The Rare-Earth Elements Doping of BaGdF5 Nanophosphors for X-ray Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
2.4. Cytotoxicity and ROS Generation Assays
- CellROX Green (CRG)—a probe for mitochondrial and nuclear ROS;
- CellROX Orange (CRO)—a probe for cytosolic ROS;
- 7-AAD—a control dye for gating viable cells.
3. Results and Discussion
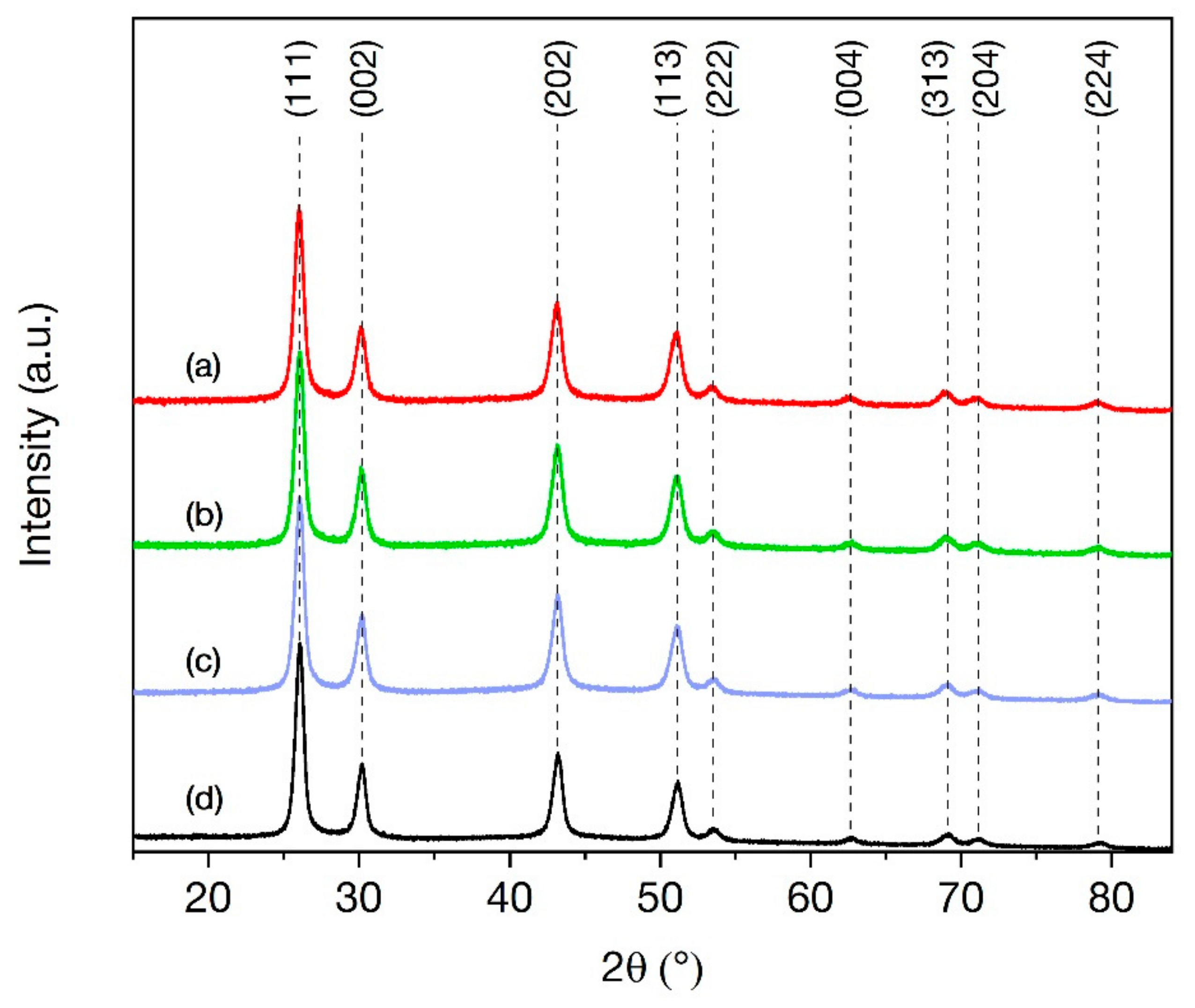
3.1. X-ray Diffraction
3.2. X-ray Fluorescence (XRF)
3.3. High-Resolution TEM (HRTEM)
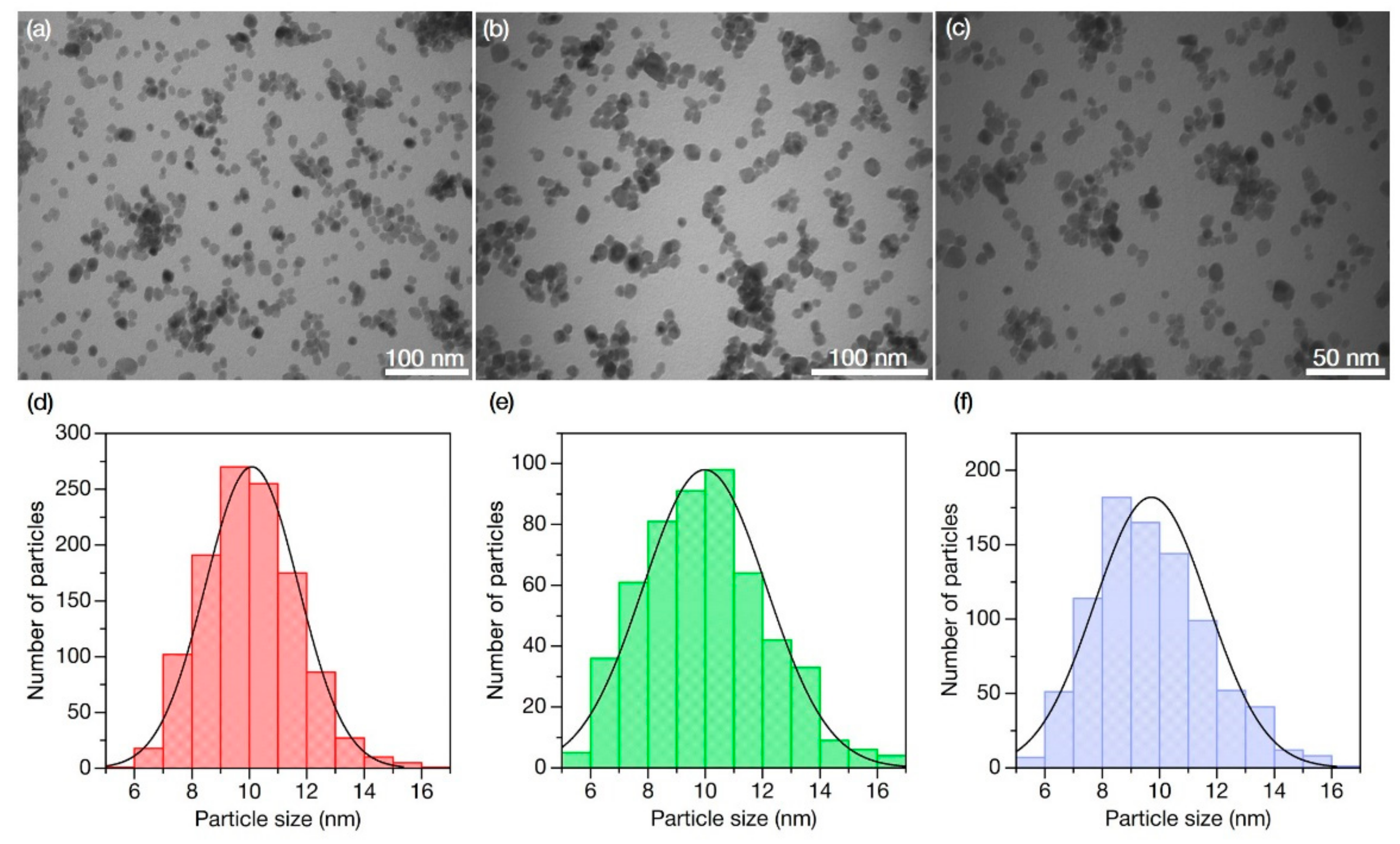
3.4. Transmission Electron Microscopy (TEM)
3.5. Zeta-Potential
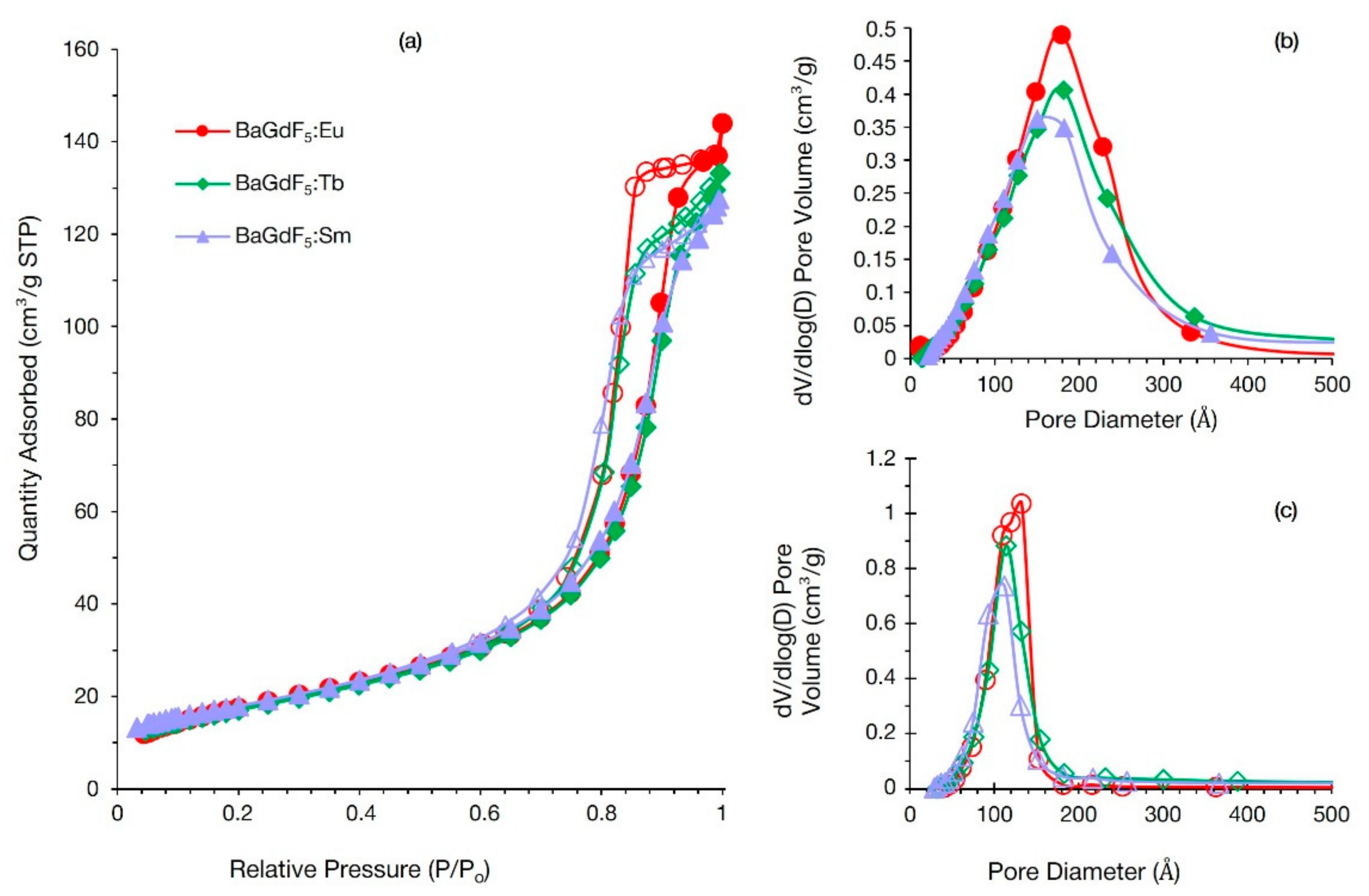
3.6. Nitrogen Adsorption
3.7. Fourier Transform Infrared (FTIR) Spectroscopy
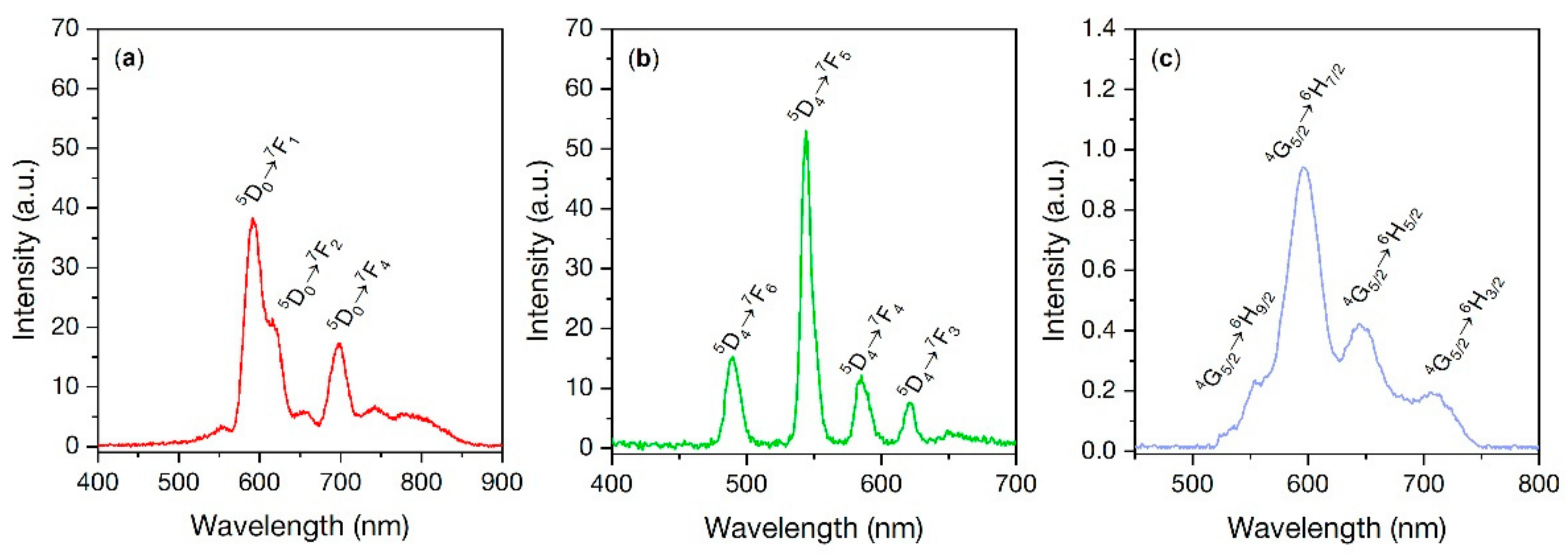
3.8. X-ray Excited Optical Luminescence (XEOL)
3.9. Cytotoxicity Analysis
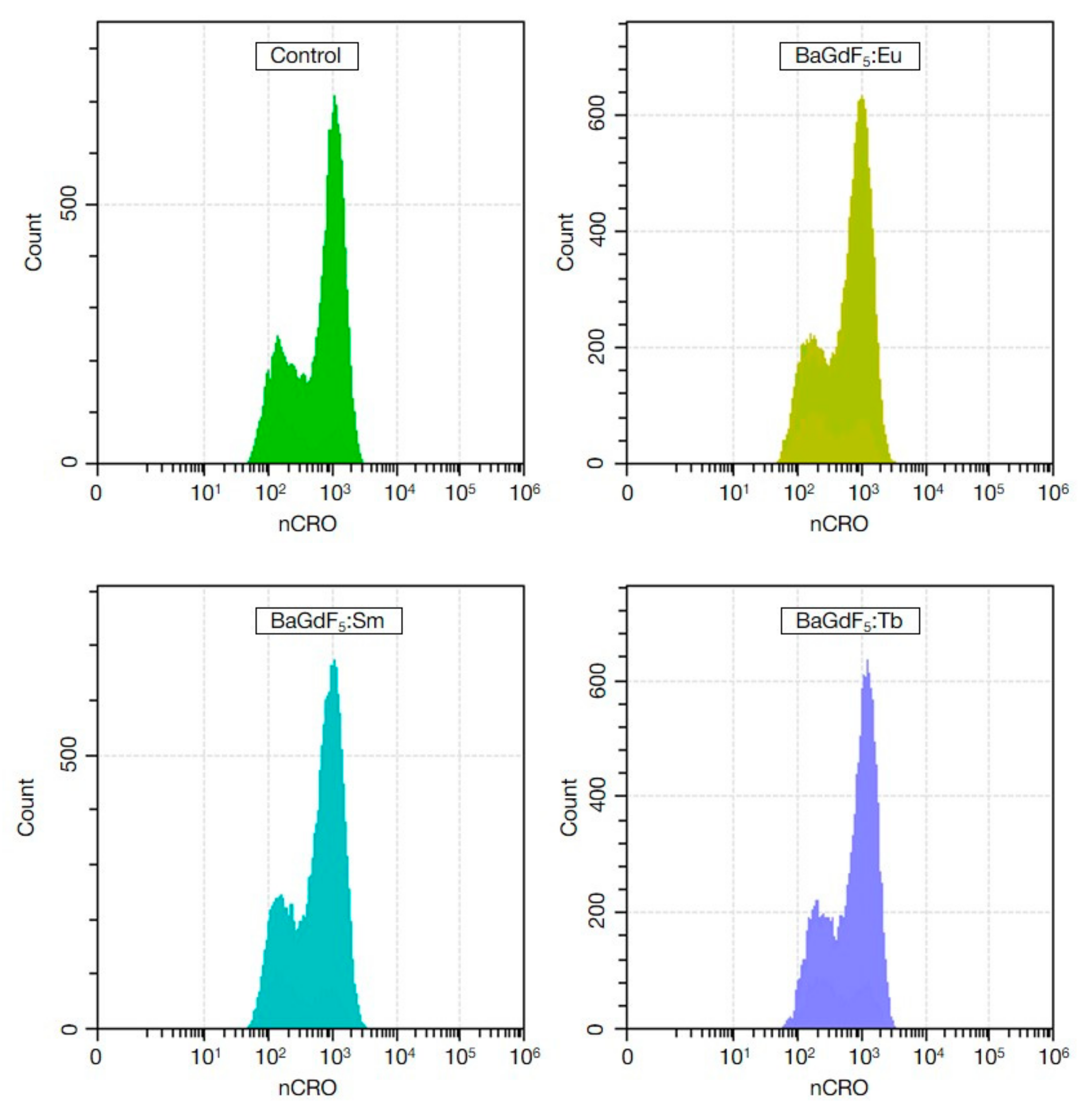
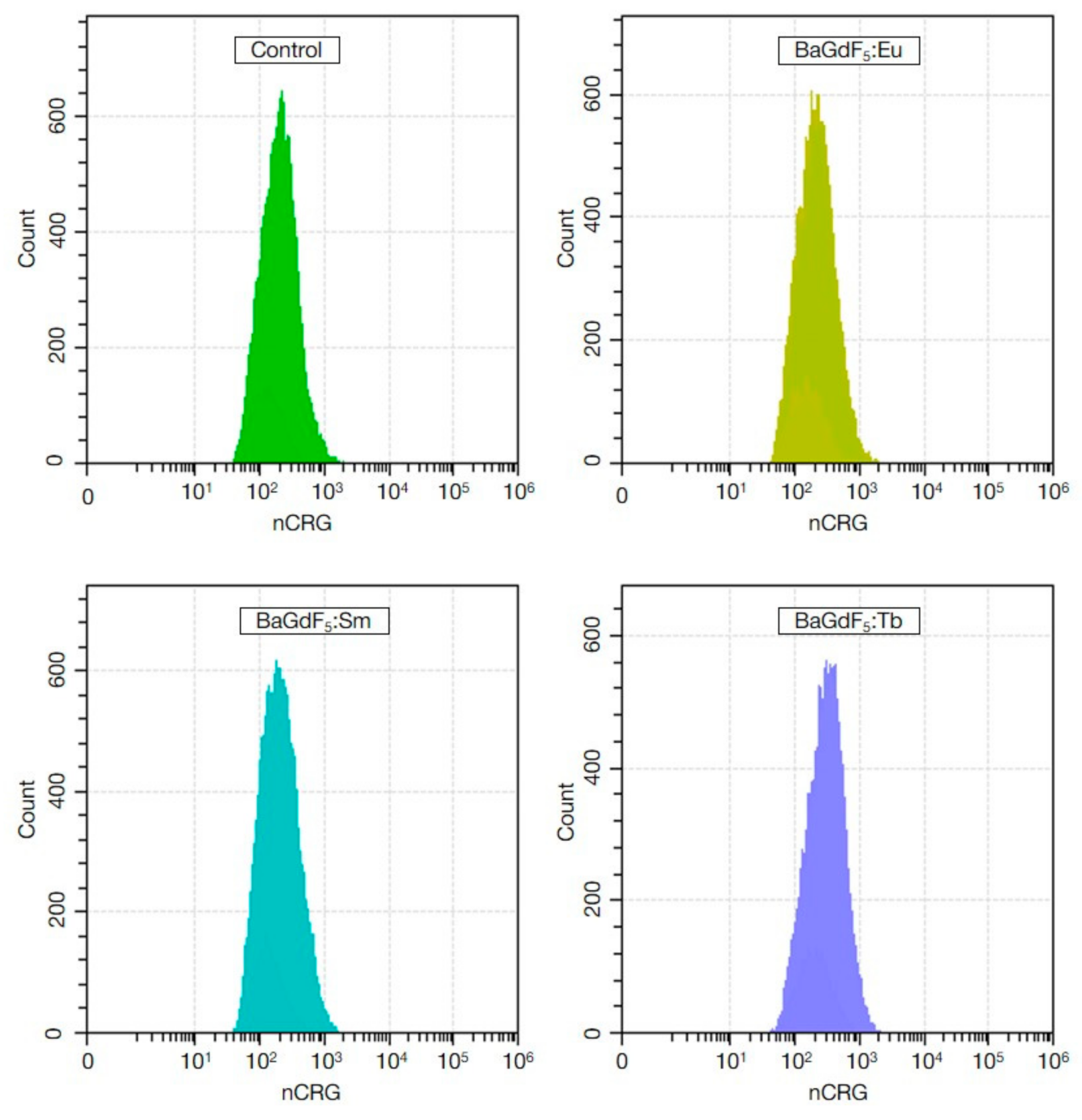
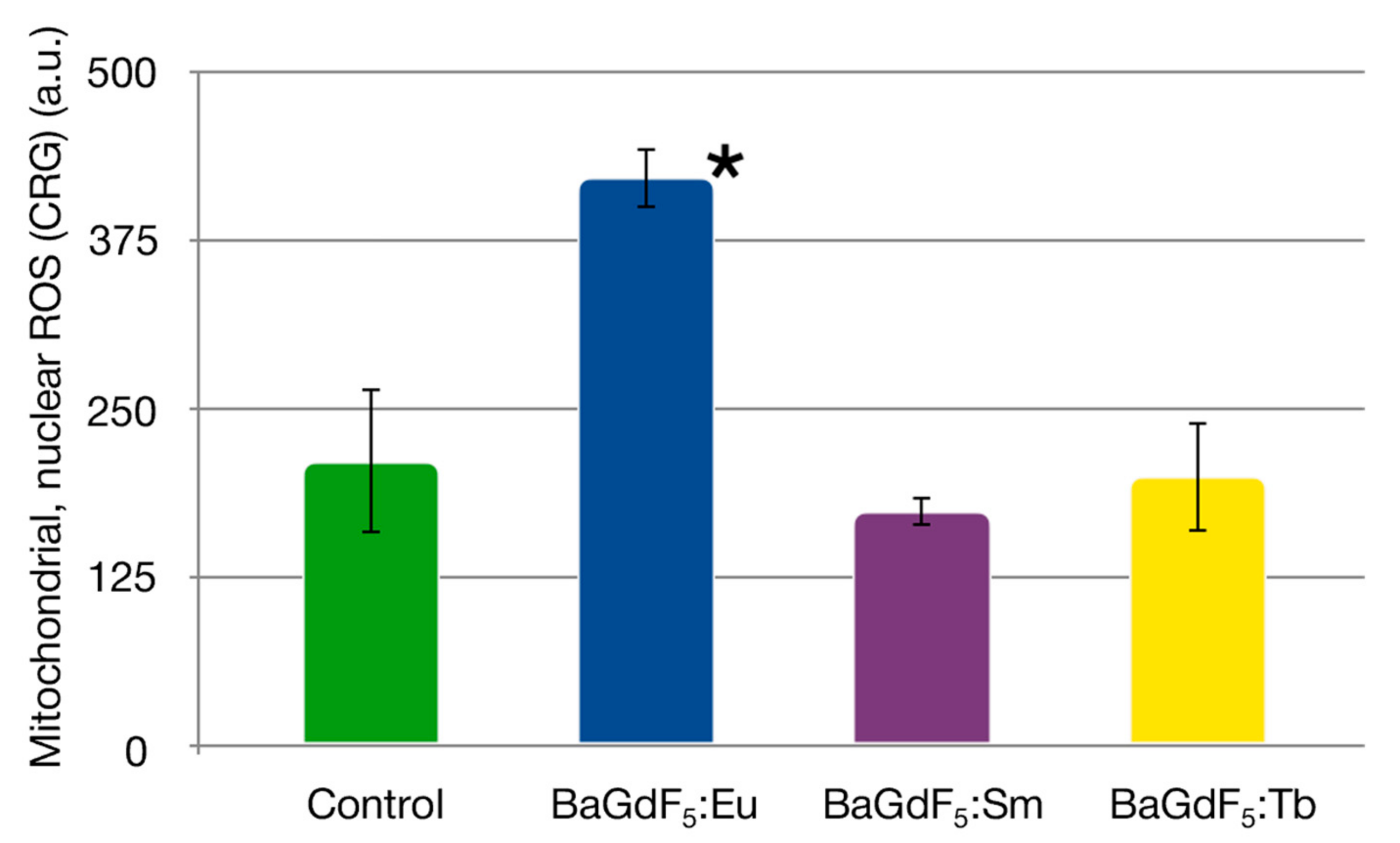
3.10. ROS Generation (In Vitro)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, N.; Wang, Y.; Shen, D.; Kang, Q.; Zhao, R.; Chen, L. Self-assembly of nanoparticles by human serum albumin and photosensitizer for targeted near-infrared emission fluorescence imaging and effective phototherapy of cancer. J. Mater. Chem. B 2019, 7, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Dai, C.; Cao, P.; Sun, D.; Ouyang, R.; Miao, Y. The role of reactive oxygen species in tumor treatment. RSC Adv. 2020, 10, 7740–7750. [Google Scholar] [CrossRef]
- Sapre, A.A.; Novitskaya, E.; Vakharia, V.; Cota, A.; Wrasidlo, W.; Hanrahan, S.M.; Derenzo, S.; Makale, M.T.; Graeve, O.A. Optimized Scintillator YAG:Pr Nanoparticles for X-ray Inducible Photodynamic Therapy. Mater. Lett. 2018, 228, 49–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; Andre, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Chen, M.H.; Jenh, Y.J.; Wu, S.K.; Chen, Y.S.; Hanagata, N.; Lin, F.H. Non-invasive Photodynamic Therapy in Brain Cancer by Use of Tb3+-Doped LaF3 Nanoparticles in Combination with Photosensitizer Through X-ray Irradiation: A Proof-of-Concept Study. Nanoscale Res. Lett. 2017, 12, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhmetyev, V.V.; Dorokhina, A.M.; Keskinova, M.V.; Mjakin, S.V.; Vlasenko, A.B.; Lebedev, L.A.; Malygin, V.V.; Sychov, M.M. Synthesis and characterization of finely dispersed phosphors doped with rare-earth metal ions for enhanced photodynamic therapy of cancer. Chem. Pap. 2019, 74, 787–797. [Google Scholar] [CrossRef]
- Lee, G.; Struebing, C.; Wagner, B.; Summers, C.; Ding, Y.; Bryant, A.; Thadhani, N.; Shedlock, D.; Star-Lack, J.; Kang, Z. Synthesis and characterization of a BaGdF5:Tb glass ceramic as a nanocomposite scintillator for x-ray imaging. Nanotechnology 2016, 27, 205203. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mancebo, D.; Becerro, A.I.; Corral, A.; Balcerzyk, M.; Ocana, M. Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes. Nanomaterials 2019, 9, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Yin, T.; Huang, P.; Gao, G.; Shapter, J.G.; Shen, Y.; Zhang, J.; Cui, D. Hydrothermal Synthesis of Monodispersed BaGdF5:Yb/Er Nanoparticles for CT and MR Imaging. J. Chin. Chem. Soc. 2016, 63, 977–984. [Google Scholar] [CrossRef]
- Guan, H.; Sheng, Y.; Song, Y.; Zheng, K.; Xu, C.; Xie, X.; Dai, Y.; Zou, H. White light-emitting, tunable color luminescence, energy transfer and paramagnetic properties of terbium and samarium doped BaGdF5 multifunctional nanomaterials. RSC Adv. 2016, 6, 73160–73169. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Eu3+/Tb3+ doped cubic BaGdF5 multifunctional nanophosphors: Multicolor tunable luminescence, energy transfer and magnetic properties. J. Lumin. 2017, 186, 6–15. [Google Scholar] [CrossRef]
- Yanes, A.C.; del-Castillo, J.; Ortiz, E. Energy transfer and tunable emission in BaGdF5:RE3+ (RE= Ce, Tb, Eu) nano-glass-ceramics. J. Alloys Compd. 2019, 773, 1099–1107. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, L.; Li, X.; He, A. Manipulating upconversion emission of cubic BaGdF5:Ce3+/Er3+/Yb3+ nanocrystals through controlling Ce3+ doping. J. Alloys Compd. 2017, 721, 374–382. [Google Scholar] [CrossRef]
- He, F.; Li, C.; Zhang, X.; Chen, Y.; Deng, X.; Liu, B.; Hou, Z.; Huang, S.; Jin, D.; Lin, J. Optimization of upconversion luminescence of Nd3+-sensitized BaGdF5-based nanostructures and their application in dual-modality imaging and drug delivery. Dalton Trans. 2016, 45, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Song, Y.; Zheng, K.; Sheng, Y.; Zou, H. BaGdF5:Dy3+,Tb3+,Eu3+ multifunctional nanospheres: Paramagnetic, luminescence, energy transfer, and tunable color. Phys. Chem. Chem. Phys. 2016, 18, 13861–13873. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Chen, G.; Yang, C. Sensing using rare-earth-doped upconversion nanoparticles. Theranostics 2013, 3, 331–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef]
- Becerro, A.I.; Gonzalez-Mancebo, D.; Cantelar, E.; Cusso, F.; Stepien, G.; de la Fuente, J.M.; Ocana, M. Ligand-Free Synthesis of Tunable Size Ln:BaGdF5 (Ln = Eu3+ and Nd3+) Nanoparticles: Luminescence, Magnetic Properties, and Biocompatibility. Langmuir 2016, 32, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Teo, R.D.; Termini, J.; Gray, H.B. Lanthanides: Applications in Cancer Diagnosis and Therapy. J. Med. Chem. 2016, 59, 6012–6024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Dai, Y.; Liu, J.; Zhou, Y.; Chen, Y.; Li, C.; Ma, P.; Lin, J. Ultra-small BaGdF5-based upconversion nanoparticles as drug carriers and multimodal imaging probes. Biomaterials 2014, 35, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, T.; Mrowczynska, L.; Szczeszak, A.; Sniadecki, Z.; Runowski, M.; Idzikowski, B.; Lis, S. Synthesis, characterization, and cytotoxicity in human erythrocytes of multifunctional, magnetic, and luminescent nanocrystalline rare earth fluorides. J. Nanopart. Res. 2015, 17, 399. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Kang, X.; Shang, M.; Li, G.; Peng, C.; Li, C.; Lin, J. Size and shape controllable synthesis and luminescent properties of BaGdF5:Ce3+/Ln3+ (Ln = Sm, Dy, Eu, Tb) nano/submicrocrystals by a facile hydrothermal process. Nanoscale 2011, 3, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Sudheendra, L.; Das, G.K.; Li, C.; Stark, D.; Cena, J.; Cherry, S.; Kennedy, I.M. NaGdF4:Eu3+ Nanoparticles for Enhanced X-ray Excited Optical Imaging. Chem. Mater. 2014, 26, 1881–1888. [Google Scholar] [CrossRef]
- Kirsanova, D.Y.; Butova, V.V.; Polyakov, V.A.; Zolotukhin, P.V.; Belanova, A.A.; Legostaev, V.M.; Kuchma, E.A.; Gadzhimagomedova, Z.M.; Soldatov, A.V. X-ray nanophosphors based on BaGdF5 for X-ray photodynamic therapy in oncology. Nanotechnologies Russ. 2020, 15, 105–111. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Für. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Wyckoff, R.W.G. Second edition. Interscience Publishers, New York, New York Fluorite structure. Cryst. Struct. 1963, 1, 239–444. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Wang, Y.; Zhang, J.; Dong, P. Crystal structure and up- and down-conversion properties of Yb3+, Ho3+ codoped BaGdF5 solid-solution with different morphologies. CrystEngComm 2012, 14, 3131–3141. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-Ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Kirsanova, D.Y.; Gadzhimagomedova, Z.M.; Maksimov, A.Y.; Soldatov, A.V. Nanomaterials for Deep Tumor Treatment. Mini Rev. Med. Chem. 2020, 21, 677–688. [Google Scholar] [CrossRef]
- Banski, M.; Noculak, A.; Misiewicz, J.; Podhorodecki, A. Rice oil as a green source of capping ligands for GdF3 nanocrystals. New J. Chem. 2016, 40, 7928–7934. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; He, Y.; Liu, J.; Feng, J.; Sun, Z.; Lei, P.; Yuan, Q.; Zhang, H. Core–shell BaYbF5:Tm@BaGdF5:Yb,Tm nanocrystals for in vivo trimodal UCL/CT/MR imaging. RSC Adv. 2016, 6, 14283–14289. [Google Scholar] [CrossRef]
- Xu, C.; Ma, M.; Yang, L.; Zeng, S.; Yang, Q. Upconversion luminescence and magnetic properties of ligand-free monodisperse lanthanide doped BaGdF5 nanocrystals. J. Lumin. 2011, 131, 2544–2549. [Google Scholar] [CrossRef]
- Chieng, B.; Ibrahim, N.; Yunus, W.; Hussein, M. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, H.; Wang, J.; Yang, Y.; Wu, D.; Zhang, Y.; Zhang, Y.; Zhou, Z.; Yang, S. Graphene oxide-BaGdF5 nanocomposites for multi-modal imaging and photothermal therapy. Biomaterials 2015, 42, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.V.d.S.; Montes, P.J.; Valerio, M.E.G.; Jackson, R.A. The optical properties of Eu3+ doped BaAl2O4: A computational and spectroscopic study. Opt. Mater. 2012, 34, 1434–1439. [Google Scholar] [CrossRef]
- Jürgensen, A. XEOL spectroscopy of lanthanides in aqueous solution. Can. J. Chem. 2017, 95, 1198–1204. [Google Scholar] [CrossRef] [Green Version]
- Gaedtke, C.; Williams, G.V.M.; Janssens, S.; Raymond, S.G.; Clarke, D.J. The effect of ionizing radiation on the luminescence properties of Eu3+ and Sm3+ doped LaF3 nanoparticles. Phys. Status Solidi C 2012, 9, 2247–2250. [Google Scholar] [CrossRef]



| Sample | Elemental Composition (at.%) by XRF | |||||
|---|---|---|---|---|---|---|
| Ba | Gd | Eu | Tb | Sm | F | |
| BaGdF5:Eu | 11.64 | 14.9 | 1.37 | - | - | 72.09 |
| BaGdF5:Tb | 12.13 | 14.39 | - | 1.51 | - | 71.97 |
| BaGdF5:Sm | 12.75 | 14.41 | - | - | 1.02 | 71.81 |
| Group | Analyzed Parameter | |||
|---|---|---|---|---|
| CRO Channel | CRG Channel | |||
| M ± SD 1, a.u. | pMW 2 | M ± SD, a.u. | pMW | |
| Control | 578.70 ± 114,77 | - | 211.28 ± 53,93 | - |
| BaGdF5:Eu | 507.60 ± 14,77 | 0.353 | 421.75 ± 22.31 | <0.001 |
| BaGdF5:Tb | 658.55 ± 98.45 | 0.212 | 199.78 ± 40.77 | 1.000 |
| BaGdF5:Sm | 638.64 ± 36.31 | 0.402 | 174.13 ± 10.65 | 0.212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirsanova, D.; Polyakov, V.; Butova, V.; Zolotukhin, P.; Belanova, A.; Gadzhimagomedova, Z.; Soldatov, M.; Pankin, I.; Soldatov, A. The Rare-Earth Elements Doping of BaGdF5 Nanophosphors for X-ray Photodynamic Therapy. Nanomaterials 2021, 11, 3212. https://doi.org/10.3390/nano11123212
Kirsanova D, Polyakov V, Butova V, Zolotukhin P, Belanova A, Gadzhimagomedova Z, Soldatov M, Pankin I, Soldatov A. The Rare-Earth Elements Doping of BaGdF5 Nanophosphors for X-ray Photodynamic Therapy. Nanomaterials. 2021; 11(12):3212. https://doi.org/10.3390/nano11123212
Chicago/Turabian StyleKirsanova, Daria, Vladimir Polyakov, Vera Butova, Peter Zolotukhin, Anna Belanova, Zaira Gadzhimagomedova, Mikhail Soldatov, Ilia Pankin, and Alexander Soldatov. 2021. "The Rare-Earth Elements Doping of BaGdF5 Nanophosphors for X-ray Photodynamic Therapy" Nanomaterials 11, no. 12: 3212. https://doi.org/10.3390/nano11123212
APA StyleKirsanova, D., Polyakov, V., Butova, V., Zolotukhin, P., Belanova, A., Gadzhimagomedova, Z., Soldatov, M., Pankin, I., & Soldatov, A. (2021). The Rare-Earth Elements Doping of BaGdF5 Nanophosphors for X-ray Photodynamic Therapy. Nanomaterials, 11(12), 3212. https://doi.org/10.3390/nano11123212







