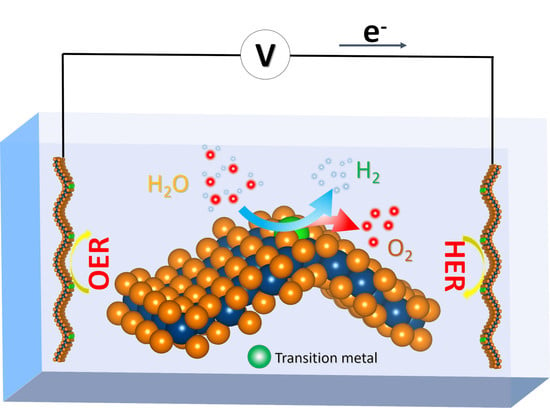
Computational Study of the Curvature-Promoted Anchoring of Transition Metals for Water Splitting
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
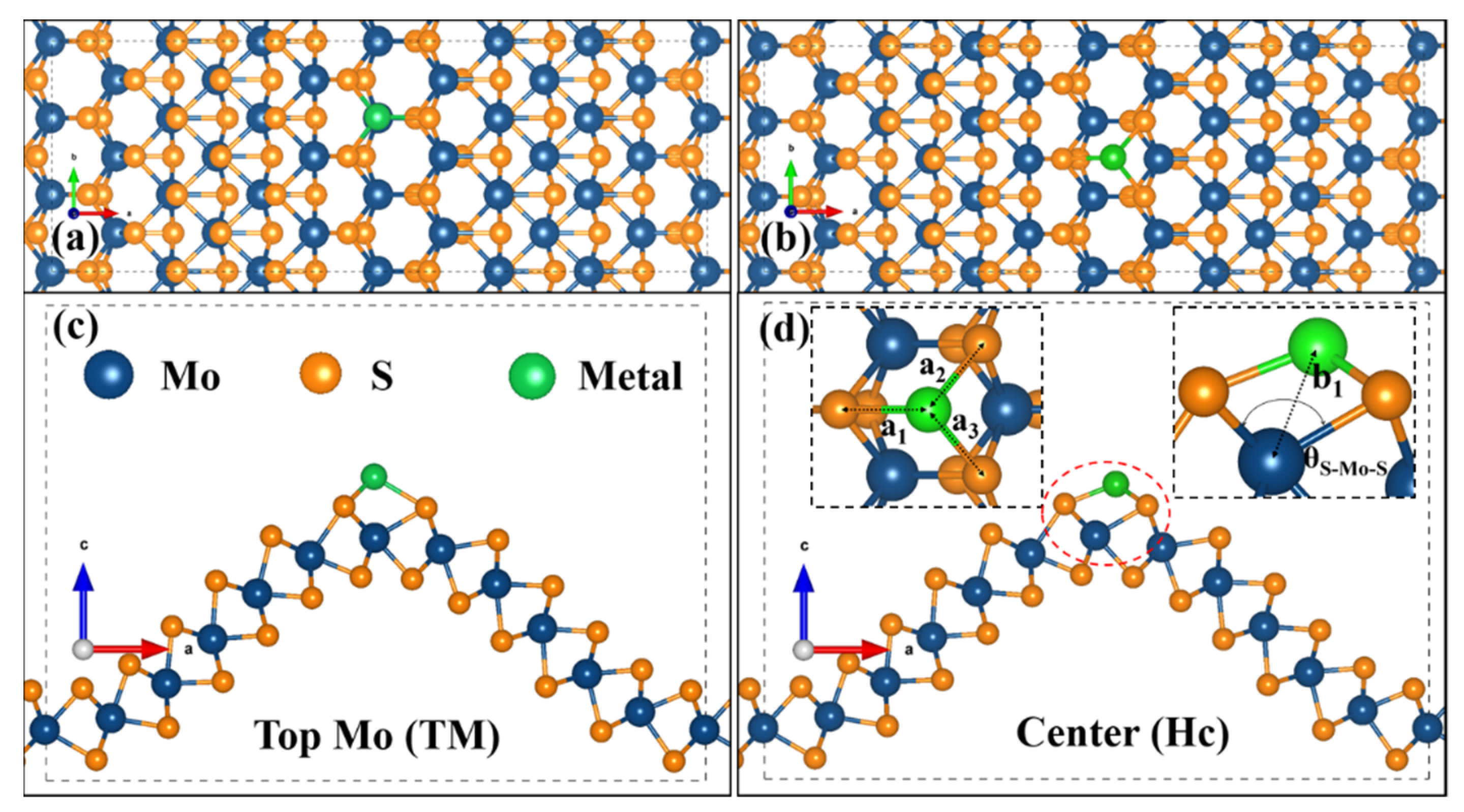
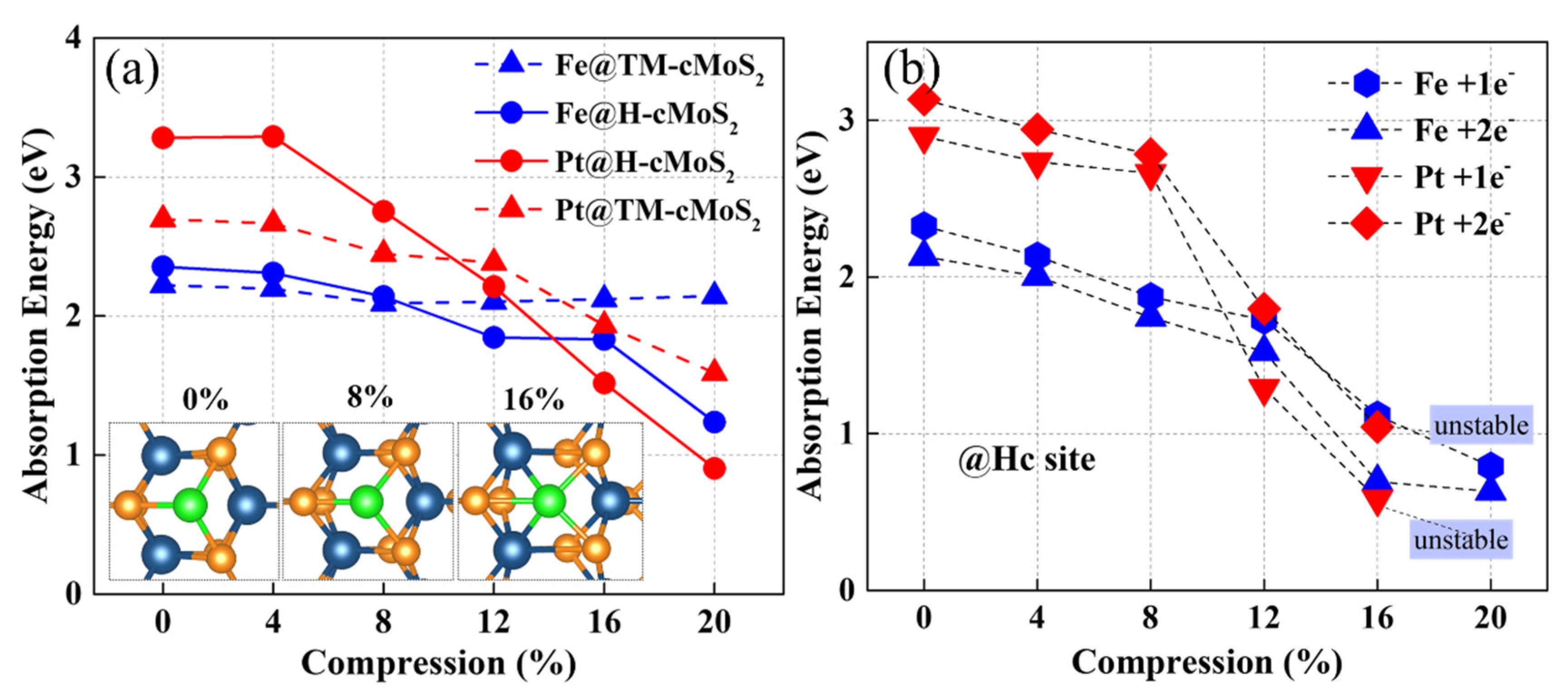
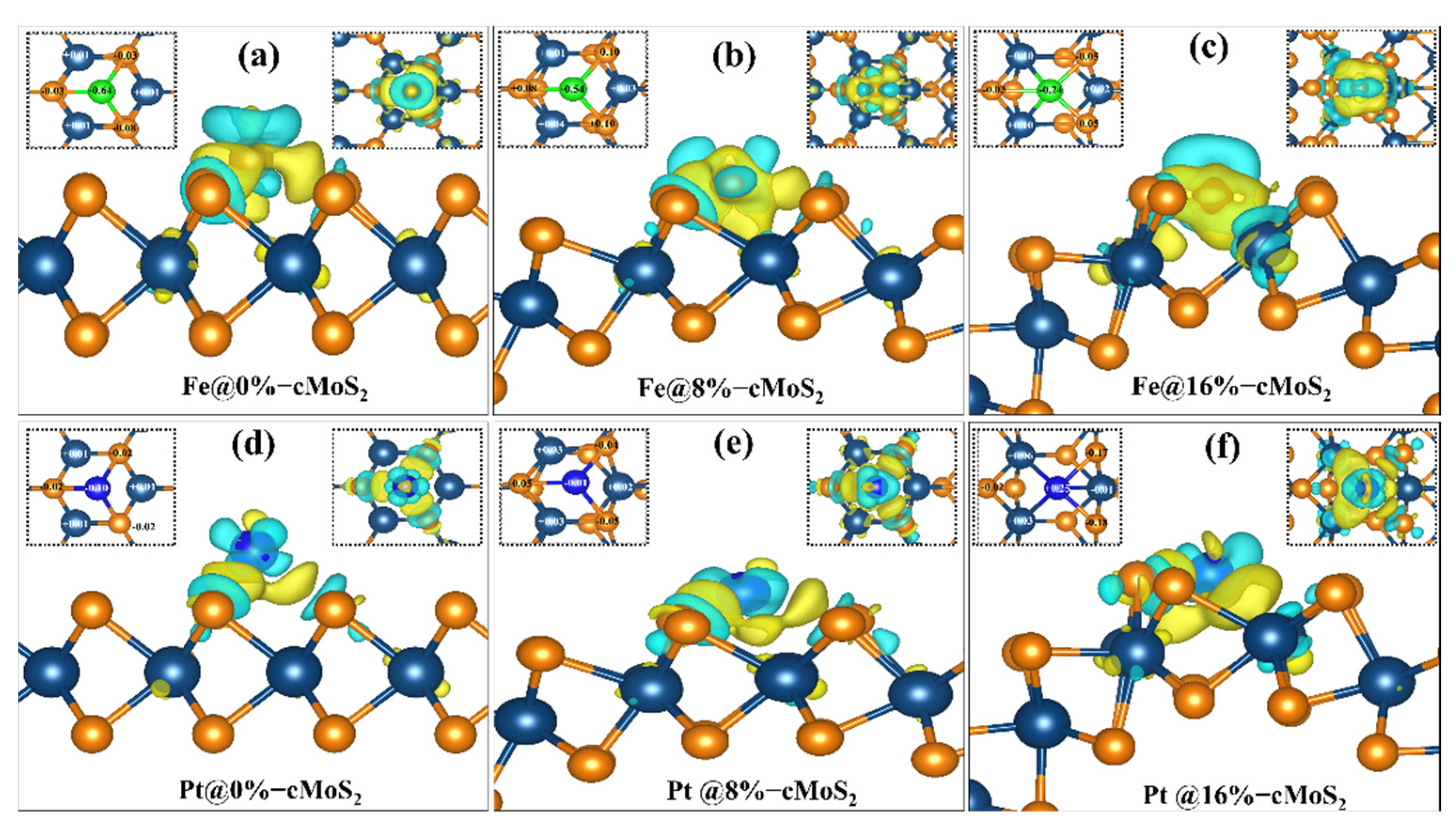
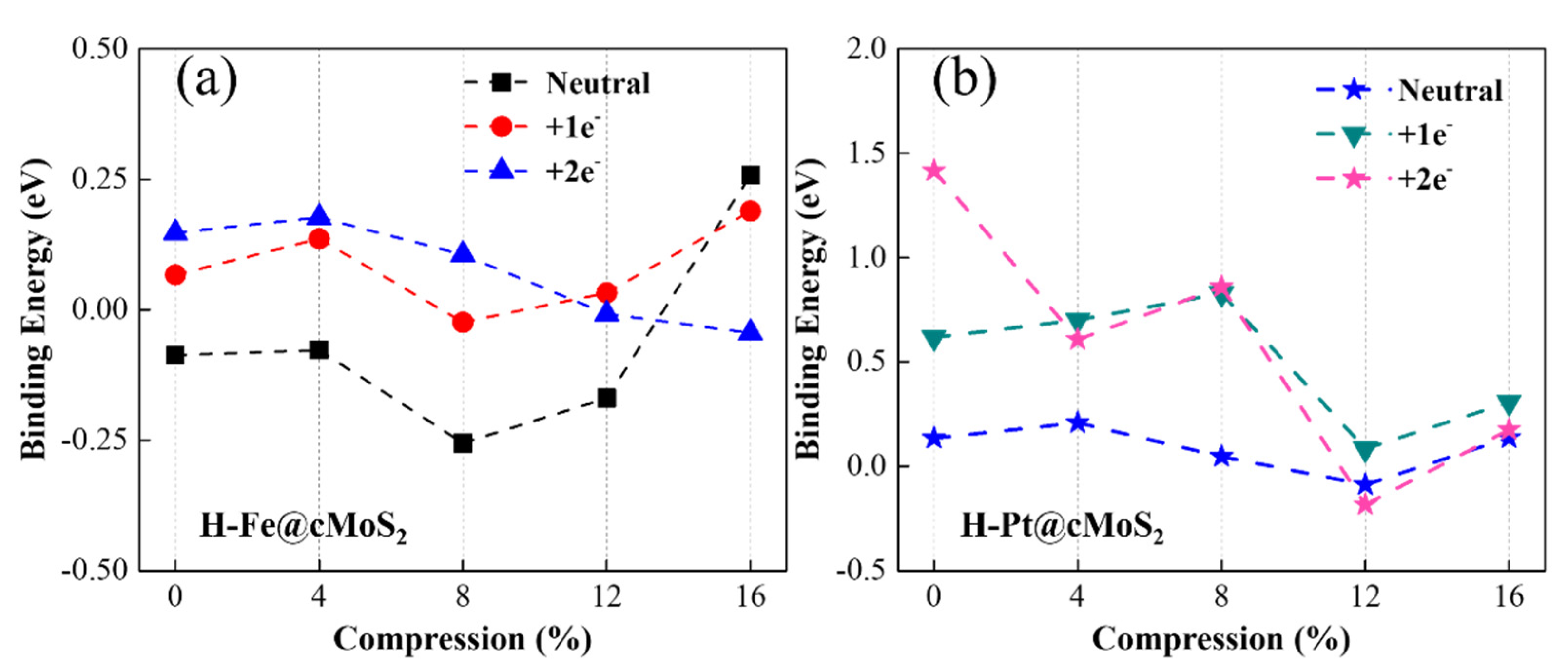
3.1. Anchoring-Activity of Metal on cMoS2
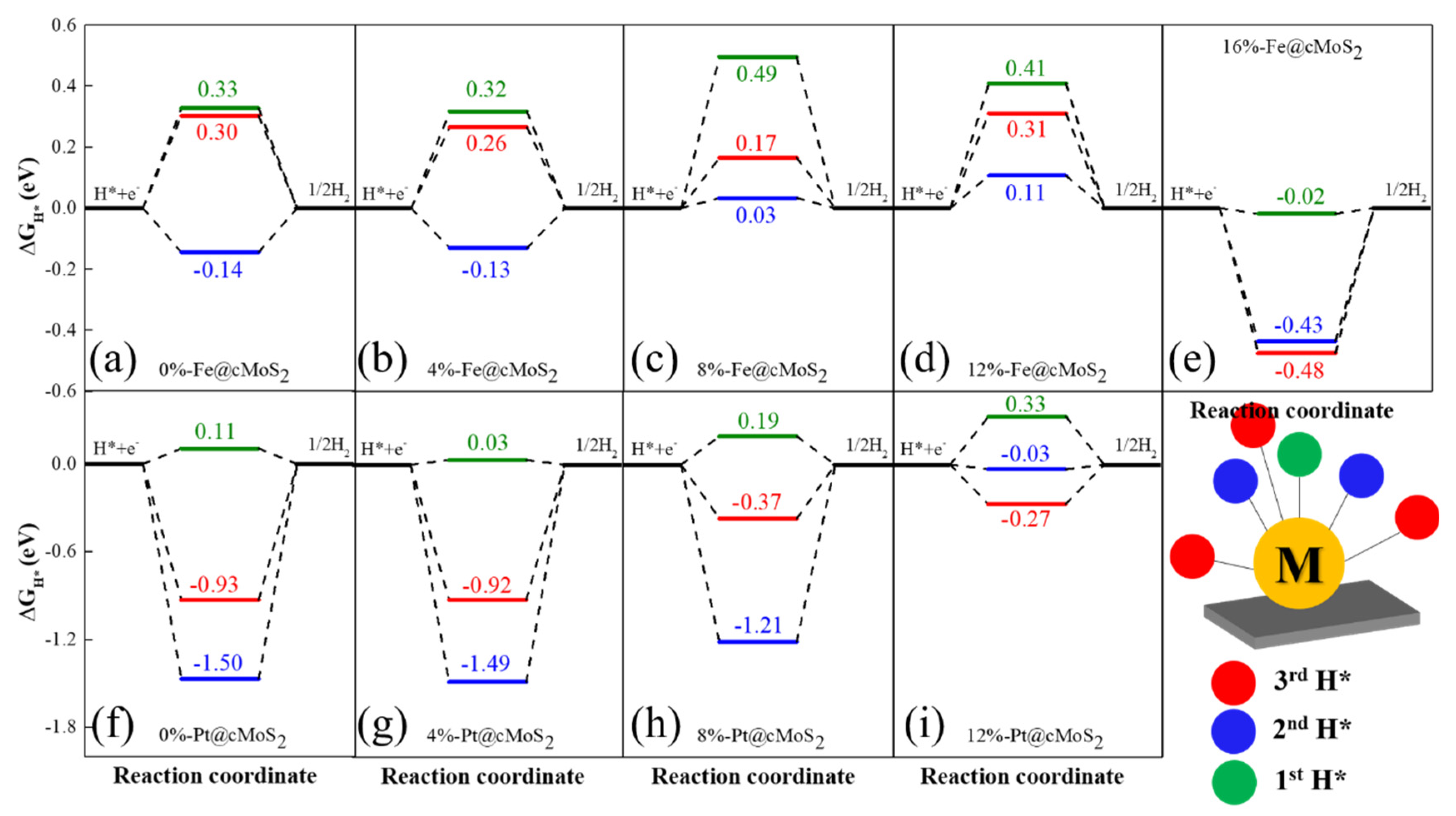
3.2. Hydrogen Evolution Reaction
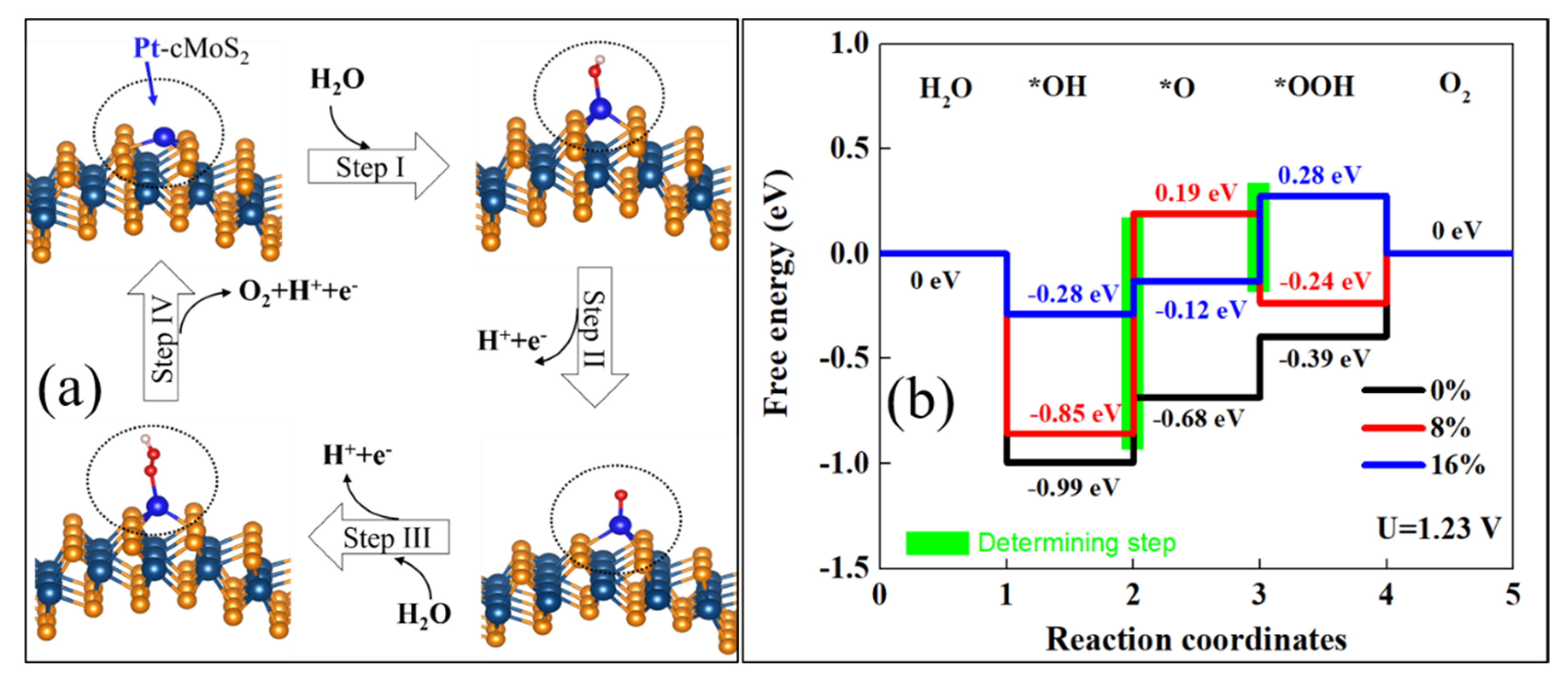
3.3. Oxygen Evolution Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Momirlan, M.; Veziroglu, T. Current status of hydrogen energy, Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Murthya, A.; Madhavana, J.; Muruganb, K. Recent advances in hydrogen evolution reaction catalysts on carbon/carbon-based supports in acid media. J. Power Sources 2018, 398, 9–26. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, J.; Gao, J. Interface Chemistry of Platinum-Based Materials for Electrocatalytic Hydrogen Evolution in Alkaline Conditions. In Methods for Electrocatalysis; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Zou, X.X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent Strategies for Improving the Catalytic Activity of 2D TMD Nanosheets Toward the Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Yan, C.; Handoko, A.D.; Seh, Z.W.; Li, H.; Liu, Z. Ultrathin two-dimensional materials for photo- and electrocatalytic hydrogen evolution. Mater. Today 2018, 21, 749–770. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [Green Version]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Novoselov, K.; Fu, Q.; Zheng, N.; Tian, N.Z.Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Wu, D.; Zhang, X.; Zhao, X.; Zhou, Z. Computational Screening of 2D Materials and Rational Design of Heterojunctions for Water Splitting Photocatalysts. Small Methods 2018, 2, 1700359. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.-R.; Xu, W.-X.; Wang, C.; Wang, F.-B.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Rawal, T.B.; Rahman, T.S. Single-Layer MoS2 with Sulfur Vacancies: Structure and Catalytic Application. J. Phys. Chem. C 2014, 118, 5346–5351. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Alarawi, A.A.; Ramalingam, V.; He, J.-H. Recent advances in emerging single atom confined two-dimensional materials for water splitting applications. Mater. Today Energy 2019, 11, 1–23. [Google Scholar] [CrossRef]
- He, T.; Zhang, C.; Du, A. Single-atom supported on graphene grain boundary as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Eng. Sci. 2019, 194, 58–63. [Google Scholar] [CrossRef]
- Ma, D.; Li, T.; Zhang, X.; He, C.; Tang, Y.; Yang, Z. Modulating electronic, magnetic and chemical properties of MoS2 monolayer sheets by substitutional doping with transition metals. Appl. Surf. Sci. 2016, 364, 181–189. [Google Scholar] [CrossRef]
- Saab, M.; Raybaud, P. Tuning the Magnetic Properties of MoS2 Single Nanolayers by 3d Metals Edge Doping. J. Phys. Chem. C 2016, 120, 10691–10697. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Modulating the Electronic Structure of Single-Atom Catalysts on 2D Nanomaterials for Enhanced Electrocatalytic Performance. Small Methods 2019, 3, 1800438. [Google Scholar] [CrossRef]
- Qi, K.; Yu, S.; Wang, Q.; Zhang, W.; Fan, J.; Zheng, W.; Cui, X. Decoration of the Inert Basal Plane of Defect-Rich MoS2 with Pd Atoms for Achieving Pt-Similar HER Activity. J. Mater. Chem. A 2016, 4, 4025–4031. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable Hydrogen Evolution Reaction Catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Fei, H.; Dong, J.; Arellano-Jiménez, M.J.; Ye, G.; Kim, N.D.; Samuel, E.L.G.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Matta, S.; Will, G.; Du, A. Transition-metal single atoms anchored on graphdiyne as high-efficiency electrocatalysts for water splitting and oxygen reduction. Small Methods 2019, 3, 1800419. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-Atom Catalysts: From Design to Application. Electrochem. Energy Rev. 2019, 2, 539–573. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector Augmented-wave Method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kong, Y.; Ai, H.; Wang, W.; Xie, X.; Lo, K.H.; Wang, S.; Pan, H. Waved 2D Transition-Metal Disulfides for Nanodevices and Catalysis: A First-Principle Study. ACS Appl. Nano Mater. 2020, 3, 2804–2812. [Google Scholar] [CrossRef]
- Laursen, A.B.; Varela, A.S.; Dionigi, F.; Fanchiu, H.; Miller, C.; Trinhammer, O.L.; Rossmeisl, J.; Dahl, S. Electrochemical Hydrogen Evolution: Sabatier’s Principle and the Volcano Plot. J. Chem. Educ. 2012, 89, 1595–1599. [Google Scholar] [CrossRef]
- Nørskov, J.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Joshi, Y.V.; Ghosh, P.; Venkataraman, P.S.; Delgass, W.N.; Thomson, K.T. Electronic Descriptors for the Adsorption Energies of Sulfur-Containing Molecules on Co/MoS2, Using DFT Calculations. J. Phys. Chem. C 2009, 113, 9698–9709. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. Density Functional Theory Study of the Water Dissociation on Platinum Surfaces: General Trends. J. Phys. Chem. A 2014, 118, 5832–5840. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Z.; Searles, D.; Chen, Y.; Lu, G.; Du, A. Charge-Controlled Switchable CO2 Capture on Boron Nitride Nanomaterials. J. Am. Chem. Soc. 2013, 135, 8246–8253. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, H.; Cheng, D. Design of high-performance MoS2 edge supported single-metal atom bifunctional catalysts for overall water splitting via a simple equation. Nanoscale 2019, 11, 20228–20237. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yu, Z.G.; Seng, H.L.; Zhang, N.; Liu, X.; Zhang, Y.-W.; Yang, W.; Gong, H. Simultaneous edge and electronic control of MoS2 nanosheets through Fe doping for an efficient oxygen evolution reaction. Nanoscale 2018, 10, 20113–20119. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 Quantum Dots as Efficient Catalyst Materials for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Xiong, Q.; Wang, Y.; Liu, P.F.; Zheng, L.R.; Wang, G.; Yang, H.G.; Wong, P.K.; Zhang, H.; Zhao, H. Cobalt Covalent Doping in MoS2 to Induce Bifunctionality of Overall Water Splitting. Adv. Mater. 2018, 30, e1801450. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, X.; Deng, L.; Shi, Z.; Liu, S.; Wei, Q.; Zhang, L.; Cheng, Y.; Zhang, L.; Lu, H.; et al. Enhanced Valley Zeeman Splitting in Fe-Doped Monolayer MoS2. ACS Nano 2020, 14, 4636–4645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombrú, D.; Faccio, R.; Mombrú, Á.W. Possible doping of single-layer MoS2 with Pt: A DFT study. Appl. Surf. Sci. 2018, 462, 409–416. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Kong, Y.; Wang, B.; Li, X.; Liu, P.; Santiago, A.R.P.; He, T. Computational Study of the Curvature-Promoted Anchoring of Transition Metals for Water Splitting. Nanomaterials 2021, 11, 3173. https://doi.org/10.3390/nano11123173
Liu W, Kong Y, Wang B, Li X, Liu P, Santiago ARP, He T. Computational Study of the Curvature-Promoted Anchoring of Transition Metals for Water Splitting. Nanomaterials. 2021; 11(12):3173. https://doi.org/10.3390/nano11123173
Chicago/Turabian StyleLiu, Weiwei, Youchao Kong, Bo Wang, Xiaoshuang Li, Pengfei Liu, Alain R. Puente Santiago, and Tianwei He. 2021. "Computational Study of the Curvature-Promoted Anchoring of Transition Metals for Water Splitting" Nanomaterials 11, no. 12: 3173. https://doi.org/10.3390/nano11123173
APA StyleLiu, W., Kong, Y., Wang, B., Li, X., Liu, P., Santiago, A. R. P., & He, T. (2021). Computational Study of the Curvature-Promoted Anchoring of Transition Metals for Water Splitting. Nanomaterials, 11(12), 3173. https://doi.org/10.3390/nano11123173