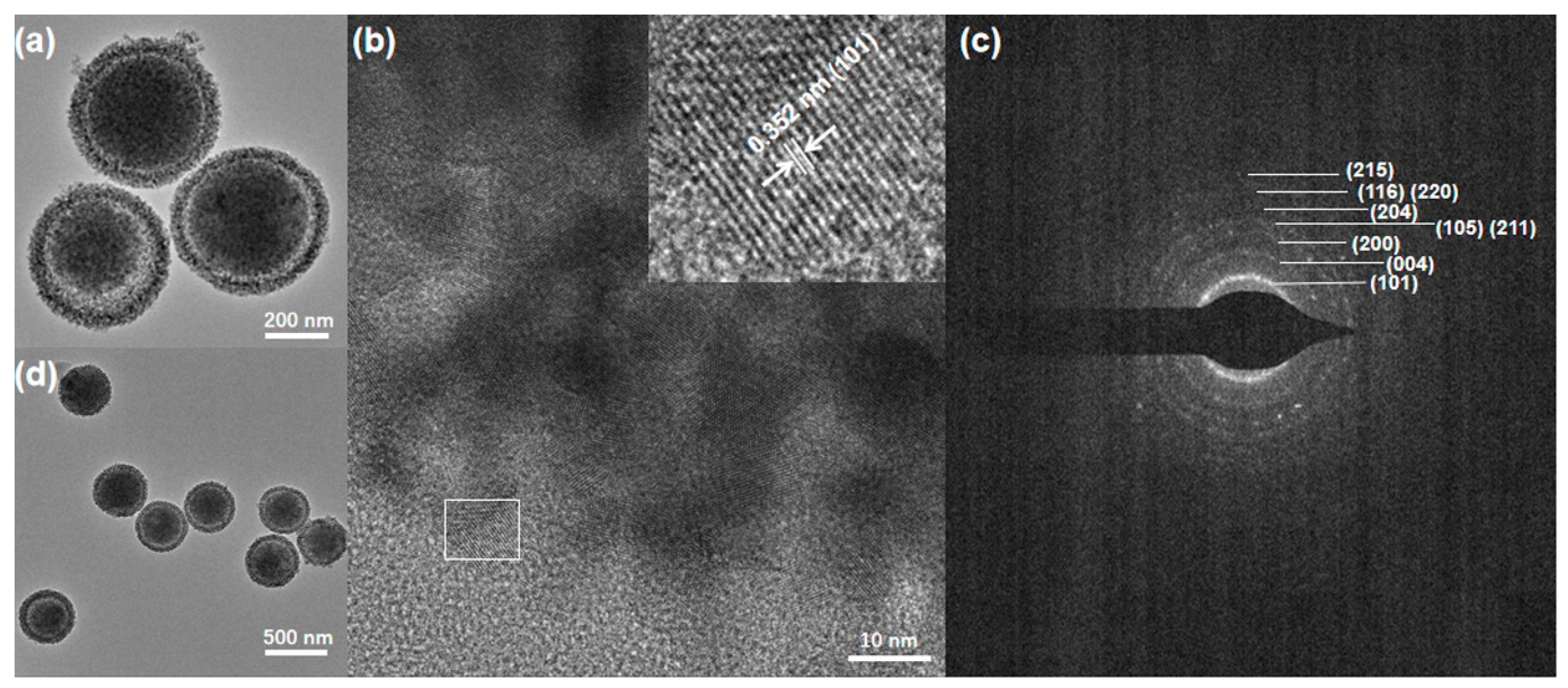
3.4. TEM Images
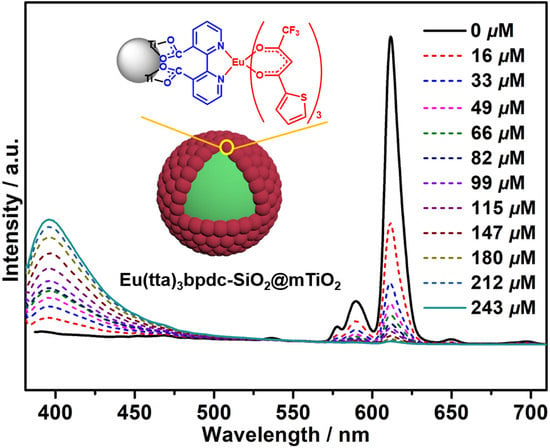
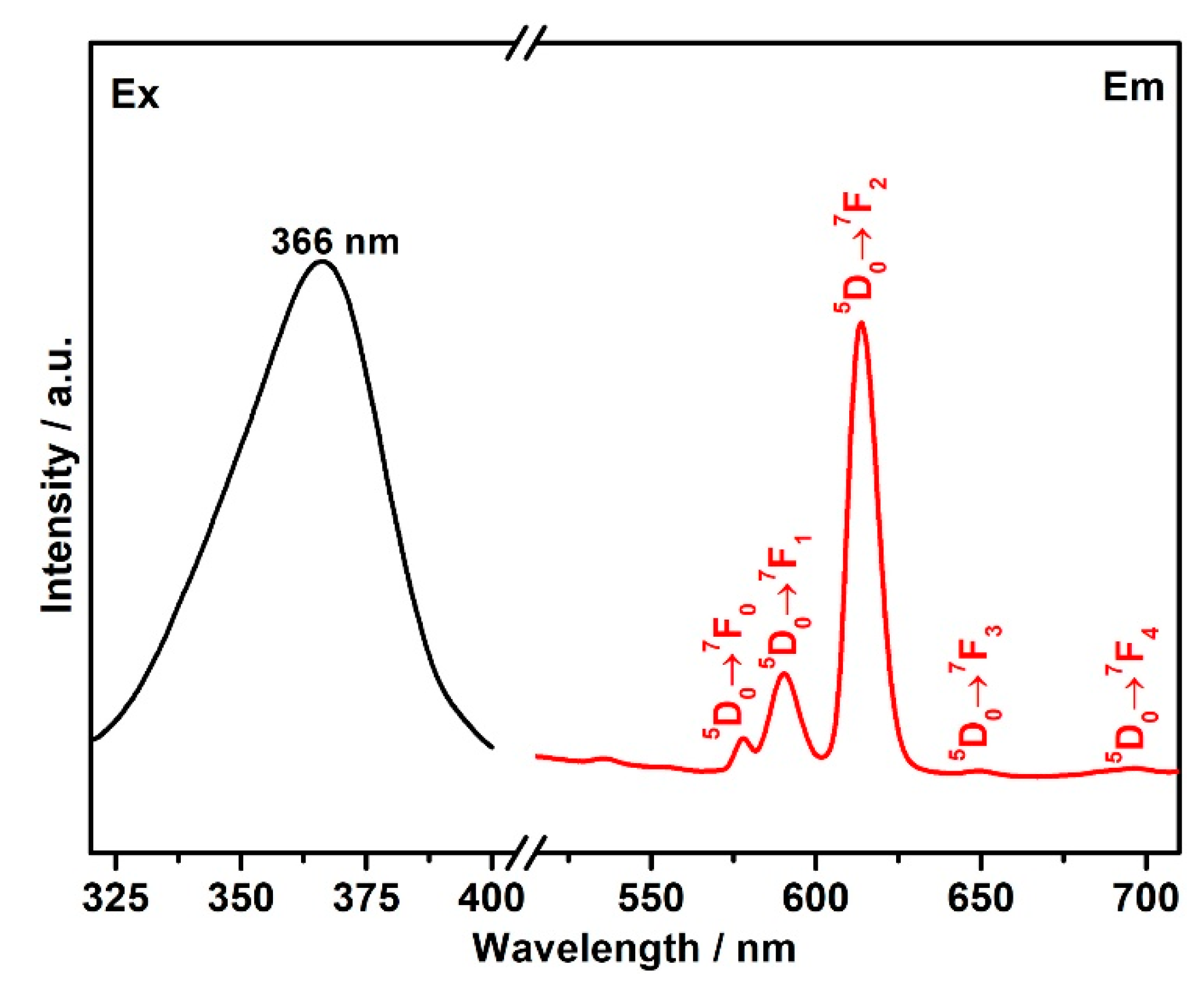
3.5. Luminescent Properties
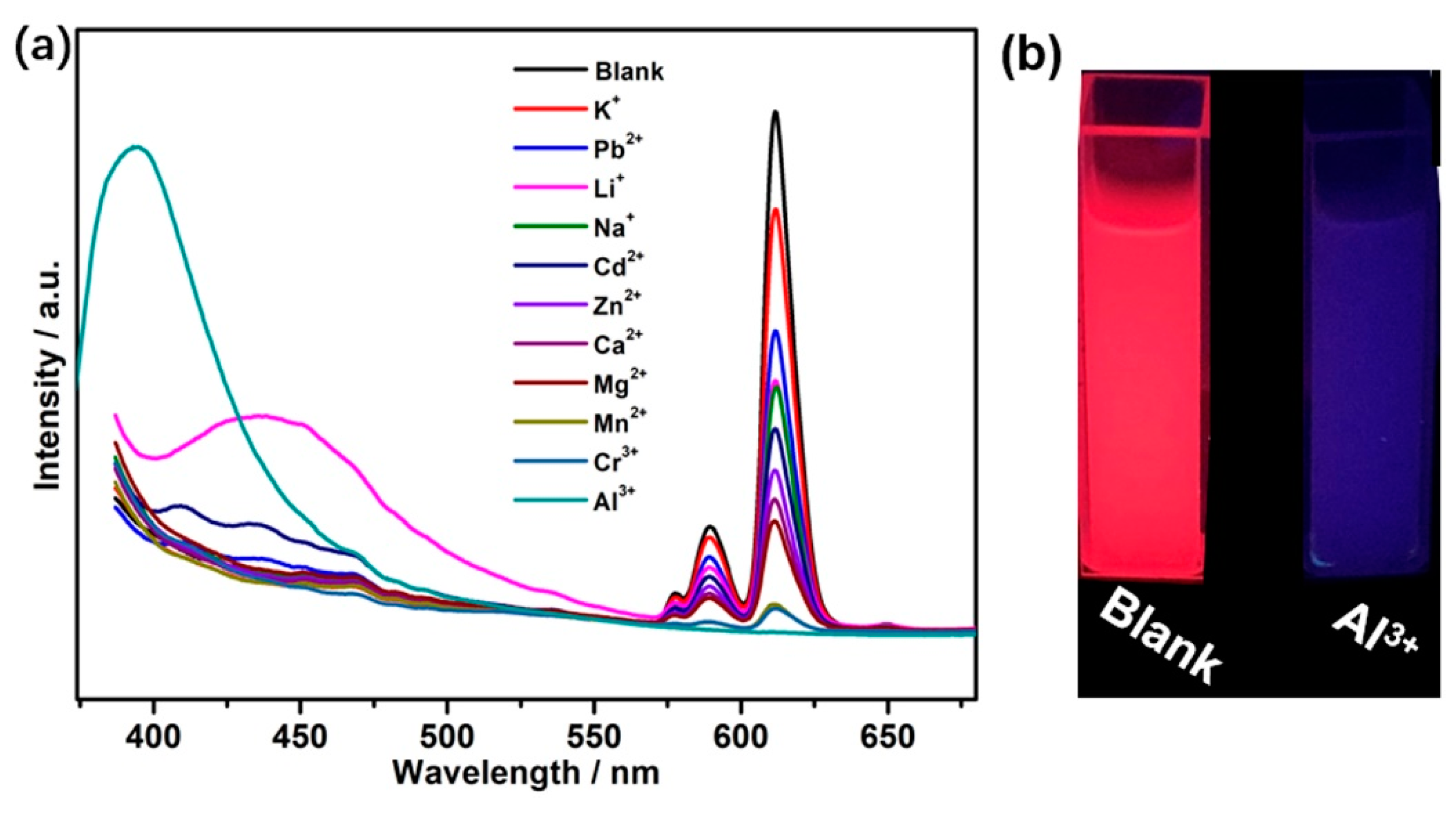
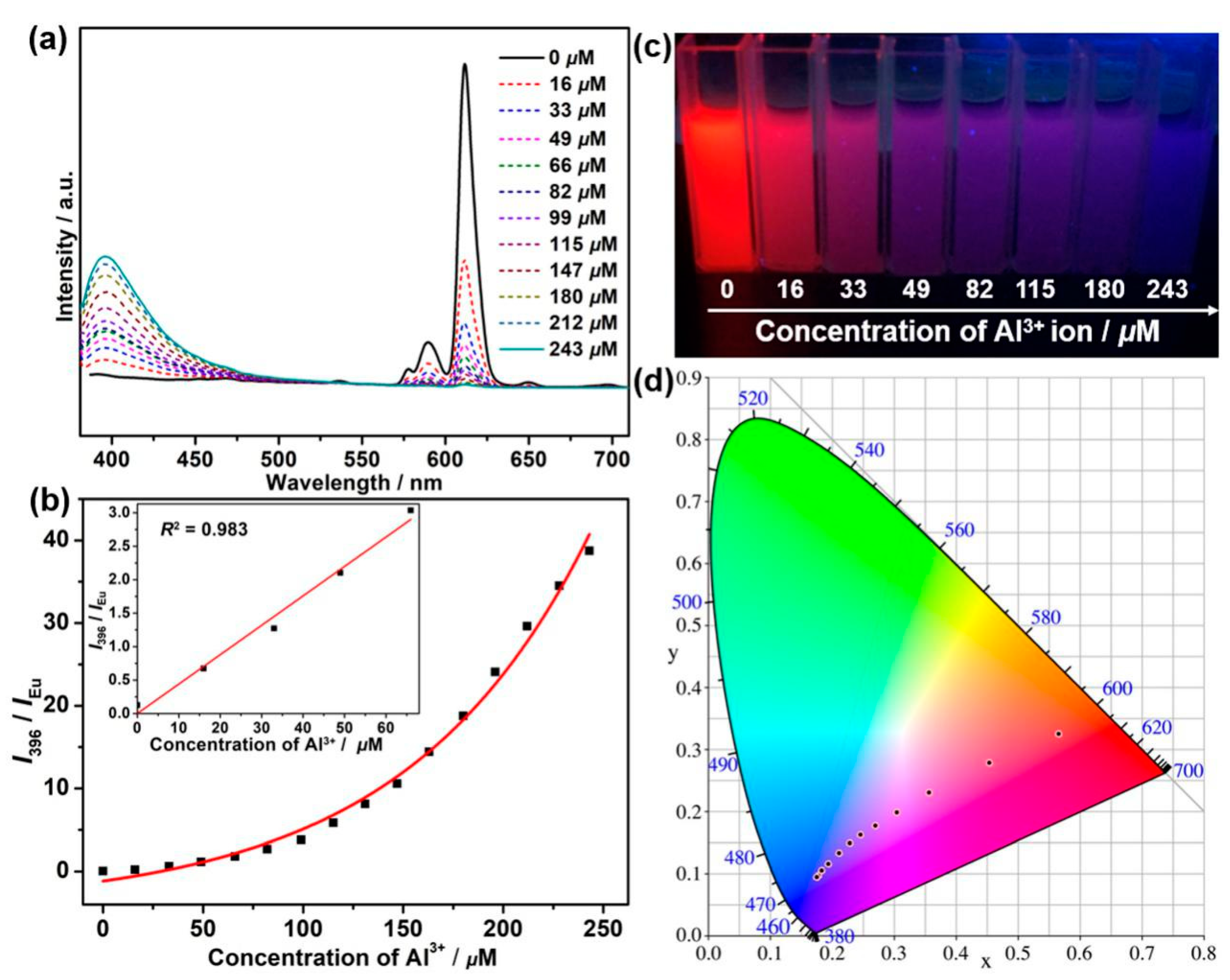
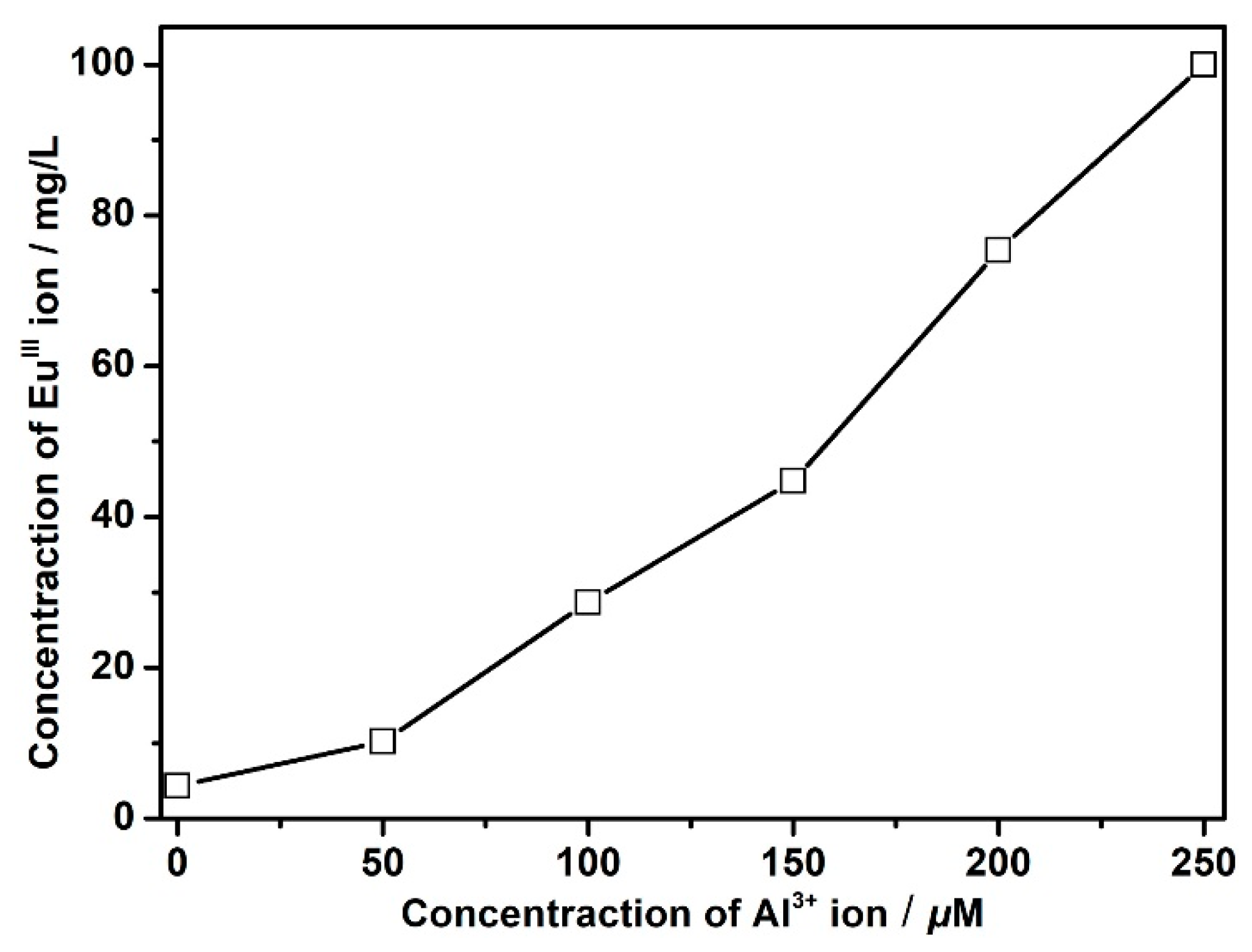
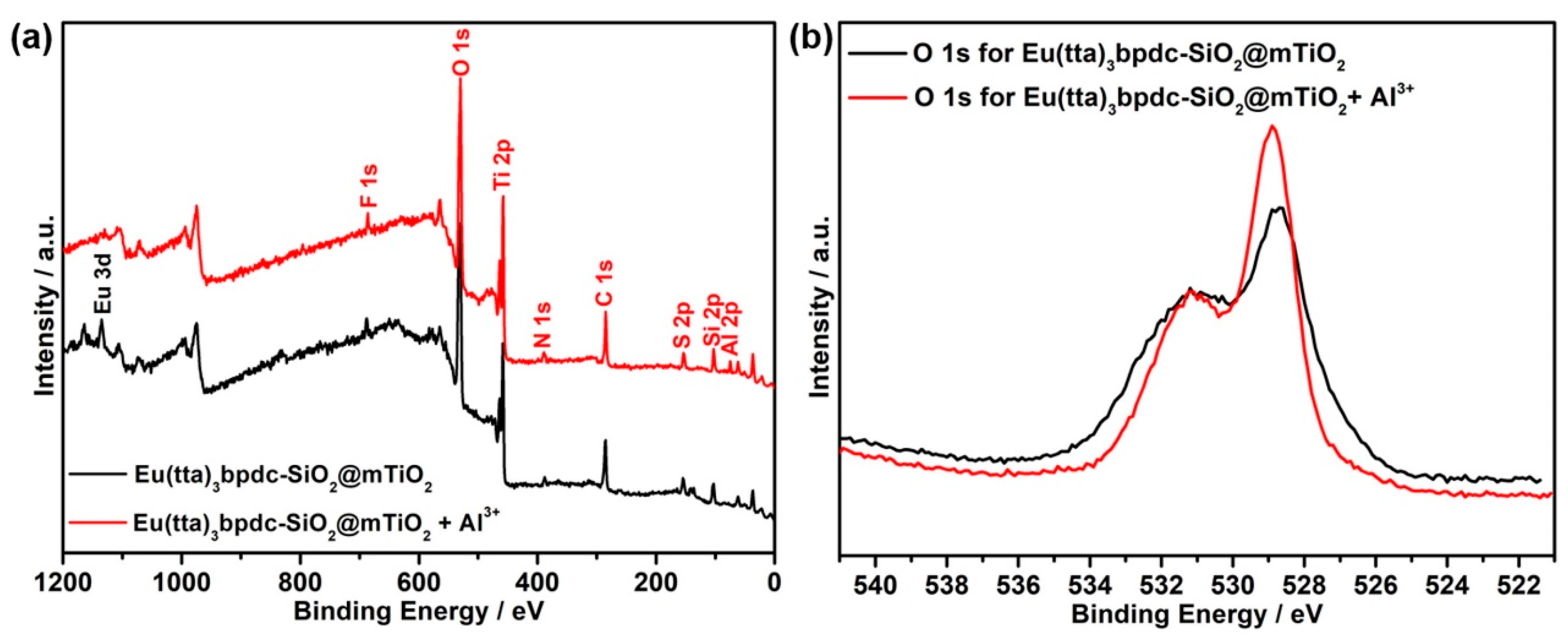
3.6. Sensing of Al3+ Cations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing. Coord. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Chen, H.; Cao, J.; Zhou, P.; Li, X.; Xie, Y.; Liu, W.; Tang, Y. Multiplex recognition and logic devices for molecular robot prototype based on an europium(iii)-cyclen system. Biosens. Bioelectron. 2018, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.-S.; Yu, J.-C.; Cui, Y.-J.; Yang, Y.; Wang, Z.-Y.; Yang, D.-R.; Qian, G.-D. Luminescent Metal-Organic Framework Films As Highly Sensitive and Fast-Response Oxygen Sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, D.; Peng, J.; Du, Q.; He, H. Ratiometric fluorescence sensing of metal-organic frameworks: Tactics and perspectives. Coord. Chem. Rev. 2020, 404, 213113. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273, 76–86. [Google Scholar] [CrossRef]
- SeethaLekshmi, S.; Ramya, A.R.; Reddy, M.L.P.; Varughesea, S. Lanthanide complex-derived white-light emitting solids: A survey on design strategies. J. Photoch. Photobiol. C 2017, 33, 109–131. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Zhou, W.; Zhu, Z.; Ma, F.; Zhou, R.; Su, F.; Zheng, L.; Ma, L.; Liang, H. Host Differential Sensitization toward Color/Lifetime-Tuned Lanthanide Coordination Polymers for Optical Multiplexin. Angew. Chem. Int. Ed. 2020, 59, 2–9. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.-M.; Xie, F.; Jin, X.; Li, J.; Yang, G.; Gu, C.; Wang, Y.; Zhang, S.X.-A. A Single-Pixel RGB Device in a Colorful Alphanumeric Electrofluorochromic Display. Adv. Mater. 2020, 32, 2003121. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, R.; Han, Q.; Dong, J.; Yan, L.; Zheng, H. Tuning Red Upconversion Emission in Single LiYF4:Yb3+/Ho3+ Microparticle. J. Phys. Chem. C 2015, 119, 2349–2355. [Google Scholar] [CrossRef]
- Jose, B.A.S.; Matsushita, S.; Akagi, K. Lyotropic Chiral Nematic Liquid Crystalline Aliphatic Conjugated Polymers Based on Disubstituted Polyacetylene Derivatives That Exhibit High Dissymmetry Factors in Circularly Polarized Luminescence. J. Am. Chem. Soc. 2012, 134, 19795–19807. [Google Scholar] [CrossRef]
- Li, G.G.; Hou, Z.Y.; Peng, C.; Wang, W.X.; Cheng, Z.Y.; Li, C.X.; Lian, H.Z.; Lin, J. Electrospinning Derived One-Dimensional LaOCl: Ln3+ (Ln = Eu/Sm, Tb, Tm) Nanofibers, Nanotubes and Microbelts with Multicolor-Tunable Emission Properties. Adv. Funct. Mater. 2010, 20, 3446–3456. [Google Scholar] [CrossRef]
- He, G.J.; Guo, D.; He, C.; Zhang, X.L.; Zhao, X.W.; Duan, C.Y. A color-tunable europium complex emitting three primary colors and white light. Angew. Chem. Int. Ed. 2009, 48, 6132–6135. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Xu, H.; Yue, Y.F.; Guo, Z.Y.; Yu, J.C.; Chen, Z.X.; Gao, J.K.; Yang, Y.; Qian, G.D.; Chen, B.L. A luminescent mixed-lanthanide metal-organic framework thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Zhang, J.H.; Sun, Z.M. Tunable emission based on lanthanide(III) metal-organic frameworks: An alternative approach to white light. J. Mater. Chem. 2012, 22, 8868–8873. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Li, Y.; Yu, X. Full-color emission of a Eu3+-based mesoporous hybrid material modulated by Zn2+ ions: Emission color changes for Zn2+ sensing via an ion exchange approach. Dalton Trans. 2019, 48, 10547–10556. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.; Yu, T. Eu3+ based mesoporous hybrid material with tunable multicolor emission modulated by fluoride ion: Application for selective sensing toward fluoride ion. J. Mater. Chem. C 2017, 5, 5411–5419. [Google Scholar] [CrossRef]
- Perl, D.P.; Brody, A.R. Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 1980, 208, 297–299. [Google Scholar] [CrossRef]
- Barcelo, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminum toxicity and resistance. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 3, 205–340. [Google Scholar] [CrossRef]
- Krejpcio, Z.; Wojciak, R.W.P.J. The Influence of Al3+ Ions on Pepsin and Trypsin Activity in Vitro. Environ. Stud. 2002, 11, 251–254. [Google Scholar]
- Goswami, S.; Paul, S.; Manna, A. Selective “naked eye” detection of Al(iii) and PPi in aqueous media on a rhodamine-isatin hybrid moiety. RSC Adv. 2013, 3, 10639–10643. [Google Scholar] [CrossRef]
- Kashyap, K.S.; Kumar, A.; Hira, S.K.; Dey, S. Recognition of Al3+ through the off-on mechanism as a proficient driving force for the hydrolysis of BODIPY conjugated Schiff base and its application in bio-imaging. Inorg. Chim. Acta 2019, 498, 119157. [Google Scholar] [CrossRef]
- Kim, H.; Manivannan, R.; Son, Y.-A. A Chromone Based Fluorescent Probe for the Effective Detection of Aluminium Ion. J. Nanosci. Nanotechnol. 2020, 20, 2840–2846. [Google Scholar] [CrossRef]
- Park, J.; Angupillai, S.; Son, Y.-A. A Highly Sensitive Fluorescent Probe for Selective Detection of Al3+ Cation by Switching the Solvent from Aprotic to Protic Environment. Mol. Cryst. Liq. Cryst. 2015, 622, 103–113. [Google Scholar] [CrossRef]
- Thangaraja, S.E.; Antonya, E.J.; Selvanb, G.T.; Selvakumarb, P.M.; Enocha, I.V.M.V. A New Fluorenone-Based Turn-on Fluorescent Al3+ Ion Sensor. J. Anal. Chem. 2019, 74, 87–92. [Google Scholar] [CrossRef]
- Ma, D.; Chen, C.; Chen, M.; Zhu, S.; Wu, Y.; Li, Z.; Li, Y.; Zhou, L. A hydrostable Cadmium-Organic Framework for Highly Selective and Sensitive Luminescence Sensing of Al3+ Ion. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1829–1837. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, Y.; Huang, D.; Su, M.; Wang, K.; Hong, M. A highly sensitive and selective fluorescent sensor for detection of Al3+ using a Europium(III) quinolinecarboxylate. Inorg Chem. 2014, 53, 6497–6499. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Fan, C.; Pu, S. A novel fluorescent sensor for Al3+ and Zn2+ based on a new europium complex with a 1,10-phenanthroline ligand. J. Rare Earth 2021, 39, 460–468. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, Z.; Pang, X.; Yu, X. Highly selective luminescent sensing of Cu2+ in aqueous solution based on a Eu(III)-centered periodic mesoporous organosilicas hybrid. Mater. Design 2019, 172, 107712. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Liu, J.; Peng, Y.-X.; Ge, X.; Shi, L.; Huang, W. Multicolor (Vis-NIR) mesoporous silica nanospheres linked with lanthanide complexes using 2-(5-bromothiophen)imidazo[4,5-f][1,10]phenanthroline for in vitro bioimaging. Dalton Trans. 2015, 44, 237–246. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Li, J.; Lou, X.W. A universal cooperative assembly-directed method for coating of mesoporous TiO2 nanoshells with enhanced lithium storage properties. Sci. Adv. 2016, 2, 1501554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; Huang, Y.; Xiao, J.; Wang, L.; Lin, Z.; Li, W.; Liu, H.; Gu, Q.; Liu, H.K.; Chou, S.-L. Phosphorus-Modulation-Triggered Surface Disorder in Titanium Dioxide Nanocrystals Enables Exceptional Sodium-Storage Performance. Angew. Chem. Int. Ed. 2019, 58, 4022–4026. [Google Scholar] [CrossRef]
- Liu, P.; Li, H.R.; Wang, Y.G.; Liu, B.Y.; Zhang, W.J.; Wang, Y.J.; Yan, W.D.; Zhang, H.J.; Schubert, U. Europium complexes immobilization on titania via chemical modification of titanium alkoxide. J. Mater. Chem. 2008, 18, 735–737. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Humphry-Baker, R.; Liska, P.; Grätzel, M. Investigation of Sensitizer Adsorption and the Influence of Protons on Current and Voltage of a Dye-Sensitized Nanocrystalline TiO2 Solar Cell. J. Phys. Chem. B 2003, 107, 8981–8987. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley and Sons: Hoboken, NJ, USA, 1978. [Google Scholar]
- Bai, C.; Wei, F.-H.; Hu, H.-M.; Yan, L.; Wang, X.; Xue, G.-L. New highly luminescent europium (III) complex covalently bonded with titania-based host via using a terpyridine carboxylate derivative linker for fluorescence sensing. J. Lumin. 2020, 227, 117545. [Google Scholar] [CrossRef]
- Wei, F.; Bai, C.; Hu, H.-M.; He, S.; Wang, X.; Xue, G. Novel luminescent europium-centered hybrid material covalently grafted with organically modified titania via 2-substituted imidazophenanthroline for fluorescence sensing. J. Rare Earth 2021, 39, 666–673. [Google Scholar] [CrossRef]
- Aleem, A.R.; Liu, J.; Wang, J.; Wang, J.; Zhao, Y.; Wang, Y.; Wang, Y.; Wang, W.; Rehman, F.; Kipper, M.J.; et al. Selective Sensing of Cu2+ and Fe3+ Ions with Vis-Excitation using Fluorescent Eu3+-Induced Aggregates of Polysaccharides (EIAP) in Mammalian Cells and Aqueous Systems. J. Hazard. Mater. 2020, 399, 122991. [Google Scholar] [CrossRef]
- Yu, X.D.; Wang, Z.Y.; Li, Y.J.; Geng, L.J.; Ren, J.J.; Feng, G.L. Fluorescent and Electrochemical Supramolecular Coordination Polymer Hydrogels Formed from Ion-Tuned Self-Assembly of Small Bis-Terpyridine Monomer. Inorg. Chem. 2017, 56, 7512–7518. [Google Scholar] [CrossRef]
- Orudzhev, F.; Ramazanov, S.; Sobola, D.; Isaev, A.; Wang, C.; Magomedova, A.; Kadiev, M.; Kaviyarasu, K. Atomic Layer Deposition of Mixed-Layered Aurivillius Phase on TiO2 Nanotubes: Synthesis, Characterization and Photoelectrocatalytic Properties. Nanomaterials 2020, 10, 2183. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Zhang, J.Z.; Feng, J.; Liu, J.; Zhao, Y.; Shi, L. Visible and near-infrared luminescent mesoporous titania mi-crospheres functionalized with lanthanide complexes: Microstructure and luminescence with visible excitation. RSC Adv. 2014, 4, 28481–28489. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, C.; He, S.; Hu, H.-M.; Zeng, H.; Zou, F.; Wang, J.-J. Europium(III) Complex-Functionalized SiO2@mTiO2 Nanospheres for Al3+-Modulated Multicolor Emission. Nanomaterials 2021, 11, 2886. https://doi.org/10.3390/nano11112886
Bai C, He S, Hu H-M, Zeng H, Zou F, Wang J-J. Europium(III) Complex-Functionalized SiO2@mTiO2 Nanospheres for Al3+-Modulated Multicolor Emission. Nanomaterials. 2021; 11(11):2886. https://doi.org/10.3390/nano11112886
Chicago/Turabian StyleBai, Chao, Shi He, Huai-Ming Hu, Hui Zeng, Feng Zou, and Ji-Jiang Wang. 2021. "Europium(III) Complex-Functionalized SiO2@mTiO2 Nanospheres for Al3+-Modulated Multicolor Emission" Nanomaterials 11, no. 11: 2886. https://doi.org/10.3390/nano11112886
APA StyleBai, C., He, S., Hu, H.-M., Zeng, H., Zou, F., & Wang, J.-J. (2021). Europium(III) Complex-Functionalized SiO2@mTiO2 Nanospheres for Al3+-Modulated Multicolor Emission. Nanomaterials, 11(11), 2886. https://doi.org/10.3390/nano11112886