Colloidal Cu-Zn-Sn-Te Nanocrystals: Aqueous Synthesis and Raman Spectroscopy Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu-Zn-Sn-Te NCs
- (I)
- Preparation of the tin precursor stock solution. The tin precursor stock solution was prepared similarly to the procedure described previously in Ref. [35]. A stock aqueous 0.5 M solution of SnCl2 in 4.0 M NaOH was prepared by slowly pouring an aqueous 1.0 M suspension of SnCl2 × 2H2O into an aqueous 8.0 M solution of NaOH (volumic ratio of the suspension and NaOH solutions were 1:1) and was left for 24 h at room temperature.
- (II)
- Preparation of a tellurium precursor solution (NaHTe). It starts with NaBH4 reduction of Te to sodium telluride (NaHTe) liberating H2 and NaHTe in an aqueous medium, with subsequent dissociation of NaHTe, which releases Te anions (Te2-). The NaHTe solution is usually prepared using tellurium (Te) powder and sodium borohydride (NaBH4), which are dissolved in water or another solvent in an inert atmosphere. The reaction of the tellurium precursor can be written according to the equation.
2.3. Characterization
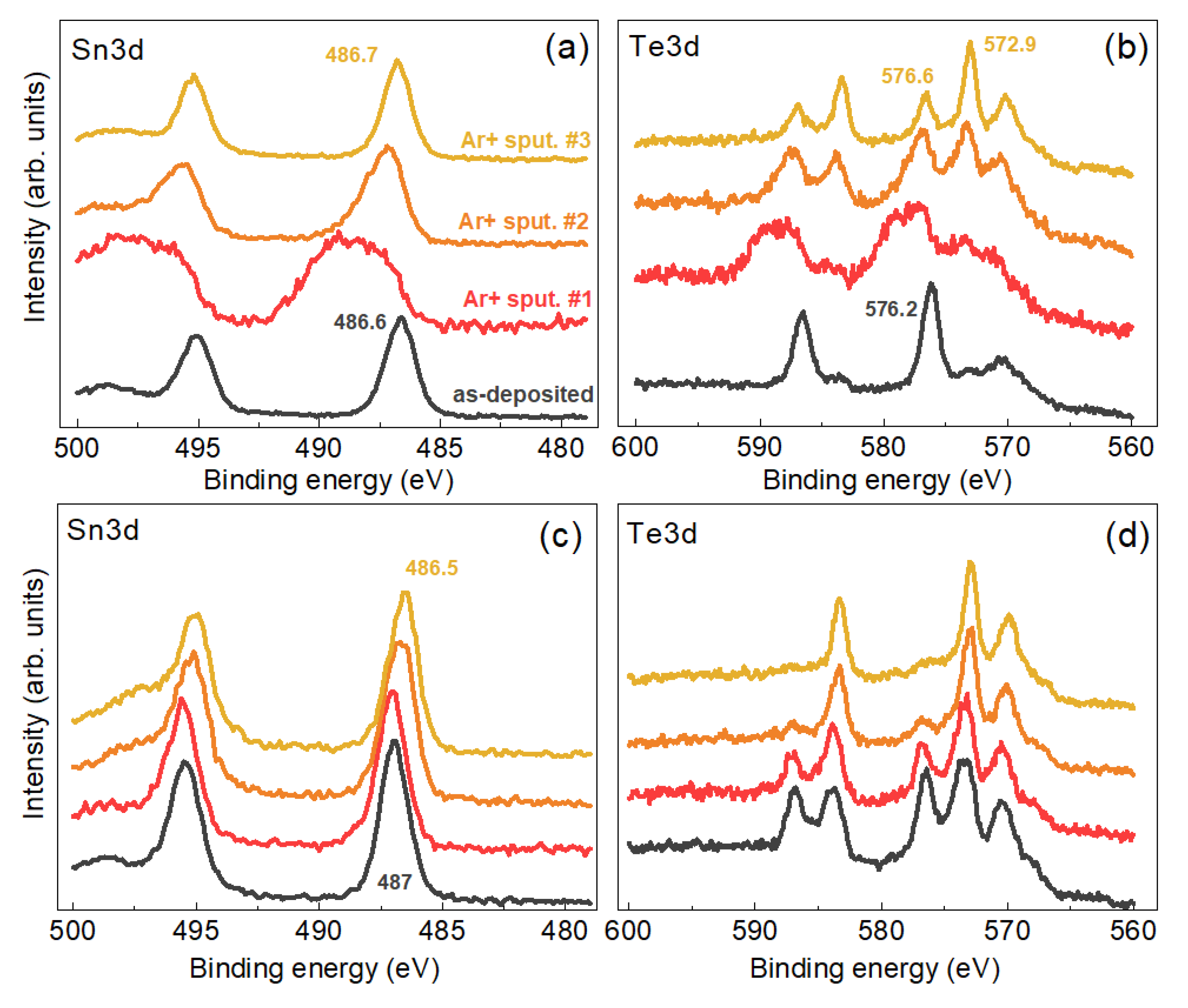
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fonoll-Rubio, R.; Andrade-arvizu, J.; Blanco-Portals, J.; Becerril-Romero, I.; Guc, M.; Saucedo, E.; Peiro, F.; Calvo-Barrio, L.; Ritzer, M.; Schnohr, C.S.; et al. Insights into Interface and Bulk Defects in a High Efficiency Kesterite-Based Device. Energy Environ. Sci. 2021, 14, 507–523. [Google Scholar] [CrossRef]
- Zhou, H.; Hsu, W.; Duan, H.; Bob, B.; Yang, W. CZTS Nanocrystals: A Promising Approach for next Generation Thin Film Photovoltaics. Energy Environ. Sci. 2013, 6, 2822–2838. [Google Scholar] [CrossRef]
- Romanyuk, Y.E.; Haass, S.G.; Giraldo, S.; Placidi, M.; Tiwari, D.; Fermin, D.J.; Hao, X.; Xin, H.; Schnabel, T.; KaukKuusik, M.; et al. Doping and Alloying of Kesterites. J. Phys. Energy 2019, 1, 044004. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, S.; Jehl, Z.; Placidi, M.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Saucedo, E. Progress and Perspectives of Thin Film Kesterite Photovoltaic Technology: A Critical Review. Adv. Mater. 2019, 31, 1806692. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Huhn, W.P.; Wessler, G.C.; Shin, D.; Saparov, B.; Mitzi, D.B.; Blum, V. I2-II-IV-VI4(I = Cu, Ag; II = Sr, Ba; IV = Ge, Sn; VI = S, Se): Chalcogenides for Thin-Film Photovoltaics. Chem. Mater. 2017, 29, 7868–7879. [Google Scholar] [CrossRef]
- Litvinchuk, A.P.; Dzhagan, V.M.; Yukhymchuk, V.O.; Valakh, M.Y.; Babichuk, I.S.; Parasyuk, O.V.; Piskach, L.V.; Gordan, O.D.; Zahn, D.R.T. Electronic Structure, Optical Properties, and Lattice Dynamics of Orthorhombic Cu2CdGeS4 and Cu2CdSiS4 Semiconductors. Phys. Rev. B 2014, 90, 165201. [Google Scholar] [CrossRef]
- Vu, T.V.; Lavrentyev, A.A.; Gabrelian, B.V.; Parasyuk, O.V.; Ocheretova, V.A.; Khyzhun, O.Y. Electronic Structure and Optical Properties of Ag2HgSnSe4: First-Principles DFT Calculations and X-Ray Spectroscopy Studies. J. Alloy. Compd. 2018, 732, 372–384. [Google Scholar] [CrossRef]
- Ritchie, C.; Sidney, A.; Chesman, R.; Jasieniak, J.; Mulvaney, P. Aqueous Synthesis of Cu2ZnSnSe4 Nanocrystals. Chem. Mater. 2019, 31, 2138–2150. [Google Scholar] [CrossRef]
- Ritchie, C.; Chesman, A.; Styles, M.J.; Jasieniak, J.; Mulvaney, P. Aqueous Synthesis of High-Quality Cu2ZnSnS4 Nanocrystals and Their Thermal Annealing Characteristics. Langmuir 2018, 34, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sturm, C.; Kleinke, H. Chalcogenides as Thermoelectric Materials. J. Solid State Chem. 2019, 270, 273–279. [Google Scholar] [CrossRef]
- Yang, H.; Fan, W.; Vaneski, A.; Susha, A.S.; Teoh, W.Y.; Rogach, A.L. Heterojunction Engineering of CdTe and CdSe Quantum Dots on TiO2 Nanotube Arrays: Intricate Effects of Size- Dependency and Interfacial Contact on Photoconversion Efficiencies. Adv. Funct. Mater. 2012, 22, 2821–2829. [Google Scholar] [CrossRef]
- Gaponik, N.; Rogach, A.L. Thiol-Capped CdTe Nanocrystals: Progress and Perspectives of the Related Research Fields. Phys. Chem. Chem. Phys. 2010, 12, 8685–8693. [Google Scholar] [CrossRef]
- Valakh, M.Y.; Litvinchuk, A.P.; Dzhagan, V.M.; Yukhymchuk, V.O.; Havryliuk, Y.O.; Guc, M.; Bodnar, I.V.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Zahn, D.R.T. Optical Properties of Quaternary Kesterite-Type Cu2Zn(Sn1-xGex)S4 Crystalline Alloys: Raman Scattering, Photoluminescence and First-Principle Calculations. RSC Adv. 2016, 6, 67756–67763. [Google Scholar] [CrossRef] [Green Version]
- Stroyuk, O.; Raevskaya, A.; Spranger, F.; Selyshchev, O.; Dzhagan, V.; Solonenko, D.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. Mercury-Indium-Sulfide Nanocrystals: A New Member of the Family of Ternary in Based Chalcogenides Mercury-Indium-Sulfide Nanocrystals: A New Member of the Family of Ternary in Based Chalcogenides. J. Chem. Phys. 2019, 151, 144701. [Google Scholar] [CrossRef]
- Khyzhun, O.Y.; Ocheretova, V.A.; Fedorchuk, A.O.; Parasyuk, O.V. X-Ray Spectroscopy Study of the Electronic Structure of Non-Centrosymmetric Ag2CdSnS4 Single Crystal. Opt. Mater. (Amst.) 2014, 36, 1396–1401. [Google Scholar] [CrossRef]
- Adhikary, A.; Mohapatra, S.; Lee, S.H.; Hor, Y.S.; Adhikari, P.; Ching, W.-Y.; Choudhury, A. Metallic Ternary Telluride with Sphalerite Superstructure. Inorg. Chem. 2016, 55, 2114–2122. [Google Scholar] [CrossRef]
- Wei, K.; Nolas, G.S. Synthesis, Characterization and Alloying of Cu2ZnSnQ4 ( Q = S, Se and Te ) Nanocrystals. J. Solid State Chem. 2015, 226, 215–218. [Google Scholar] [CrossRef]
- Sturm, C. Thermoelectric Properties of Zinc-Doped Cu5Sn2Se7 and Cu5Sn2Te7. Dalt. Trans. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Khabibullin, A.R.; Wei, K.; Ge, Z.; Martin, J.; Salvador, J.R.; Woods, L.M.; Nolas, G.S.; Salvador, J.R.; Woods, L.M.; et al. Synthesis, Transport Properties, and Electronic Structure of Cu2CdSnTe4. Appl. Phys. Lett. 2019, 104, 252107. [Google Scholar] [CrossRef] [Green Version]
- Gabrelian, B.V.; Lavrentyev, A.A.; Vu, T.V.; Kalmykova, K.F.; Ananchenko, L.N.; Tkach, V.A.; Parasyuk, O.V.; Khyzhun, O.Y. Valence-Band Electronic Structure and Main Optical Properties of Cu2HgGeTe4: Theoretical Simulation within a DFT Framework and Experimental XPS Study. Mater. Today Commun. 2020, 23, 100828. [Google Scholar] [CrossRef]
- Gullu, H.H.; Yildiz, D.E.; Sürücü, Ö.B.; Terlemezoglu, M. Temperature Dependence of Electrical Properties in In/Cu2ZnSnTe4/Si/Ag Diodes. Bull. Mater. Sci. 2019, 42, 45. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, B.R.; Peng, W.; Gomes, L.C.; Gorai, P.; Zhu, T.; Smiadak, D.M.; Je, G.; Stevanovic, V.; Ertekin, E.; Zevalkink, A.; et al. Ultralow Thermal Conductivity in Diamond-Like Semiconductors: Selective Scattering of Phonons from Antisite Defects. Chem. Mater. 2018, 30, 3395–3409. [Google Scholar] [CrossRef]
- Babichuk, I.S.; Semenenko, M.O.; Caballero, R.; Datsenko, O.I.; Golovynskyi, S.; Qiu, R.; Li, B.; Qu, J.; Leon, M. Raman Mapping of MoS2 at Cu2ZnSnS4 / Mo Interface in Thin Film. Sol. Energy 2020, 205, 154–160. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Oliva, F.; Guc, M.; Giraldo, S.; Saucedo, E.; Pérez-Rodríguez, A.; Izquierdo-Roca, V. Defect Characterisation in Cu2ZnSnSe4 Kesterites via Resonance Raman Spectroscopy and the Impact on Optoelectronic Solar Cell Properties. J. Mater. Chem. A 2019, 7, 13293–13304. [Google Scholar] [CrossRef]
- Márquez, J.; Neuschitzer, M.; Dimitrievska, M.; Gunder, R.; Haass, S.; Werner, M.; Romanyuk, Y.E.; Schorr, S.; Pearsall, N.M.; Forbes, I. Systematic Compositional Changes and Their Influence on Lattice and Optoelectronic Properties of Cu2ZnSnSe4 Kesterite Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 144, 579–585. [Google Scholar] [CrossRef]
- Dzhagan, V.; Kapush, O.; Budzulyak, S.; Mazur, N.; Havryliuk, Y.; Litvinchuk, A. Colloidal Cu2ZnSnS4 -Based and Ag-Doped Nanocrystals: Synthesis and Raman Spectroscopy Study. Phys. Chem. Solid State 2021, 2, 260–268. [Google Scholar] [CrossRef]
- Guc, M.; Schorr, S.; Gurieva, G.; Guc, M.; Dimitrievska, M. Point Defects, Compositional Fluctuations, and Secondary Phases in Non-Stoichiometric Kesterites. J. Phys. Energy 2020, 2, 012002. [Google Scholar]
- Selyshchev, O.; Havryliuk, Y.; Valakh, M.Y.; Yukhymchuk, V.O.; Raievska, O.; Stroyuk, O.L.; Dzhagan, V.; Zahn, D.R.T. Raman and X-ray Photoemission Identification of Colloidal Metal Sulfides as Potential Secondary Phases in Nanocrystalline Cu2ZnSnS4 Photovoltaic Absorbers. ACS Appl. Nano Mater. 2020, 3, 5706–5717. [Google Scholar] [CrossRef]
- Souna, A.J.; Wei, K.; Nolas, G.S. The Effect on the Optical Modes of Quaternary Chalcogenides upon Metal and Chalcogen Substitution. Appl. Phys. Lett. 2020, 116, 082103. [Google Scholar] [CrossRef]
- Pareek, D.; Balasubramaniam, K.R.; Sharma, P. Synthesis and Characterization of Kesterite Cu2ZnSnTe4 via Ball-Milling of Elemental Powder Precursors. RSC Adv. 2016, 6, 68754–68759. [Google Scholar] [CrossRef]
- Guc, M.; Izquierdo-roca, V.; Rodríguez, A.P.; Gurieva, G.; Levcenko, S.; Schorr, S.; Arushanov, E. Raman Spectra of Wurtzstannite Quaternary Compounds. Phys. Stat. Sol. 2013, 10, 1075–1078. [Google Scholar] [CrossRef]
- Guc, M.; Levcenko, S.; Izquierdo-Roca, V.; Fontane, X.; Valakh, M.Y.; Arushanov, E.; Pérez-Rodríguez, A. Polarized Raman Scattering Analysis of Cu2ZnSiS4 and Cu2ZnSiSe4 Single Crystals. J. Appl. Phys. 2013, 114, 173507. [Google Scholar] [CrossRef]
- Levcenko, S.; Nateprov, A.; Kravtsov, V.; Guc, M.; Pérez-rodríguez, A.; Izquierdo-roca, V.; Fontané, X.; Arushanov, E. Structural Study and Raman Scattering Analysis of Cu2ZnSiTe4 Bulk Crystals. Opt. Express 2014, 22, A1936. [Google Scholar] [CrossRef] [PubMed]
- Rinc, C.; Nieves, L.; Delgado, G.E.; Marcano, G.; Cu, I.T.; Such, K. Structural Characterization of the High-Temperature Modification of the Cu2ZnGeTe4 Quaternary Semiconductor Compound. Phys. Stat. Sol. 2016, 253, 1195–1201. [Google Scholar]
- Stroyuk, O.; Raevskaya, A.; Selyshchev, O.; Dzhagan, V.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. “Green” Aqueous Synthesis and Optical Characterization of Colloidal Cu2ZnSnS4 Nanocrystal Inks. Sci. Rep. 2018, 8, 13677. [Google Scholar] [CrossRef]
- Kapush, O.A.; Trishchuk, L.I.; Tomashik, V.N.; Tomashik, Z.F.; Boruk, S.D. Effect of Solvent Nature on the Stability of Highly Dispersed and Nanosized Cadmium Telluride. Russ. J. Inorg. Chem. 2013, 58, 1166–1171. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons: Chicheste, UK, 1983. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.Z.; Payne, M.C. First Principles Methods Using CASTEP. Z. Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Litvinchuk, A.P. Optical Properties and Lattice Dynamics of Cu2ZnGeSe4 Quaternary Semiconductor: A Density-Functional Study. Phys. Stat. Sol. B 2016, 253, 323–328. [Google Scholar] [CrossRef]
- Babichuk, I.S.; Golovynskyi, S.; Brus, V. Secondary Phases in Cu2ZnSnS4 Films Obtained by Spray Pyrolysis at Different Substrate Temperatures and Cu Contents. Mater. Lett. 2018, 216, 173–175. [Google Scholar] [CrossRef]
- Havryliuk, Y.; Valakh, M.Y.; Dzhagan, V.; Greshchuk, O.; Yukhymchuk, V.; Raevskaya, A.; Stroyuk, O.; Selyshchev, O.; Gaponik, N.; Zahn, D.R.T. Raman Characterization of Cu2ZnSnS4 Nanocrystals: Phonon Confinement Effect and Formation of CuxS Phases. RSC Adv. 2018, 8, 30736–30746. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, J.; Utama, M.I.B.; Peng, B.; De La Mata, M.; Arbiol, J.; Xiong, Q. Exciton-Phonon Coupling in Individual ZnTe Nanorods Studied by Resonant Raman Spectroscopy. Phys. Rev. B 2012, 85, 085418. [Google Scholar] [CrossRef]
- Yi, Y.; Marmon, J.K.; Chen, Y.; Zhang, F.; Sheng, T.; Wijewarnasuriya, P.S.; Zhang, H.; Zhang, Y. Intrinsic Exciton-Phonon Coupling and Tuning in ZnTe Nanowires Probed by Resonant Raman Scattering. Phys. Rev. Appl. 2020, 13, 011001. [Google Scholar] [CrossRef]
- Vinogradov, V.S.; Dzhagan, V.N.; Zavaritskaya, T.N.; Kucherenko, I.V.; Mel’nik, N.N.; Novikova, N.N.; Janik, E.; Wojtowicz, T.; Plyashechnik, O.S.; Zahn, D.R.T. Optical Phonons in the Bulk and on the Surface of ZnO and ZnTe/ZnO Nanowires in Raman Spectra. Phys. Solid State 2012, 54, 2083–2090. [Google Scholar] [CrossRef]
- Salmón-Gamboa, J.U.; Barajas-Aguilar, A.H.; Ruiz-Ortega, L.I.; Garay-Tapia, A.M.; Jiménez-Sandoval, S.J. Vibrational and Electrical Properties of Cu2 − xTe Films: Experimental Data and First Principle Calculations. Sci. Rep. 2018, 8, 8093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcano, G.; Power, C.; Rincón, C.; Molina, I.; Nieves, L. Raman Spectra of the Orthorhombic Semiconductor Compound Cu2SnTe3. Solid State Commun. 2011, 151, 451–455. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Li, W.S.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Raman Spectra of CuO Nanocrystals. J. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Litvinchuk, A.P.; Möller, A.; Debbichi, L.; Krüger, P.; Iliev, M.N.; Gospodinov, M. Second-Order Raman Scattering in CuO. J. Phys. Condens. Matter. 2013, 25, 105402. [Google Scholar] [CrossRef]
- Benz, J.; Hering, K.P.; Kramm, B.; Polity, A.; Klar, P.J.; Siah, S.C. The Influence of Nitrogen Doping on the Electrical Andvibrational Properties of Cu2O. Phys. Stat. Sol. 2017, 254, 1600421. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N.; Selyshchev, O.; Dzhagan, V.; Schulze, S.; Zahn, D.R.T. Origin of the Broadband Photoluminescence of Pristine and Cu+/Ag+-Doped Ultrasmall CdS and CdSe/CdS Quantum Dots. J. Phys. Chem. C 2018, 122, 10267–10277. [Google Scholar] [CrossRef]
- Dzhagan, V.; Kempken, B.; Valakh, M.; Parisi, J.; Kolny-Olesiak, J.; Zahn, D.R.T. Probing the Structure of CuInS2-ZnS Core-Shell and Similar Nanocrystals by Raman Spectroscopy. Appl. Surf. Sci. 2017, 395, 24–28. [Google Scholar] [CrossRef]
- Manjón, F.J.; Gallego-Parra, S.; Rodríguez-Hernández, P.; Muñoz, A.; Drasar, C.; Muñoz-Sanjosé, V. Oliver Oeckler Anomalous Raman Modes in Tellurides. J. Mater. Chem. C 2021. [Google Scholar] [CrossRef]
- Azhniuk, Y.M.; Lopushansky, V.V.; Hutych, Y.I.; Prymak, M.V.; Gomonnai, A.V.; Zahn, D.R.T. Precipitates of Selenium and Tellurium in II-VI Nanocrystal-Doped Glass Probed by Raman Scattering. Phys. Stat. Sol. B 2011, 248, 674–679. [Google Scholar] [CrossRef]
- Azhniuk, Y.M.; Hutych, Y.I.; Lopushansky, V.V.; Prymak, M.V.; Gomonnai, A.V.; Zahn, D.R.T. Raman Spectra of Quaternary CdS1-x-ySexTey Nanocrystals Embedded in Borosilicate Glass. Int. J. Spectrosc. 2012, 2012, 495896. [Google Scholar] [CrossRef]
- Azhniuk, Y.M.; Gomonnai, A.V.; Hutych, Y.I.; Lopushansky, V.V.; Prots, L.A.; Turok, I.I.; Zahn, D.R.T. Evidence for Formation of Se Molecular Clusters during Precipitation of CdSe1-XSx Nanoparticles in Glass. Appl. Phys. A 2009, 95, 473–477. [Google Scholar] [CrossRef]
- Apte, A.; Bianco, E.; Krishnamoorthy, A.; Yazdi, S.; Rao, R.; Glavin, N.; Kumazoe, H.; Varshney, V.; Roy, A.; Shimojo, F.; et al. Polytypism in Ultrathin Tellurium. 2D Mater. 2019, 6, 015013. [Google Scholar] [CrossRef] [Green Version]
- Poborchii, V.V. Raman Spectra of Sulfur, Selenium or Tellurium Clusters Confined in Nano-Cavities of Zeolite A. Solid State Commun. 1998, 107, 513–518. [Google Scholar] [CrossRef]
- Artamonov, V.V.; Valakh, M.Y.; Strel’chuk, V.V.; Baidullaeva, A.; Mozol’, P.E. Raman Scattering by Tellurium Films on CdTe Single Crystals. J. Appl. Spectrosc. 1988, 48, 653–655. [Google Scholar] [CrossRef]
- Yannopoulos, S.N. Structure and Photo - Induced Effects in Elemental Chalcogens: A Review on Raman Scattering. J. Mater. Sci. Mater. Electron. 2020, 31, 7565–7595. [Google Scholar] [CrossRef]
- Brodsky, M.H.; Smith, J.E. The Raman Spectrum of Amorphous Tellurium. Phys. Stat. Sol. 1972, 52, 609–614. [Google Scholar] [CrossRef]
- Barnakova, Y.A.; Kasuyaa, A. Raman and Absorption Spectra of the Zeolites A and X Containing Selenium and Tellurium in the Nanopores. Mater. Sci. Eng. A 1996, 217/218, 129–134. [Google Scholar]
- Dzhagan, V.M.; Azhniuk, Y.M.; Milekhin, A.G.; Zahn, D.R.T. Vibrational Spectroscopy of Compound Semiconductor Nanocrystals. J. Phys. D Appl. Phys. 2018, 51, 503001. [Google Scholar] [CrossRef]
- Holah, G.D.; Schenk, A.A.; Perkowitz, S.; Tomlinson, R.D. Infrared Reflectivity of P-Type CnInTe2. Phys. Rev. B 1981, 23, 6288–6293. [Google Scholar] [CrossRef]
- Guc, M.; Litvinchuk, A.P.; Levcenko, S.; Valakh, M.Y.; Bodnar, I.V.; Dzhagan, V.M.; Izquierdo-Roca, V.; Arushanov, E.; Pérez-Rodríguez, A. Optical phonons in the wurtzstannite Cu2ZnGeS4 semiconductor: Polarized Raman spectroscopy and first-principle calculations. RSC Adv. 2016, 6, 13278–13285. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Boero, F.; Litvinchuk, A.P.; Delsante, S.; Borzone, G.; Pérez-Rodríguez, A.; Izquierdo-Roca, V. Structural Polymorphism in “Kesterite” Cu2ZnSnS4: Raman Spectroscopy and First-Principles Calculations Analysis. Inorg. Chem. 2017, 56, 3467–3474. [Google Scholar] [CrossRef]
- Litvinchuk, A.P.; Dzhagan, V.M.; Yukhymchuk, V.O.; Valakh, M.Y.; Parasyuk, O.V.; Piskach, L.V.; Wang, X.; Jacobson, A.J.; Zahn, D.R.T. Crystal Structure and Vibrational Properties of Cu2ZnSiSe4 Quaternary Semiconductor. Phys. Status Solidi Basic Res. 2016, 253, 1808–1815. [Google Scholar] [CrossRef]
- Dzhagan, V.M.; Litvinchuk, A.P.; Valakh, M.Y.; Kruszynska, M.; Kolny-Olesiak, J.; Himcinschi, C.; Zahn, D.R.T. Raman Scattering in Orthorhombic CuInS2 Nanocrystals. Phys. Stat. Sol. A 2014, 211, 195–199. [Google Scholar] [CrossRef]
- Raevskaya, A.; Rozovik, O.; Novikova, A.; Selyshchev, O.; Stroyuk, O.; Dzhagan, V.; Goryacheva, I.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. Luminescence and Photoelectrochemical Properties of Size-Selected Aqueous Copper-Doped Ag-In-S Quantum Dots. RSC Adv. 2018, 8, 7550–7557. [Google Scholar] [CrossRef] [Green Version]
- Christie, A.B.; Sutherland, I.; Walls, J.M. Studies of the Composition, Ion-Induced Reduction and Preferential Sputtering of Anodic Oxide Films on Hg0.8Cd0.2Te by XPS. Surf. Sci. 1983, 135, 225–242. [Google Scholar] [CrossRef]
- Kuriakose, L.; Simi, N.J.; Ison, V.V. CuZn2InTe4 Quantum Dots-a Novel Nanostructure Employing a Green Synthesis Route. RSC Adv. 2020, 10, 18560–18564. [Google Scholar] [CrossRef]
- Raievska, O.; Stroyuk, O.; Dzhagan, V.; Solonenko, D.; Zahn, D.R.T. Ultra-Small Aqueous Glutathione-Capped Ag–In–Se Quantum Dots: Luminescence and Vibrational Properties. RSC Adv. 2020, 10, 42178–42193. [Google Scholar] [CrossRef]






| Sample # | (NaOH), mL | Te Precursor | Nominal Amount of Te Precursor |
|---|---|---|---|
| 1 | 0.64 | NaHTe | stoichiometric |
| 2 | 0.1 | ||
| 3 | 0.64 | NaHTe | in deficit |
| 4 | 0.1 | ||
| 5 | 0.64 | Te + NaBH4 | in excess |
| 6 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhagan, V.; Kapush, O.; Mazur, N.; Havryliuk, Y.; Danylenko, M.I.; Budzulyak, S.; Yukhymchuk, V.; Valakh, M.; Litvinchuk, A.P.; Zahn, D.R.T. Colloidal Cu-Zn-Sn-Te Nanocrystals: Aqueous Synthesis and Raman Spectroscopy Study. Nanomaterials 2021, 11, 2923. https://doi.org/10.3390/nano11112923
Dzhagan V, Kapush O, Mazur N, Havryliuk Y, Danylenko MI, Budzulyak S, Yukhymchuk V, Valakh M, Litvinchuk AP, Zahn DRT. Colloidal Cu-Zn-Sn-Te Nanocrystals: Aqueous Synthesis and Raman Spectroscopy Study. Nanomaterials. 2021; 11(11):2923. https://doi.org/10.3390/nano11112923
Chicago/Turabian StyleDzhagan, Volodymyr, Olga Kapush, Nazar Mazur, Yevhenii Havryliuk, Mykola I. Danylenko, Serhiy Budzulyak, Volodymyr Yukhymchuk, Mykhailo Valakh, Alexander P. Litvinchuk, and Dietrich R. T. Zahn. 2021. "Colloidal Cu-Zn-Sn-Te Nanocrystals: Aqueous Synthesis and Raman Spectroscopy Study" Nanomaterials 11, no. 11: 2923. https://doi.org/10.3390/nano11112923
APA StyleDzhagan, V., Kapush, O., Mazur, N., Havryliuk, Y., Danylenko, M. I., Budzulyak, S., Yukhymchuk, V., Valakh, M., Litvinchuk, A. P., & Zahn, D. R. T. (2021). Colloidal Cu-Zn-Sn-Te Nanocrystals: Aqueous Synthesis and Raman Spectroscopy Study. Nanomaterials, 11(11), 2923. https://doi.org/10.3390/nano11112923







