Synthesis, Characterisation, Photocatalytic Activity, and Aquatic Toxicity Evaluation of TiO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photocatalysts Synthesis
2.2. Photocatalysts Characterisation
2.3. Photocatalytic Tests
2.4. Toxicity Effect of TiO2 Nanoparticles and Imidacloprid on Chlorella vulgaris Growth
3. Results and Discussion
3.1. Materials Characterisation
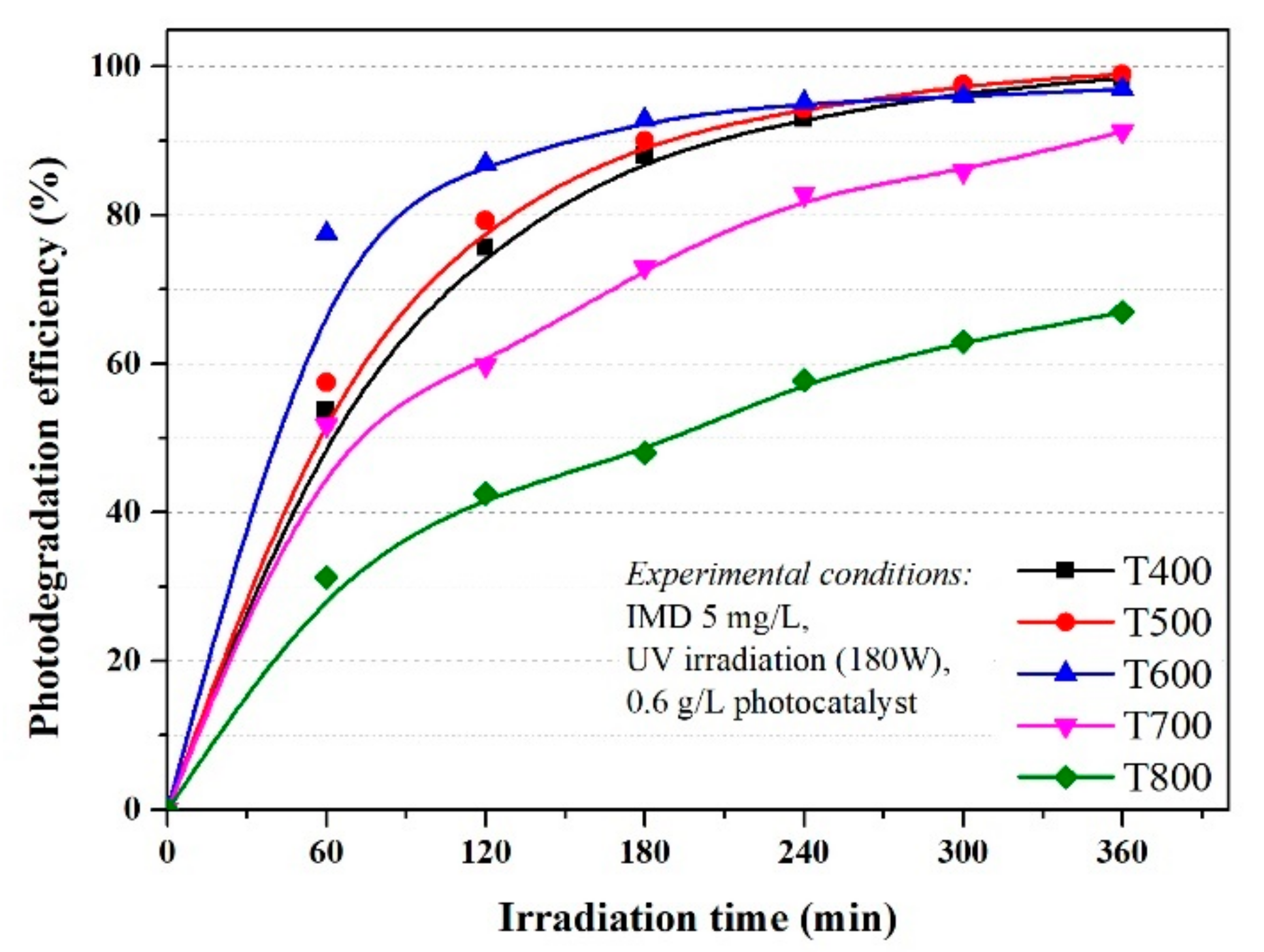
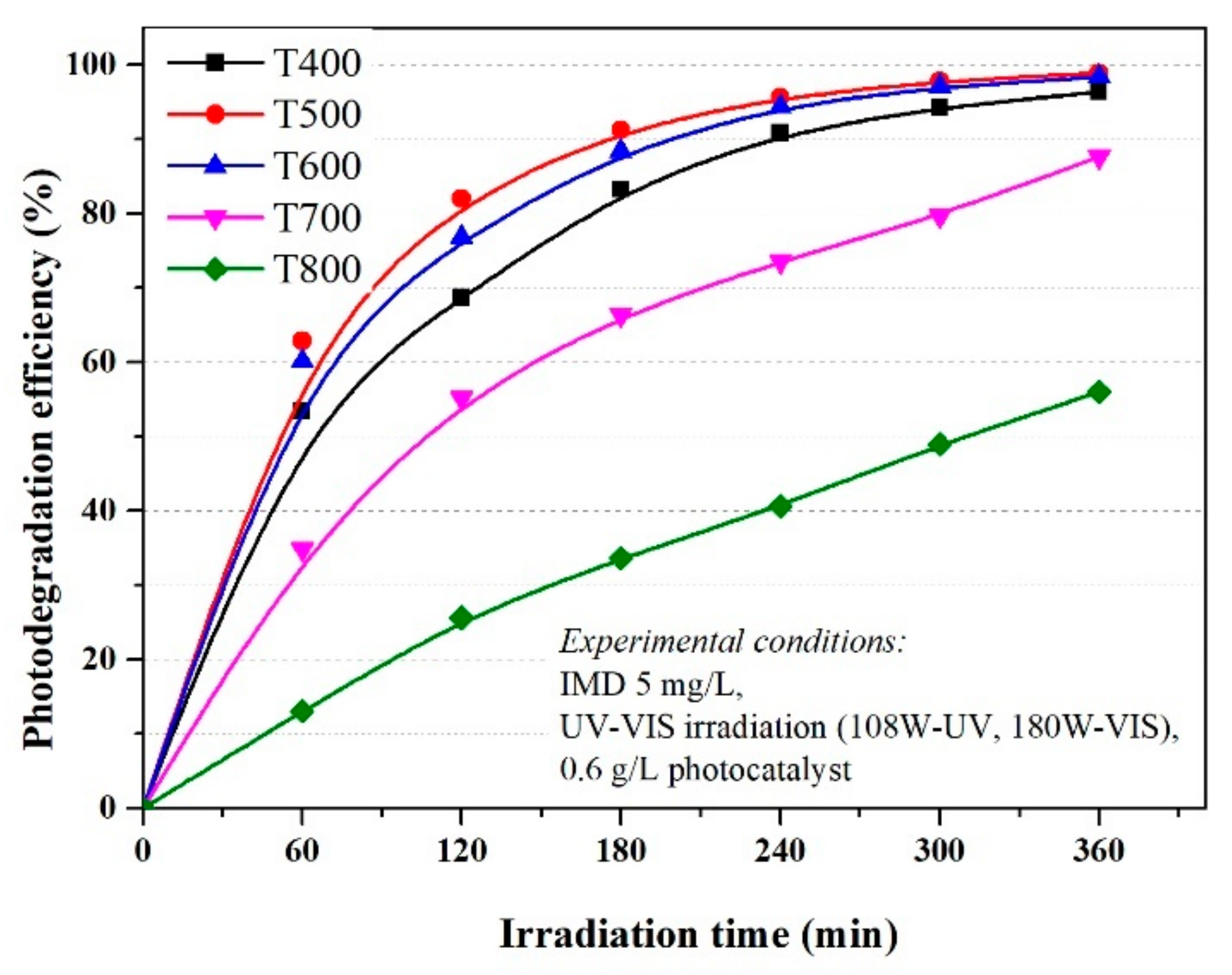
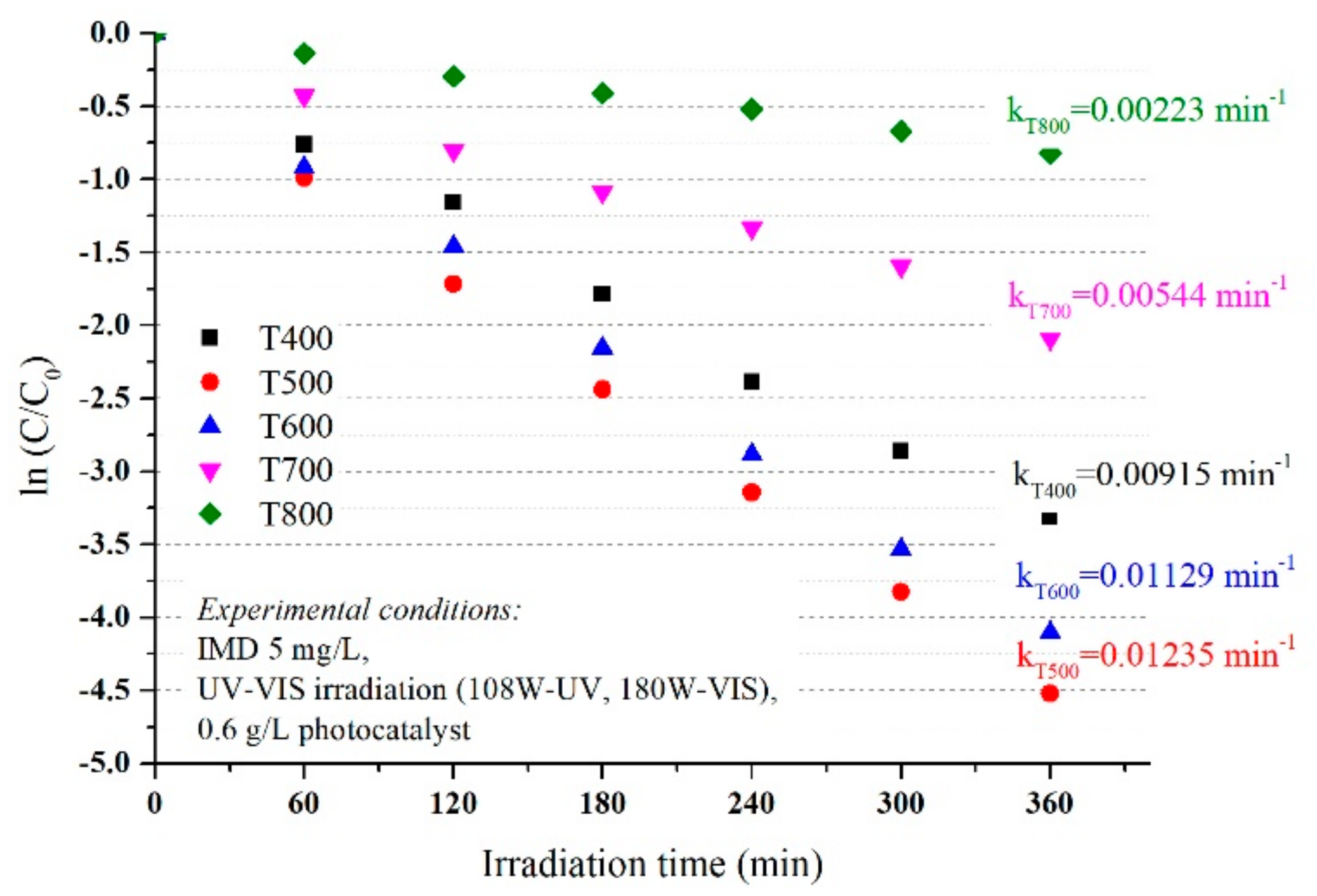
3.2. Evaluation of the Photocatalytic Activity and Kinetics of TiO2 on Imidacloprid Degradation
- −
- at low IMD concentrations, the active centres on the catalyst surface are not fully occupied by the pollutant molecules, the hydroxide HO. radicals responsible for the photodegradation form in higher concentrations and increases the efficiency of the photodegradation IMD; and
- −
- at very high concentrations of IMD, the formation of hydroxide radicals on the catalyst surface is reduced because the ions adsorbed by IMD occupies the active centres.
3.3. The Scavengers Study
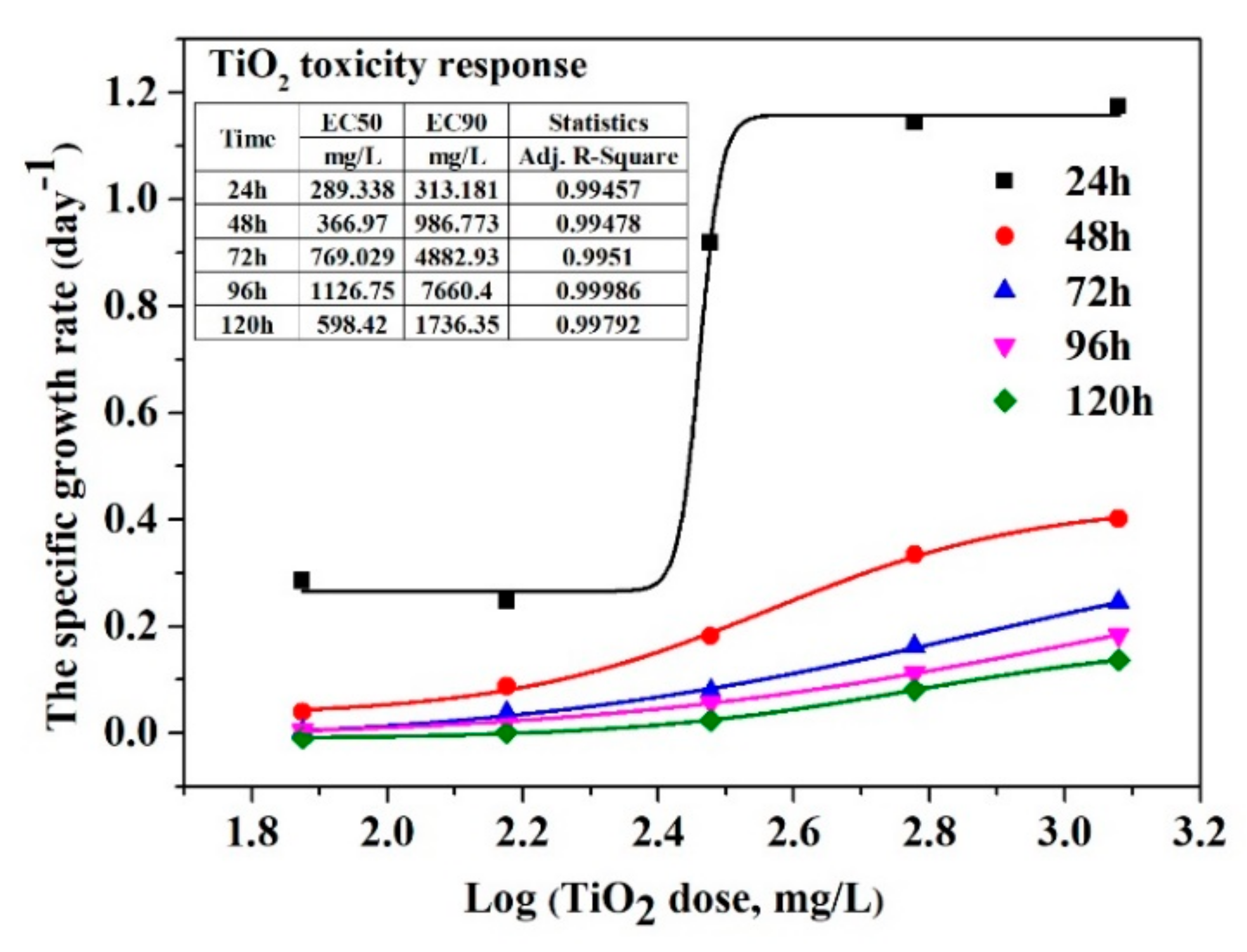
3.4. Aquatic Toxicity of Nanoparticles and Imidacloprid on Chlorella vulgaris Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifzadeh, M.S.; Abdollahzadeh, G.; Damalas, C.A.; Rezaei, R. Farmers’ criteria for pesticide selection and use in the pest control process. Agriculture 2018, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Bivehed, E.; Gustafsson, A.; Berglund, A.; Hellman, B. Evaluation of Potential DNA-Damaging Effects of Nitenpyram and Imidacloprid in Human U937-Cells Using a New Statistical Approach to Analyse Comet Data. Exp. Health 2020, 12, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Alammar, A.; Park, S.H.; Ibrahim, I.; Deepak, A.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Kaczyński, P.; Łozowicka, B.; Perkowski, M.; Zoń, W.; Hrynko, I.; Rutkowska, E.; Skibko, Z. Impact of broad-spectrum pesticides used in the agricultural and forestry sector on the pesticide profile in wild boar, roe deer and deer and risk assessment for venison consumers. Sci. Total Environ. 2021, 784, 147215. [Google Scholar] [CrossRef]
- Sarkar, M.A.; Biswas, P.K.; Roy, S.; Kole, R.K.; Chowdhury, A. Effect of pH and Type of Formulation on the Persistence of Imidacloprid in Water. Bull. Environ. Contam. Toxicol. 1999, 63, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Serrano, E.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Fast oxidation of the neonicotinoid pesticides listed in the EU Decision 2018/840 from aqueous solutions. Sep. Purif. Technol. 2020, 235, 116168. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Degradation of traditional and new emerging pesticides in water by nanomaterials: Recent trends and future recommendations. Int. J. Environ. Sci. Technol. 2018, 15, 1347–1380. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Zhao, L.; Qi, Z.; Gou, J.; Zhang, S.; Zhang, J.Z. Recent advances in ultrathin two-dimensional materials and biomedical applications for reactive oxygen species generation and scavenging. Nanoscale 2020, 12, 19516–19535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Adochite, C.; Andronic, L. Toxicity of a Binary Mixture of TiO2 and Imidacloprid Applied to Chlorella vulgaris. Int. J. Environ. Res. Public Health 2021, 18, 7785. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–55. ISBN 9780128054178. [Google Scholar]
- Augusta Ajuzieogu, C.; Obukowho Odokuma, L.; Chioma Blaise, C. Toxicity Assessment of Produced Water Using Microtox Rapid Bioassay. South Asian J. Res. Microbiol. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Alga, Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems; OECD Organisation: OECD: Paris, France, 2006; ISBN 9789264069923. [Google Scholar]
- Baruah, P.; Chaurasia, N. Ecotoxicological effects of alpha-cypermethrin on freshwater alga Chlorella sp.: Growth inhibition and oxidative stress studies. Environ. Toxicol. Pharmacol. 2020, 76, 103347. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Enesca, A.; Cazan, C.; Visa, M. TiO2–active carbon composites for wastewater photocatalysis. J. Sol-Gel. Sci. Technol. 2014, 71, 396–405. [Google Scholar] [CrossRef]
- Andronic, L.; Isac, L.; Miralles-Cuevas, S.; Visa, M.; Oller, I.; Duta, A.; Malato, S. Pilot-plant evaluation of TiO2 and TiO2-based hybrid photocatalysts for solar treatment of polluted water. J. Hazard. Mater. 2016, 320, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Adochite, C.; Andronic, L. Aquatic Toxicity of Photocatalyst Nanoparticles to Green Microalgae Chlorella vulgaris. Water 2020, 13, 77. [Google Scholar] [CrossRef]
- ISO 8692:2012. Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. Available online: https://standards.iteh.ai/catalog/standards/cen/f899663c-caa1-43f3-9ad8-5ed7304e5e4f/en-iso-8692-2012 (accessed on 23 November 2021).
- ISO 14442. Water Quality—Guidelines for Algal Growth Inhibition Tests with Poorly Soluble Materials, Volatile Compounds, Metals and Wastewater. Available online: https://www.iso.org/standard/34814.html (accessed on 23 November 2021).
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chemie Int. Ed. 2008, 47, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Gupta, R.; Bansal, A. Photocatalytic degradation of imidacloprid using semiconductor hybrid nano-catalyst: Kinetics, surface reactions and degradation pathways. Int. J. Environ. Sci. Technol. 2021, 18, 1425–1442. [Google Scholar] [CrossRef]
- Derbalah, A.; Sunday, M.; Chidya, R.; Jadoon, W.; Sakugawa, H. Kinetics of photocatalytic removal of imidacloprid from water by advanced oxidation processes with respect to nanotechnology. J. Water Health 2019, 17, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Babic, K.; Tomašic, V.; Gilja, V.; Le Cunff, J.; Gomzi, V.; Pintar, A.; Žerjav, G.; Kurajica, S.; Duplancic, M.; Zelic, I.E.; et al. Photocatalytic degradation of imidacloprid in the flat-plate photoreactor under UVA and simulated solar irradiance conditions—The influence of operating conditions, kinetics and degradation pathway. J. Environ. Chem. Eng. 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Wu, M.-C.; Hsiao, K.-C.; Chang, Y.-H.; Chan, S.-H. Photocatalytic hydrogen evolution of palladium nanoparticles decorated black TiO2 calcined in argon atmosphere. Appl. Surf. Sci. 2018, 430, 407–414. [Google Scholar] [CrossRef]
- Lou, W.; Kane, A.; Wolbert, D.; Rtimi, S.; Assadi, A.A. Study of a photocatalytic process for removal of antibiotics from wastewater in a falling film photoreactor: Scavenger study and process intensification feasibility. Chem. Eng. Process. Process Intensif. 2017, 122, 213–221. [Google Scholar] [CrossRef]
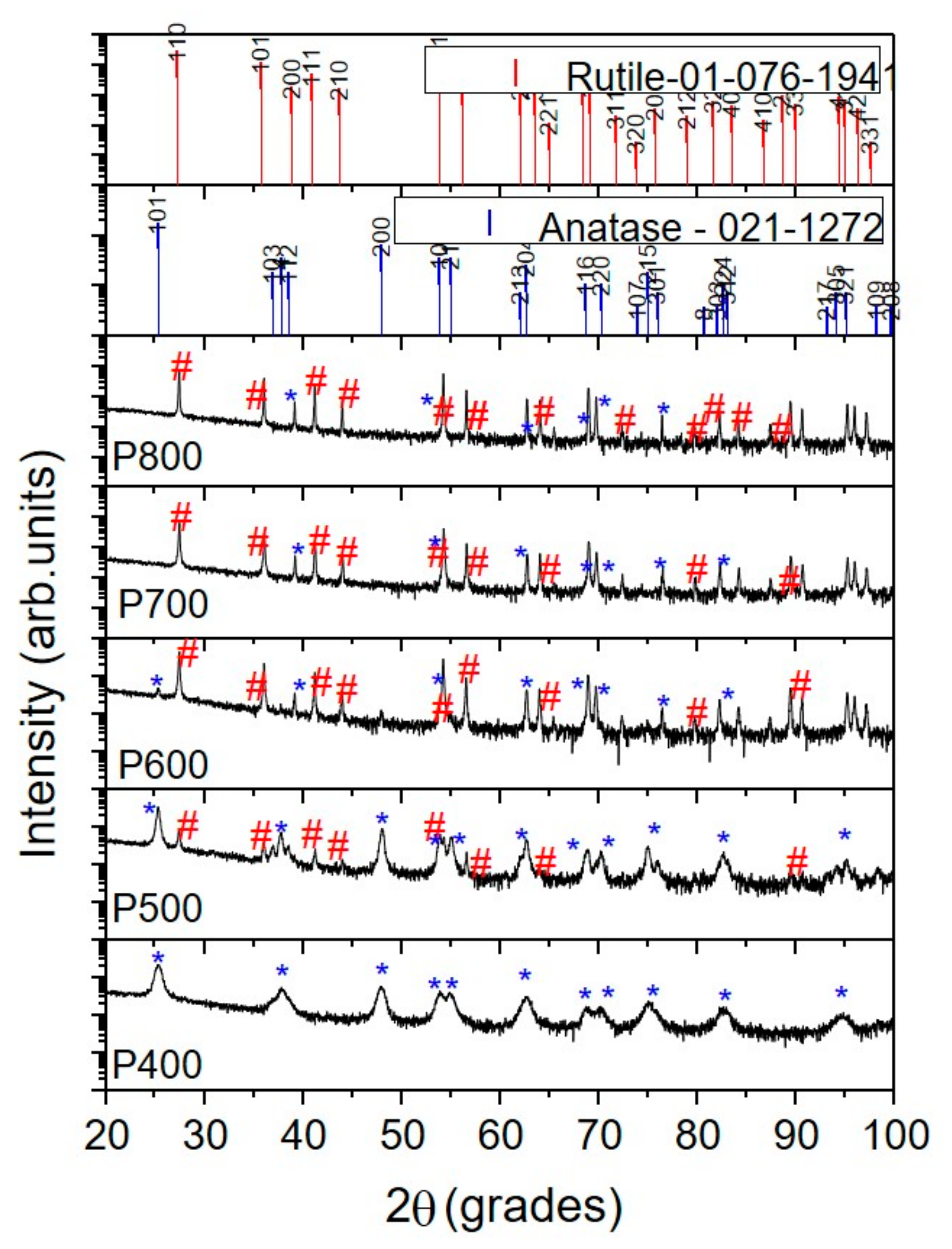
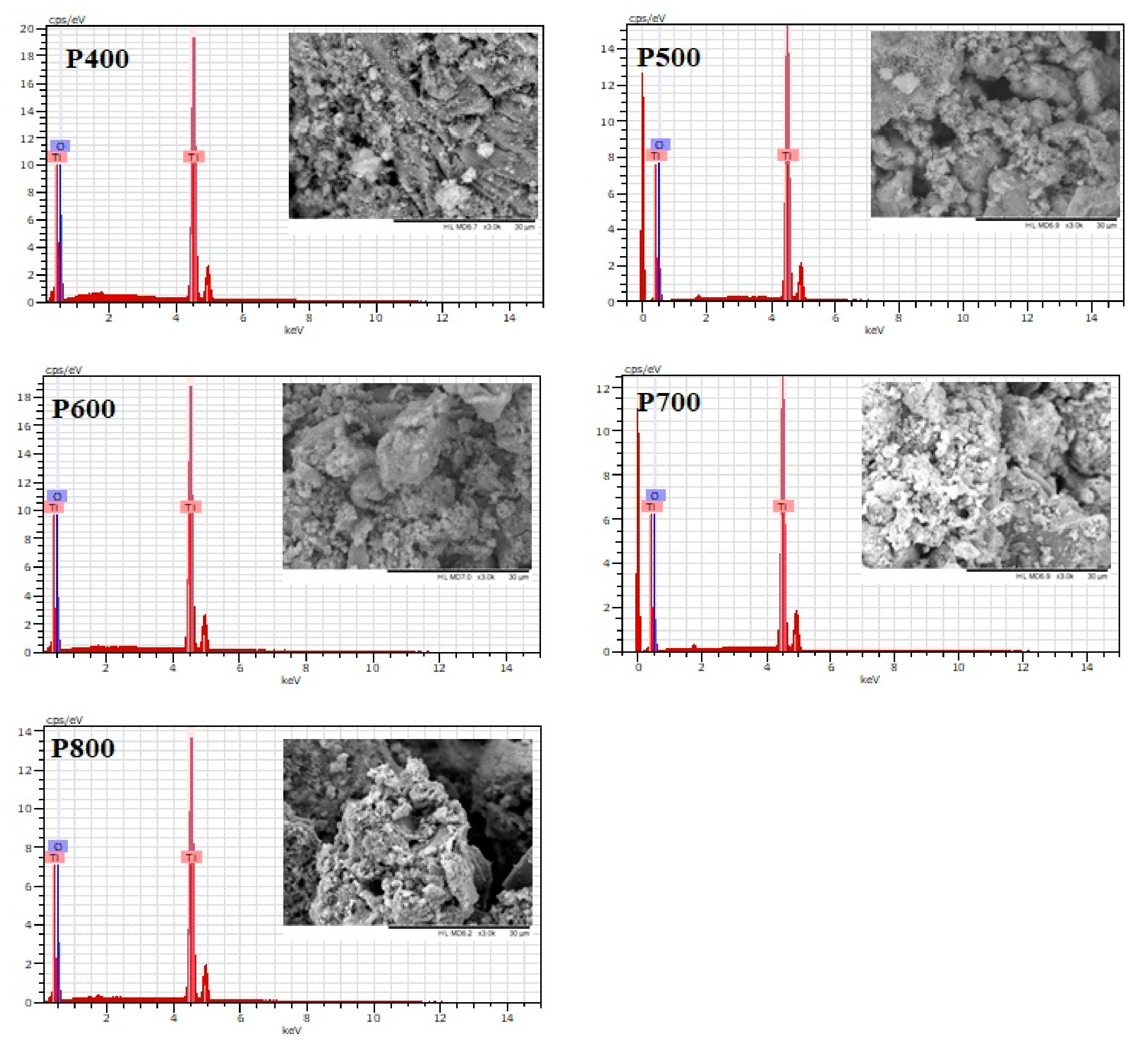



| Sample | Crystallinity (%) | Phases % | 2θ at (101) Anatase | Size (nm) | 2θ at (110) Rutile | Size (nm) |
|---|---|---|---|---|---|---|
| P400 | 74.8 | 100% anatase | 25.28 | 11.5 | - | |
| P500 | 75.2 | 98% anatase 2% rutile | 25.25 | 24.6 | 27.40 | 49 |
| P600 | 82.2 | 90% anatase 10% rutile | 25.24 | 32.4 | 27.39 | 72.5 |
| P700 | 85.7 | 100% rutile | - | - | 27.54 | 14.09 |
| P800 | 83.5 | 100% rutile | - | - | 27.39 | 10.3 |
| Sample | ANATASE | RUTILE | ||||||
|---|---|---|---|---|---|---|---|---|
| Plane (hkl) | (101) | (004) | (200) | (204) | (110) | (101) | (111) | (220) |
| P400 | 0.6345 | 0.1246 | 0.1612 | 0.0796 | - | - | - | - |
| P500 | 0.6525 | 0.0648 | 0.1860 | 0.0966 | 0.5132 | 0.2286 | 0.1423 | 0.1158 |
| P600 | 0.5139 | - | 0.1318 | 0.3541 | 0.6831 | 0.0473 | 0.1635 | 0.1059 |
| P700 | - | - | - | - | 0.7822 | 0.0365 | 0.1811 | - |
| P800 | - | - | - | - | 0.6299 | 0.2298 | 0.0961 | 0.0440 |
| Scavengers | Involved Radicals | Degradation after 6 h (%) * | Reaction Mechanism | |
|---|---|---|---|---|
| UV | UV–VIS | |||
| Without scavengers | - | 69 | 46 | - |
| formic acid 20 mM | 90 | 70 | (8) | |
| isopropyl alcohol 1 M | 43 | 37 | (9) | |
| K2Cr2O7 1 mM | 11 | 16 | (10) | |
| 1,4 benzoquinone 1 mM | 8 | 8 | (11) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronic, L.; Vladescu, A.; Enesca, A. Synthesis, Characterisation, Photocatalytic Activity, and Aquatic Toxicity Evaluation of TiO2 Nanoparticles. Nanomaterials 2021, 11, 3197. https://doi.org/10.3390/nano11123197
Andronic L, Vladescu A, Enesca A. Synthesis, Characterisation, Photocatalytic Activity, and Aquatic Toxicity Evaluation of TiO2 Nanoparticles. Nanomaterials. 2021; 11(12):3197. https://doi.org/10.3390/nano11123197
Chicago/Turabian StyleAndronic, Luminita, Alina Vladescu, and Alexandru Enesca. 2021. "Synthesis, Characterisation, Photocatalytic Activity, and Aquatic Toxicity Evaluation of TiO2 Nanoparticles" Nanomaterials 11, no. 12: 3197. https://doi.org/10.3390/nano11123197





.jpg)


