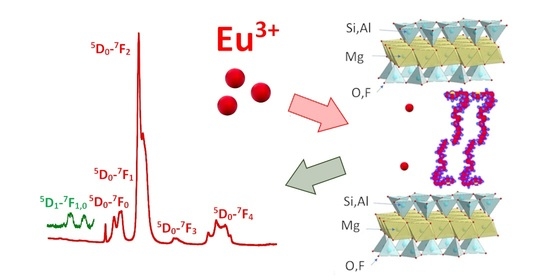
Adsorptive Capture of Ionic and Non-Ionic Pollutants Using a Versatile Hybrid Amphiphilic-Nanomica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of High Charge Micas
2.2. Synthesis of the Organo and the Amphiphilic-Mica
2.3. Adsorption of Cyclohexylamine and Eu3+Cations
2.4. Characterization
3. Results and Discussion
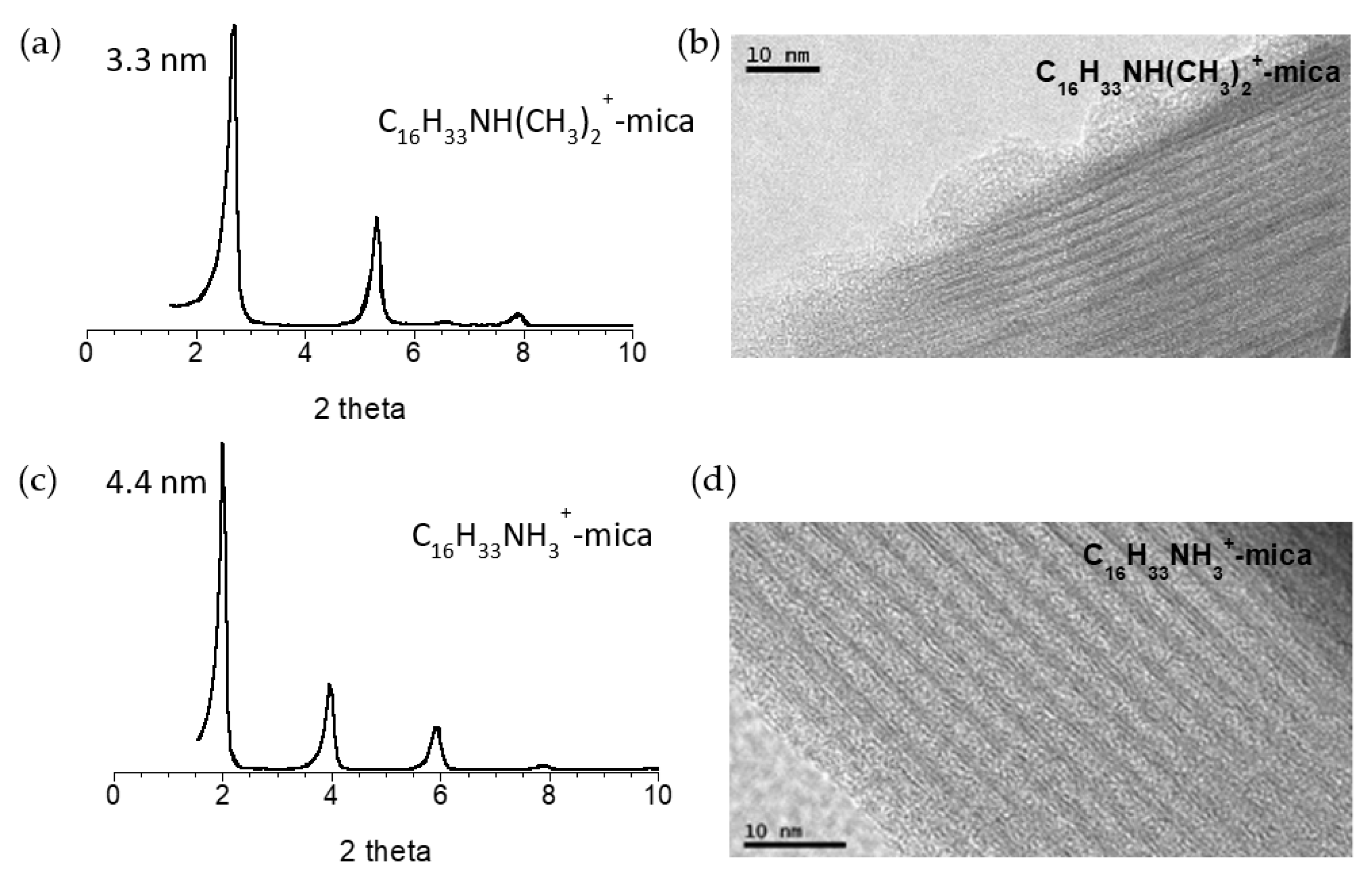
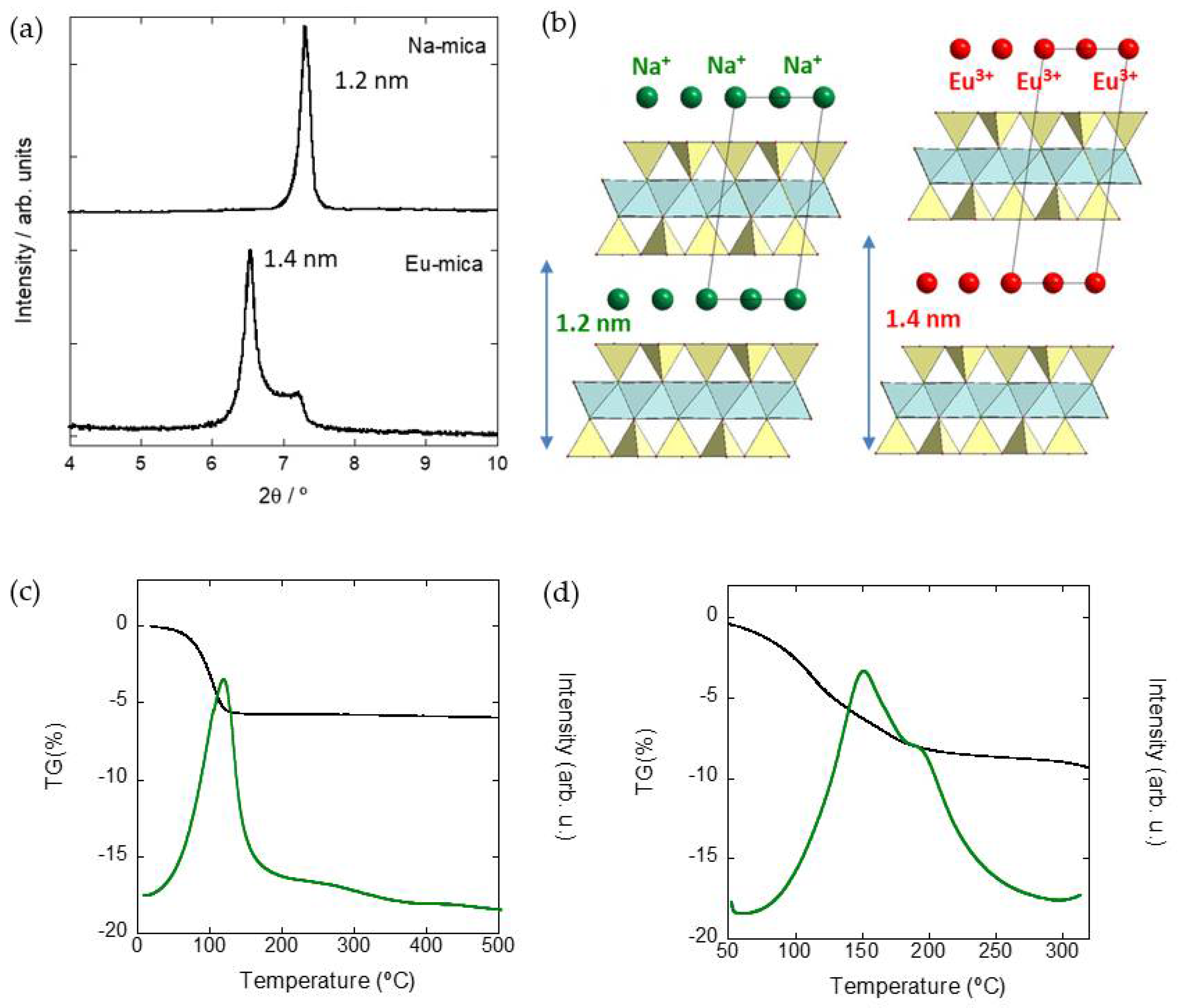
3.1. Functionalization of Na-4-Mica
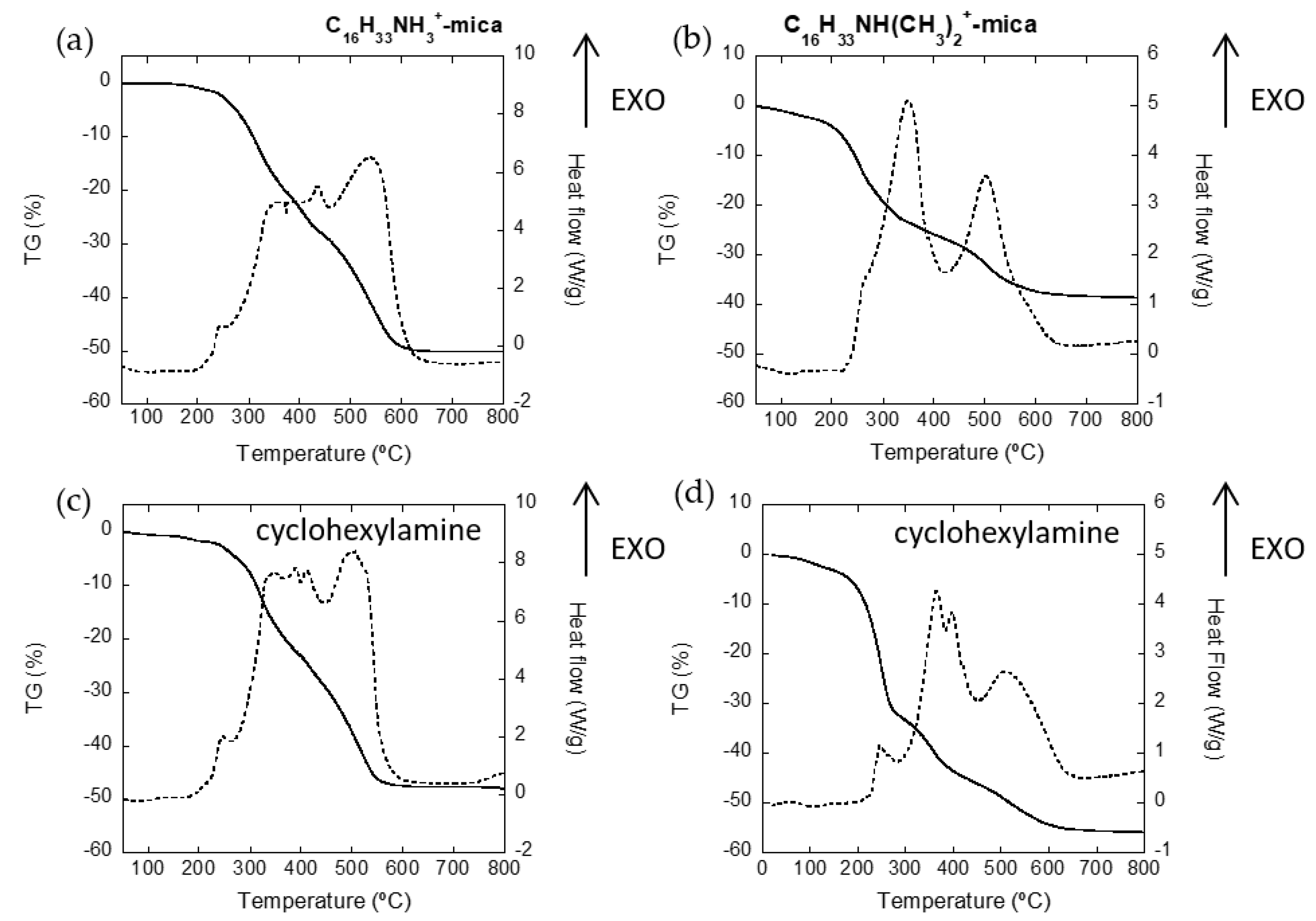
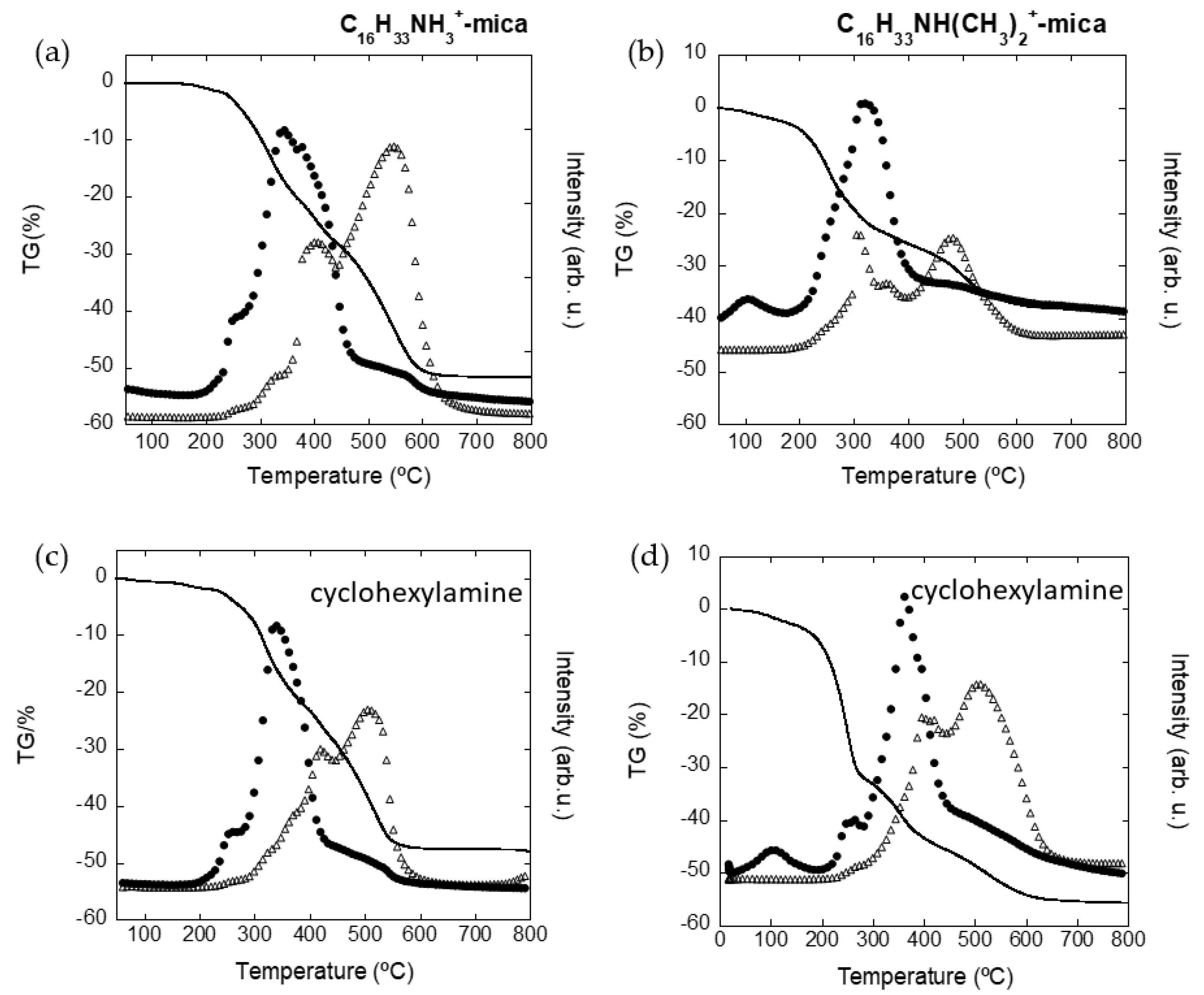
3.2. Adsorption of Cyclohexylamine
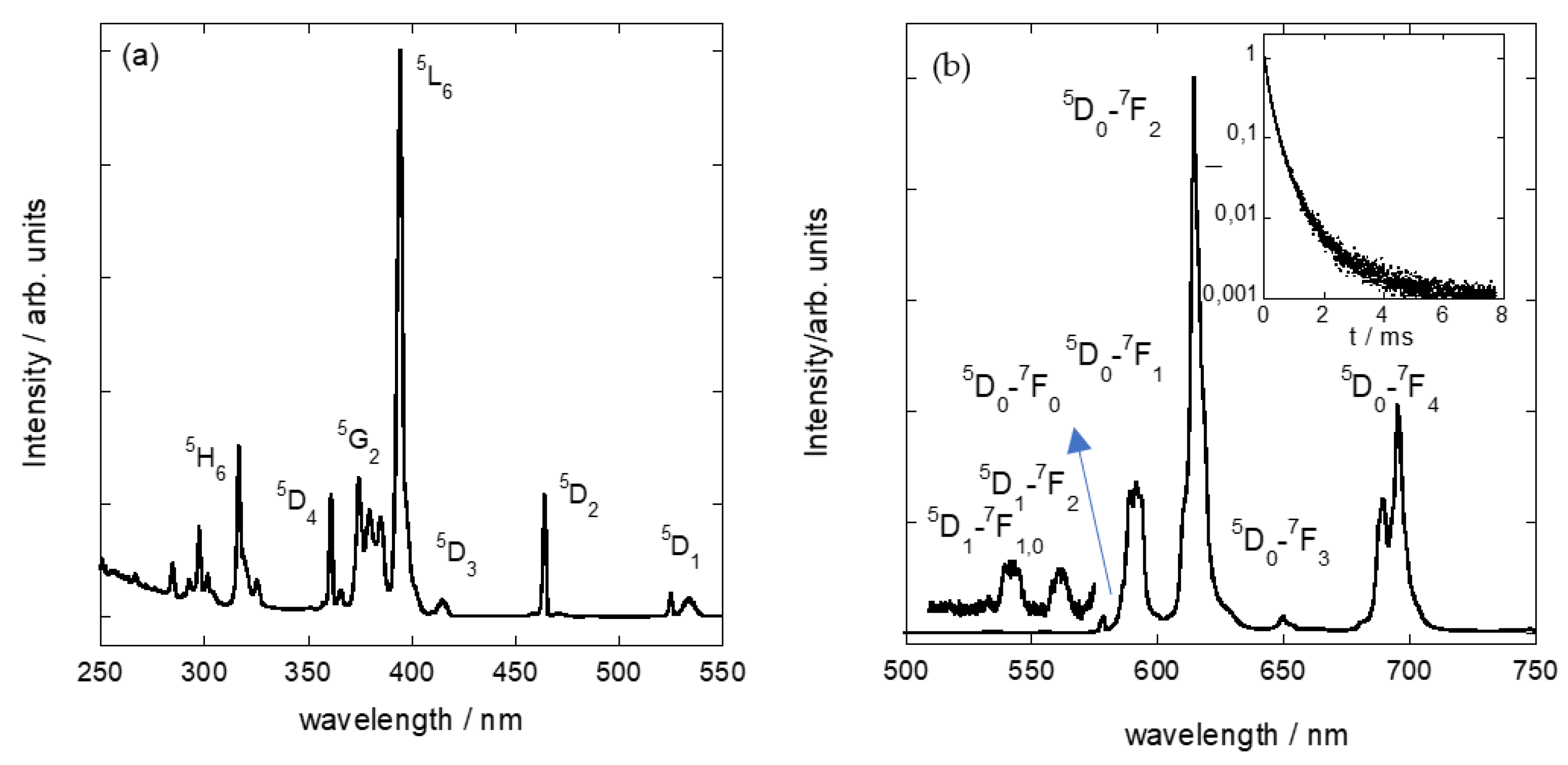
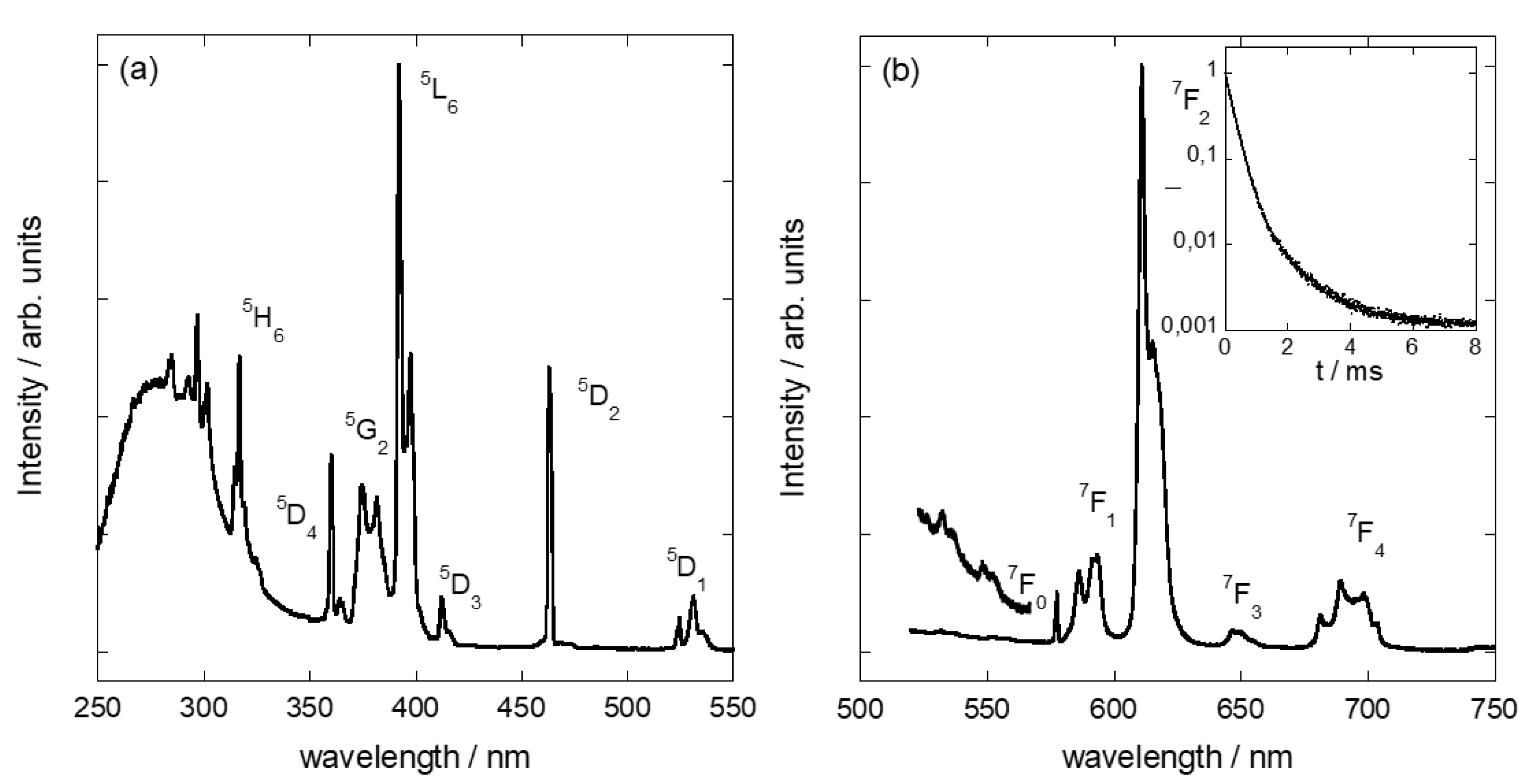
3.3. Adsorption of Eu3+Cations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide/layered silicate nanocomposite: A novel biodegradable material. Nano Lett. 2002, 2, 1093–1096. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer-layered silicate nanocomposites. Propylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming Montmorillonite surfaces using a cationic surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef] [PubMed]
- Lagaly, G. Interaction of alkylamines with different types of layered compounds. Solid State Ion. 1986, 22, 43–51. [Google Scholar] [CrossRef]
- Alba, M.D.; Castro, M.A.; Orta, M.M.; Pavón, E.; Pazos, M.C.; Valencia Rios, J.S. Formation of organo-highly charged mica. Langmuir 2011, 27, 9711–9718. [Google Scholar] [CrossRef]
- Pazos, M.C.; Castro, M.A.; Orta, M.M.; Pavón, E.; Valencia-Rios, J.S.; Alba, M.D. Synthetic high-charge organomica: Effect of the layer charge and alkyl chain length on the structure of the adsorbed surfactants. Langmuir 2012, 28, 7325–7332. [Google Scholar] [CrossRef]
- Pazos, M.C.; Cota, A.; Osuna, F.J.; Pavón, E.; Alba, M.D. Self-assembling of tetradecylammonium chain on swelling high charge micas (Na-Mica-3 and Na-Mica-2): Effect of alkylammonium concentration and mica layer charge. Langmuir 2015, 31, 4394–4401. [Google Scholar] [CrossRef] [Green Version]
- Pazos, M.C.; Castro, M.A.; Cota, A.; Osuna, F.J.; Pavón, E.; Alba, M.D. New insights into Surface-functionalized swelling high charged micas: Their adsorption performance for non-ionic organic pollutants. J. Ind. Eng. Chem. 2017, 52, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; Orta, M.D.M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Removal of priority and emerging pollutants from aqueous media by adsorption onto synthetic organo-funtionalized high-charge swelling micas. Environ. Res. 2018, 164, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Orta, M.D.M.; Martín, J.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Novel synthetic clays for the adsorption of surfactants from aqueous media. J. Environ. Manag. 2018, 206, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Orta, M.D.M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Evaluation of a modified mica and montmorillonite for the adsorption of ibuprofen from aqueous media. Appl. Clay Sci. 2019, 171, 29–37. [Google Scholar] [CrossRef]
- Osuna, F.J.; Pavón, E.; Alba, M.D. Pb2+, Cd2+ and Hg2+ removal by designed functionalized swelling high-charged micas. Sci. Total Environ. 2021, 764, 142811. [Google Scholar] [CrossRef]
- Osuna, F.J.; Pavón, E.; Pazos, M.C. Designed organomicaceous materials for efficient adsorption of iodine. J. Environ. Chem. Eng. 2021, 9, 106577. [Google Scholar] [CrossRef]
- Ijdo, W.L.; Pinnavaia, T.J. Staging of organic and inorganic gallery cations in layered silicate heterostructures. J. Solid State Chem. 1998, 139, 281–289. [Google Scholar] [CrossRef]
- Pesquera, C.; Aguado, F.; González, F.; Blanco, C.; Rodríguez, L.; Perdigón, A.C. Tunable interlayer hydrophobicity in a nanostructured high charge organo-mica. Micro. Meso. Mater. 2018, 263, 77–85. [Google Scholar] [CrossRef]
- Groenewold, G.S.; Ingram, J.C.; Gianotto, A.K.; Appelhans, A.D.; Delmore, J.E. Static secondary ionization mass spectrometry detection of cyclohexylamine on soil surfaces exposed to laboratory air. J. Am. Soc. Mass Spectrom. 1996, 7, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Martín-Rodríguez, R.; Valiente, R.; Aguado, F.; Perdigón, A.C. Highly efficient photoluminescence from isolated Eu3+ ions embedded in high-charge mica. J. Mater. Chem. C 2017, 5, 10360–10368. [Google Scholar] [CrossRef]
- Komarneni, S.; Kozai, N.; Paulus, W.J. Superselective clay for radium uptake. Nature 2001, 410, 771. [Google Scholar] [CrossRef]
- Morris, D.E.; Chisholm-Brause, C.J.; Barr, M.E.; Conradson, S.D.; Eller, P.G. Optical spectroscopic studies of the sorption of UO2+ 2 species on a reference smectite. Geochim. Cosmochim. Acta 1994, 58, 3613–3623. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B.; Geckeis, H.; Rabung, T. Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 2: Surface complexation modelling. Geochim. Cosmochim. Acta 2005, 69, 5403–5412. [Google Scholar] [CrossRef]
- Fan, Q.H.; Tan, X.L.; Li, J.X.; Wang, X.K.; Wu, W.S.; Montavon, G. Sorption of Eu(III) on attapulgite studied by batch, XPS, and EXAFS techniques. Environ. Sci. Technol. 2009, 43, 5776–5782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Lee, D.H.; Choi, C.L.; Kim, S.S.; Kim, K.S.; Choi, J. Pure Na-4-mica: Synthesis and characterization. Chem. Mater. 2002, 14, 2582–2589. [Google Scholar] [CrossRef]
- Streitwiesser, A.; Heathcock, C.H.; Kosower, E.M. Introduction to Organic Chemistry, 4th ed.; Macmillan: New York, NY, USA, 1992; p. 75. [Google Scholar]
- Yariv, S.; Borisover, M.; Lapides, S. Few introducing comments on the thermal analysis of organoclays. J. Therm. Anal. Calorim. 2011, 105, 897–906. [Google Scholar] [CrossRef]
- Dweck, J.; Barreto, E.P.; Meth, S.; Büchler, P.M. Partially exchanged organophilic bentonites. J. Therm. Anal. Calorim. 2011, 105, 907–913. [Google Scholar] [CrossRef]
- Ganguly, S.; Dana, K.; Ghatak, S. Thermogravimetric study of n-alkylammonium-intercalated montmorillonites of different cation exchange capacity. J. Therm. Anal. Calorim. 2010, 100, 71–78. [Google Scholar] [CrossRef]
- Önal, M.; Sarkaya, Y. Thermal analysis of some organoclays. J. Therm. Anal. Calorim. 2008, 91, 261–265. [Google Scholar] [CrossRef]
- Martín-Rodríguez, R.; Aguado, F.; Alba, M.D.; Valiente, R.; Perdigón, A.C. Eu3+ luminescence in high charge mica: An in situ probe for the encapsulation of radioactive waste in geological repositories. ACS Appl. Mater. Interfaces 2019, 11, 7559–7565. [Google Scholar] [CrossRef]
- Alba, M.D.; Castro, M.A.; Naranjo, M.; Pavón, E. Hydrothermal reactivity of Na-n-Micas (n = 2, 3, 4). Chem. Mater. 2006, 18, 2867–2872. [Google Scholar] [CrossRef]
- Perdigón, A.C.; Li, D.; Pesquera, C.; González, F.; Ortiz, B.; Aguado, F.; Blanco, C. Synthesis of porous clay heterostructures from high charge mica-type aluminosilicates. J. Mater. Chem. A 2013, 1, 1213–1219. [Google Scholar] [CrossRef]
- García-Jiménez, M.J.; Cota, A.; Osuna, F.J.; Pavón, E.; Alba, M.D. Influence of temperature and time on the Eu3+ reaction with synthetic Na-Mica-n (n = 2 and n = 4). Chem. Eng. J. 2016, 284, 1174–1183. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.D.; Ozin, G.A.; Olken, M.M. Laser-induced fluorescence, far-infrared spectroscopy, and luminescence quenching of europium zeolite Y: Site-selective probes of extraframework cations. J. Am. Chem. Soc. 1988, 110, 5709–5714. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium (III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Huang, X.; Zeng, Y. Synthesis and photoluminescence properties of novel highly thermal-stable red-emitting Na3Sc2(PO4)3:Eu3+ phosphors for UV-excited white-light-emitting diodes. J. Alloys Compd. 2018, 741, 300–306. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado, F.; Martín-Rodríguez, R.; Pesquera, C.; Valiente, R.; Perdigón, A.C. Adsorptive Capture of Ionic and Non-Ionic Pollutants Using a Versatile Hybrid Amphiphilic-Nanomica. Nanomaterials 2021, 11, 3167. https://doi.org/10.3390/nano11123167
Aguado F, Martín-Rodríguez R, Pesquera C, Valiente R, Perdigón AC. Adsorptive Capture of Ionic and Non-Ionic Pollutants Using a Versatile Hybrid Amphiphilic-Nanomica. Nanomaterials. 2021; 11(12):3167. https://doi.org/10.3390/nano11123167
Chicago/Turabian StyleAguado, Fernando, Rosa Martín-Rodríguez, Carmen Pesquera, Rafael Valiente, and Ana C. Perdigón. 2021. "Adsorptive Capture of Ionic and Non-Ionic Pollutants Using a Versatile Hybrid Amphiphilic-Nanomica" Nanomaterials 11, no. 12: 3167. https://doi.org/10.3390/nano11123167
APA StyleAguado, F., Martín-Rodríguez, R., Pesquera, C., Valiente, R., & Perdigón, A. C. (2021). Adsorptive Capture of Ionic and Non-Ionic Pollutants Using a Versatile Hybrid Amphiphilic-Nanomica. Nanomaterials, 11(12), 3167. https://doi.org/10.3390/nano11123167