Electrospun Ceramic Nanofibers for Photocatalysis
Abstract
:1. Introduction
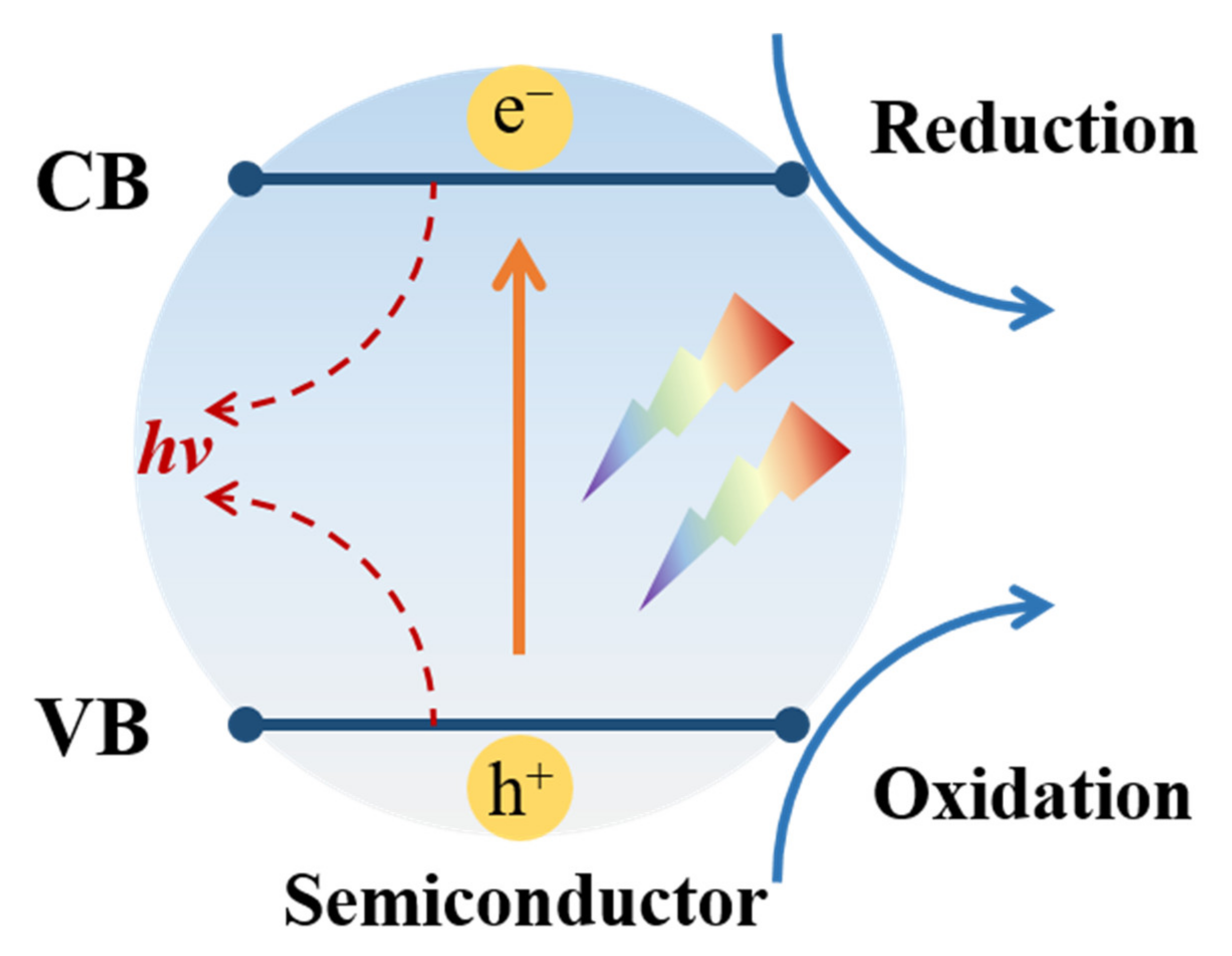
2. The Fundamental of Photocatalysis
3. Ceramic Nanofibers for Visible-Light Harvesting
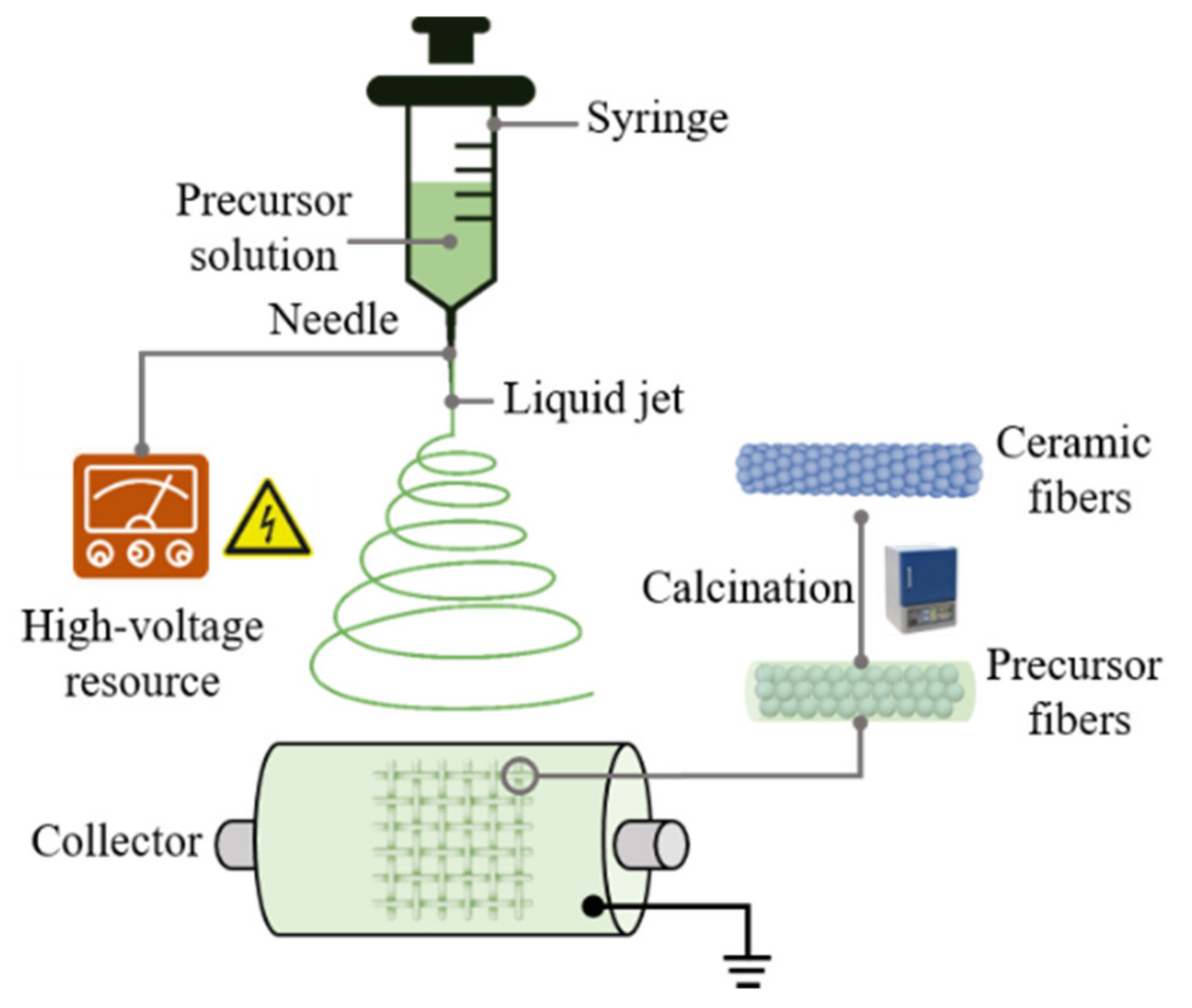
3.1. Principles of Electrospinning Ceramic Fibers
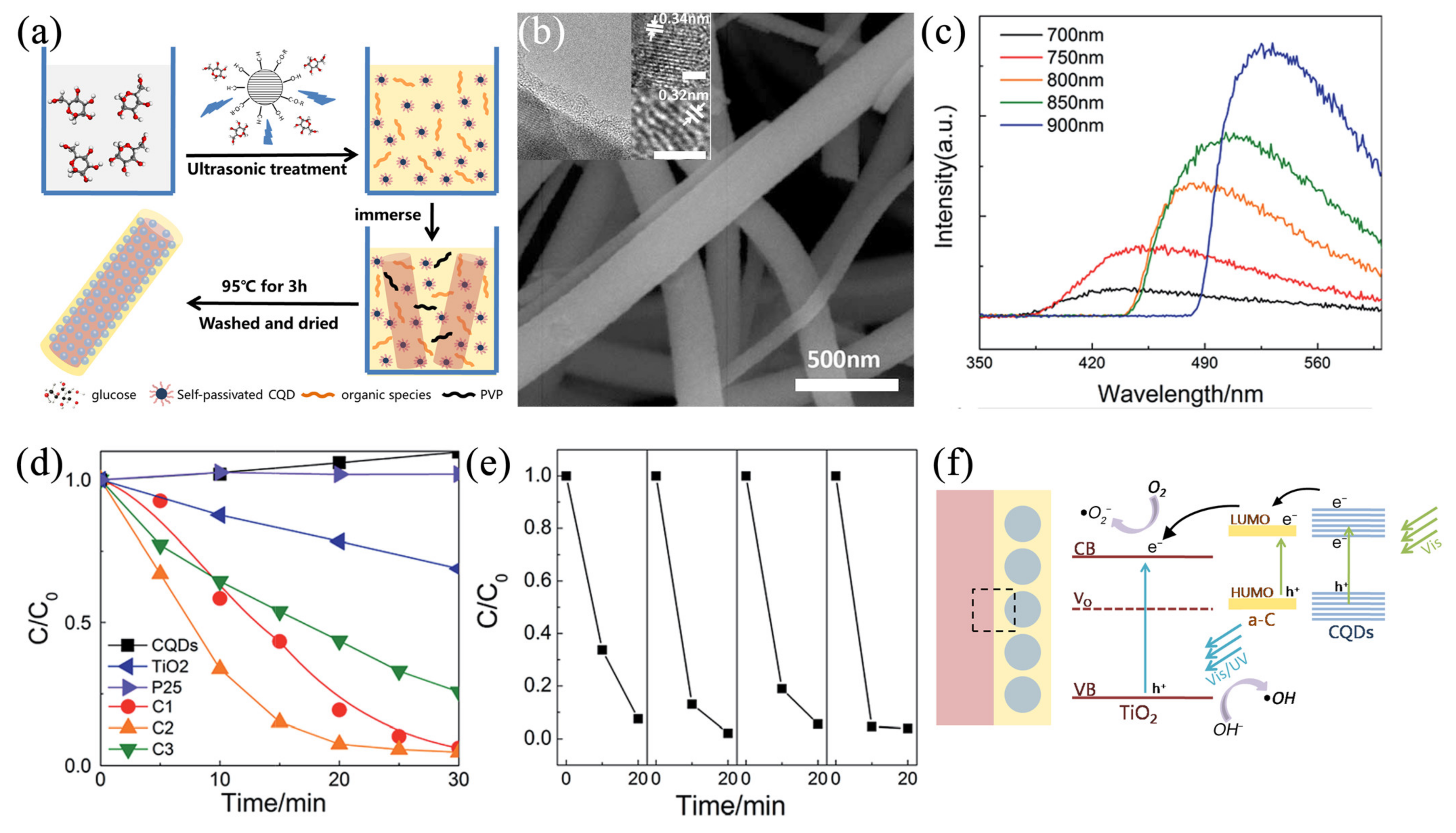
3.2. Visible Light Responsive Ceramic Nanofibers
3.3. Approaches to Expand the Light Absorption Range
3.3.1. Precursor Composition Design
3.3.2. Electrospinning Parameters
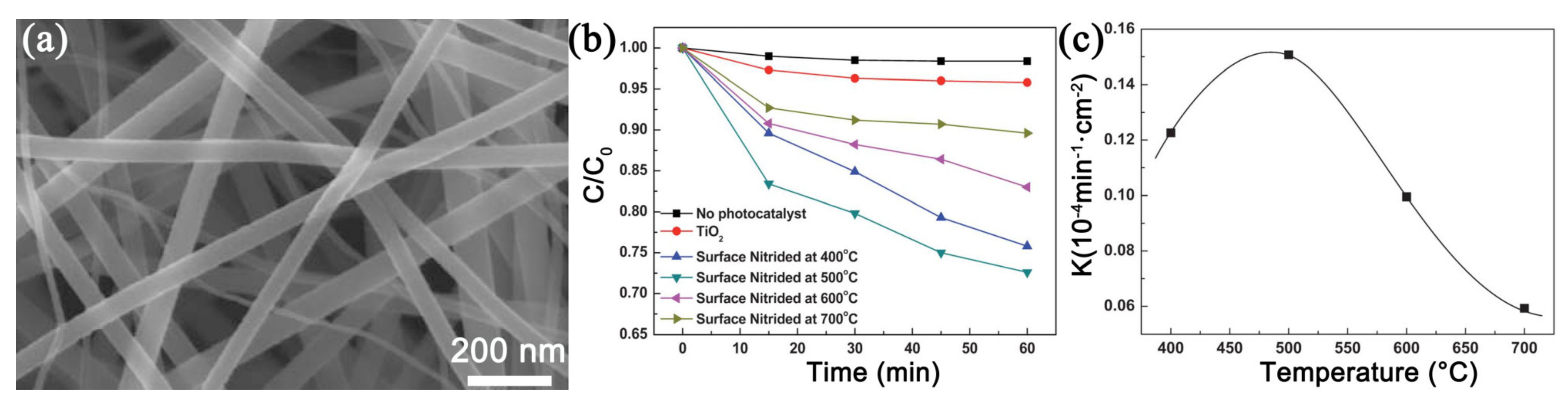
3.3.3. Heat Treatment Process
4. Ceramic Nanofibers for Efficient Carrier Separation
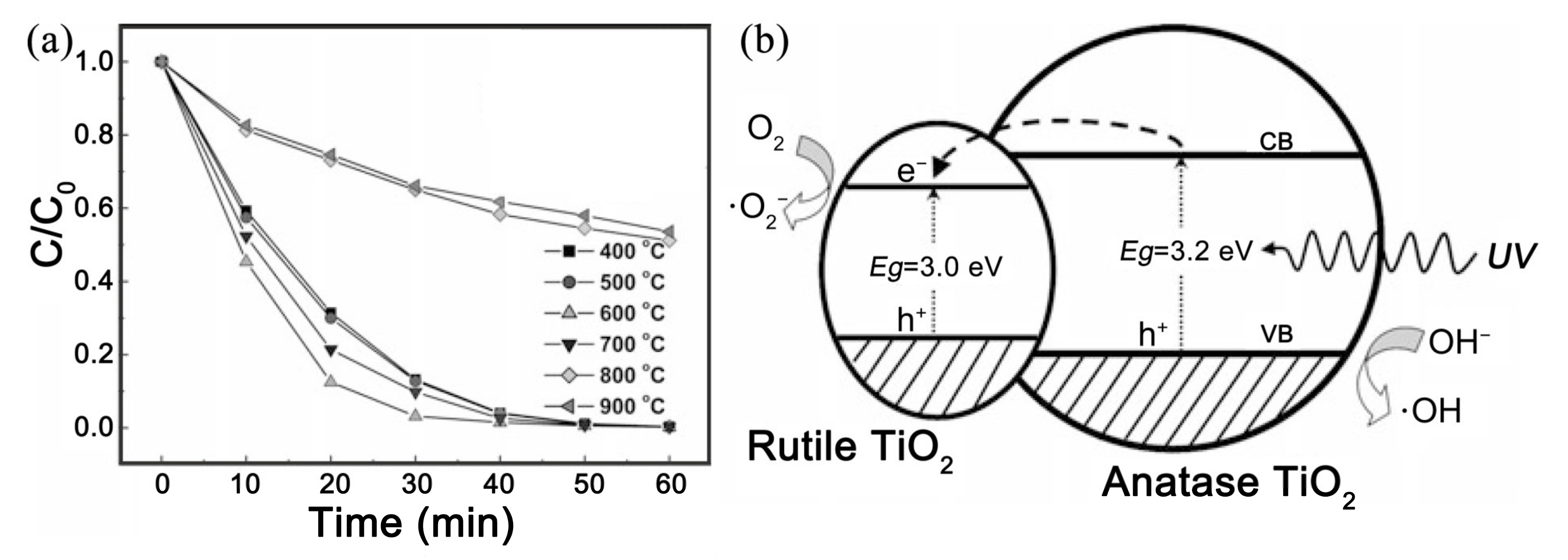
4.1. Metal–Ceramic Heterojunction
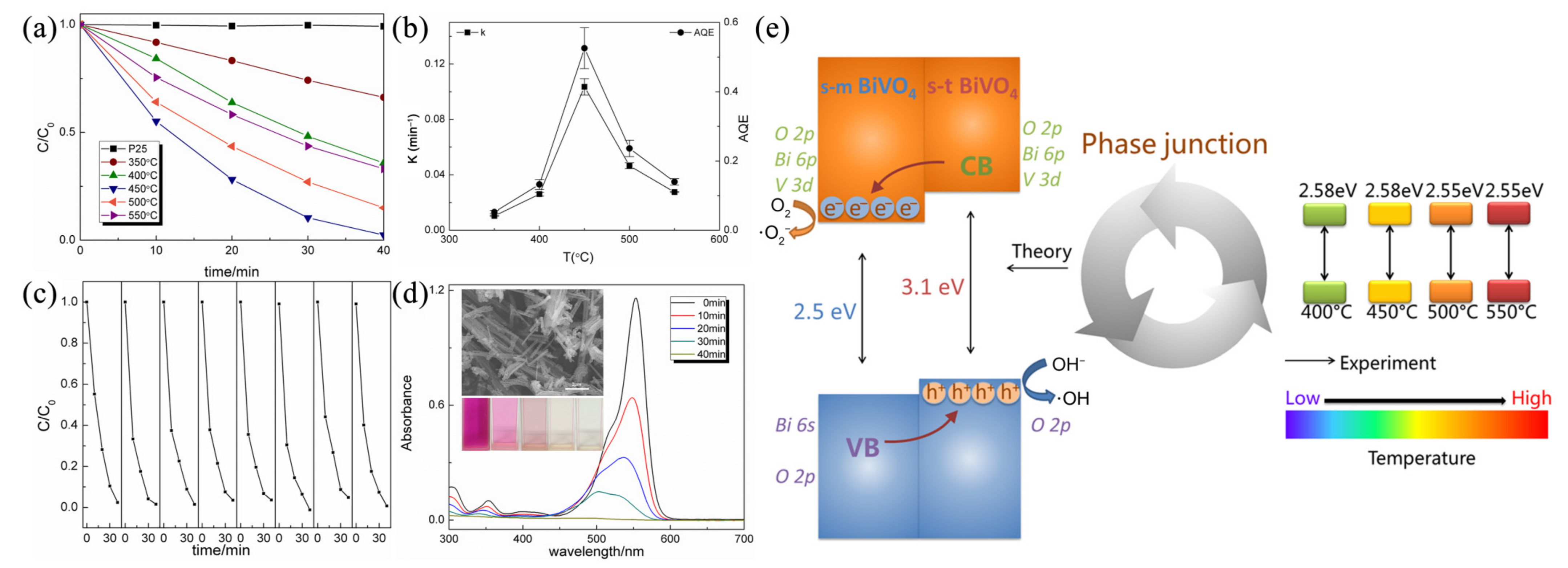
4.2. Semiconductor–Semiconductor Heterojunction
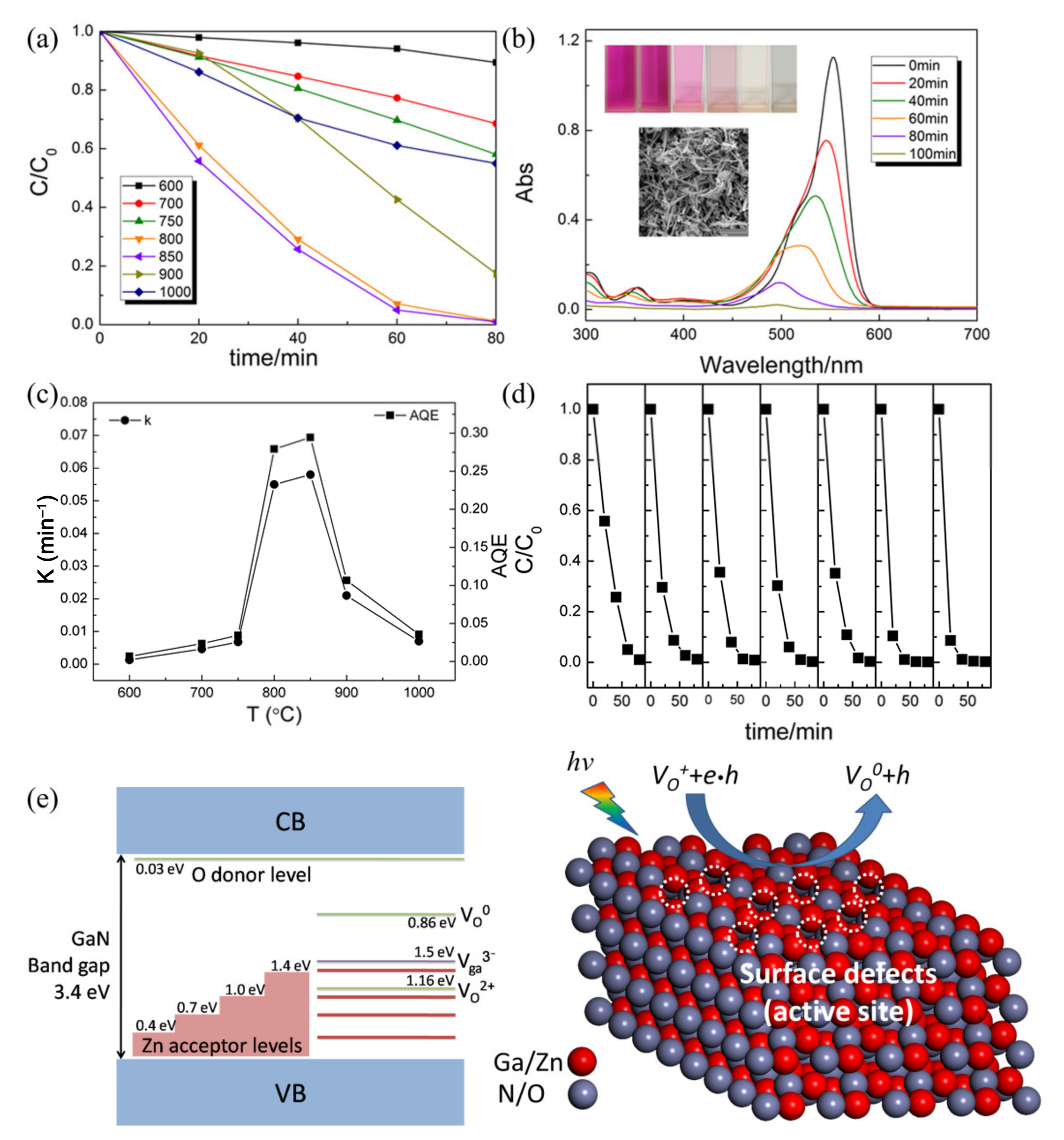
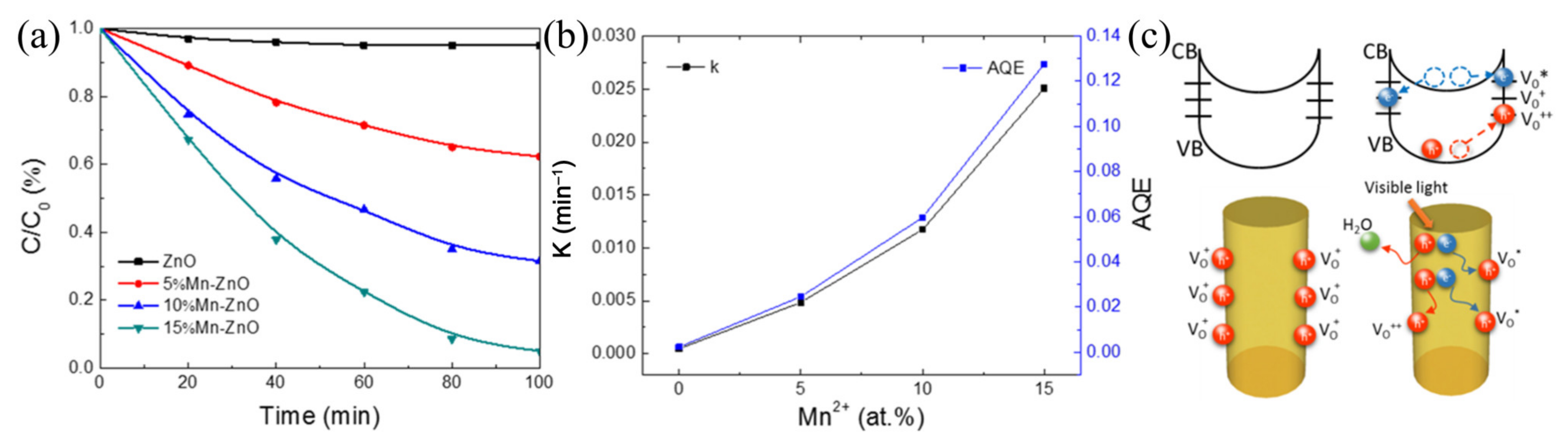
4.3. Defective Surfaces
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. 2010, 46, 5670–5703. [Google Scholar] [CrossRef]
- Pan, W.; Wu, H.; Lin, D.; Li, H.; Zhang, W. Electrospinning of nanofibers for photocatalyst. Curr. Org. Chem. 2013, 17, 1371–1381. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Cooley, J.F. Apparatus for Electrically Dispersing Fluids. U.S. Patent US692,631, 1902. [Google Scholar]
- Dan, L.; Zhu, M.; Jiang, Z.; Jiang, S.; Huang, C. Green electrospun nanofibers and their application in air filtration. Macromol. Mater. Eng. 2018, 303, 1800336. [Google Scholar]
- Wu, H.; Lin, D.; Pan, W. Fabrication, assembly, and electrical characterization of CuO nanofibers. Appl. Phys. Lett. 2006, 89, 133125. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Y.; Lin, D.; Zhang, R.; Zhang, C.; Pan, W. GaN nanofibers based on electrospinning: Facile synthesis, controlled assembly, precise doping, and application as high performance UV photodetector. Adv. Mater. 2009, 21, 227–231. [Google Scholar] [CrossRef]
- Xing, Y.; Dan, W.; Fan, Y.; Li, X.A. Low temperature synthesis of high-entropy (Y0.2Yb0.2Sm0.2Eu0.2Er0.2)2O3 nanofibers by a novel electrospinning method. J. Mater. Sci. Technol. 2022, 103, 215–220. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Su, Z.; Hu, S.; Li, H.; Yan, Y. Electrospun polyporous VN nanofibers for symmetric all-solid-state supercapacitors. J. Adv. Ceram. 2018, 7, 246–255. [Google Scholar] [CrossRef]
- Lin, D.; Pan, W.; Wu, H. Morphological control of centimeter long aluminum-doped zinc oxide nanofibers prepared by electrospinning. J. Am. Ceram. Soc. 2007, 90, 71–76. [Google Scholar] [CrossRef]
- Lin, D.; Wu, H.; Zhang, R.; Pan, W. Preparation of ZnS Nanofibers Via Electrospinning. J. Am. Ceram. Soc. 2007, 90, 3664–3666. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W. Preparation of Zinc Oxide Nanofibers by Electrospinning. J. Am. Ceram. Soc. 2006, 89, 699–701. [Google Scholar] [CrossRef]
- Li, H.; Pan, W.; Zhang, W.; Huang, S.; Wu, H. TiN nanofibers: A new material with high conductivity and transmittance for transparent conductive electrodes. Adv. Funct. Mater. 2013, 23, 209–214. [Google Scholar] [CrossRef]
- Xing, Y.; Cheng, J.; Zhang, M.; Zhao, M.; Ye, L.; Pan, W. A novel inorganic Ni-La2O3 composite with superfast and versatile water purification behavior. ACS Appl. Mater. Inter. 2018, 10, 43723–43729. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-electrospinning and its application for tissue engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, J.; Xing, Y.; Shahid, M.; Nishijima, H.; Pan, W. Stretchable platinum network-based transparent electrodes for highly sensitive wearable electronics. Small 2017, 13, 1604291. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, H.; Zhang, R.; Pan, W. Enhanced photocatalysis of electrospun Ag-ZnO heterostructured nanofibers. Chem. Mater. 2009, 21, 3479–3484. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Aldana, J.; Wang, Y.A.; Peng, X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 2001, 123, 8844–8850. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Rohj, R.K.; Hossain, A.; Mahadevan, P.; Sarma, D.D. Band gap reduction in ferroelectric BaTiO3 through heterovalent Cu-Te co-doping for visible-light photocatalysis. Front. Chem. 2021, 9, 682979. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Shi, X.; Wang, P.; Wang, L.; Xie, H.; Li, Z.; Qu, L.; Ye, L. Synthesis of one-dimensional Bi5O7Br0.5I0.5 solid solution for effective real oilfield wastewater treatment via exciton photocatalytic process. J. Taiwan Inst. Chem. E 2018, 91, 358–368. [Google Scholar] [CrossRef]
- Liu, M.; Jin, X.; Li, S.; Billeau, J.B.; Peng, T.; Li, H.; Zhao, L.; Zhang, Z.; Claverie, J.P.; Razzari, L.; et al. Enhancement of Scattering and Near Field of TiO2-Au Nanohybrids Using a Silver Resonator for Efficient Plasmonic Photocatalysis. ACS Appl. Mater. Inter. 2021, 13, 34714–34723. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.; Sierra, M.; Mendez-Ramos, J.; Acosta-Mora, P.; Ruiz-Morales, J.; Esparza, P. Solar degradation of contaminants in water: TiO2 solar photocatalysis assisted by up-conversion luminescent materials. Sol. Energy Mater. Sol. Cells 2016, 155, 194–201. [Google Scholar] [CrossRef]
- Emeline, A.V.; Rudakova, A.V.; Mikhaylov, R.V.; Bulanin, K.M.; Bahnemann, D.W. Photoactive heterostructures: How they are made and explored. Catalysts 2021, 11, 294. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H.G. Defect-mediated electron-hole separation in semiconductor photocatalysis. Inorg. Chem. Front. 2018, 5, 1240–1254. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet Effect of Single-Crystalline Ag3PO4 Sub-Microcrystals on Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The Role of Polarization in Photocatalysis. Angew. Chem. 2019, 58, 10061–10073. [Google Scholar] [CrossRef]
- Lin, D.; Wu, H.; Zhang, R.; Zhang, W.; Pan, W. Facile Synthesis of Heterostructured ZnO-ZnS Nanocables and Enhanced Photocatalytic Activity. J. Am. Ceram. Soc. 2010, 93, 3384–3389. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Y.; Xing, Y.; Shahid, M.; Pan, W. A stable and highly efficient visible-light photocatalyst of TiO2 and heterogeneous carbon core-shell nanofibers. RSC Adv. 2017, 7, 15330–15336. [Google Scholar] [CrossRef] [Green Version]
- Linsebigler, A.L.; Lu, G.; John, T.; Yates, J. Photocatalysis on TiOn Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energ. Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, e1901997. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W.; Lin, D.; Li, H. Electrospinning of ceramic nanofibers: Fabrication, assembly and applications. J. Adv. Ceram. 2012, 1, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Lin, D.; Pan, W. High Performance Surface-Enhanced Raman Scattering Substrate Combining Low Dimensional and Hierarchical Nanostructures. Langmuir 2010, 26, 6865–6868. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, W.; Li, B.; Pan, W. Diameter-Dependent Photocatalytic Activity of Electrospun TiO2 Nanofiber. J. Am. Ceram. Soc. 2010, 93, 2503–2506. [Google Scholar] [CrossRef]
- Yi, S.; Sun, S.; Zhang, Y.; Zou, Y.; Dai, F.; Si, Y. Scalable fabrication of bimetal modified polyacrylonitrile (PAN) nanofibrous membranes for photocatalytic degradation of dyes. J. Colloid Interface Sci. 2020, 559, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Fan, L.; Xiong, X.; Zhu, C.; Wu, C.; Dai, G. Electrospun CuWO4 nanofibers for visible light photocatalysis. Mater. Lett. 2019, 251, 23–25. [Google Scholar] [CrossRef]
- Lv, C.; Sun, J.; Chen, G.; Zhou, Y.; Li, D.; Wang, Z.; Zhao, B. Organic salt induced electrospinning gradient effect: Achievement of BiVO4 nanotubes with promoted photocatalytic performance. Appl. Catal. B Environ. 2017, 208, 14–21. [Google Scholar] [CrossRef]
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic degradation of methylene blue dye by porous zinc oxide nanofibers prepared via electrospinning: When defects become merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Wang, Z.; Dong, M.; Cui, L. Facile fabrication of Mn2+-doped ZnO photocatalysts by electrospinning. R. Soc. Open Sci. 2020, 7, 191050. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, J.; Yu, S.; Alcocer, E.J.; Shahid, M.; Wang, Z.; Pan, W. Synergistic effect of N-decorated and Mn2+ doped ZnO nanofibers with enhanced photocatalytic activity. Sci. Rep. 2016, 6, 32711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhang, W.; Huang, S.; Pan, W. Enhanced visible-light-driven photocatalysis of surface nitrided electrospun TiO2 nanofibers. Nanoscale 2012, 4, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, Y.; Xing, Y.; Shahid, M.; Pan, W. Surface defects decorated zinc doped gallium oxynitride nanowires with high photocatalytic activity. Appl. Catal. B Environ. 2017, 209, 53–61. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, H.; Lin, D.; Pan, W. Photocatalytic and Magnetic Properties of the Fe-TiO2/SnO2 Nanofiber Via Electrospinning. J. Am. Ceram. Soc. 2010, 93, 605–608. [Google Scholar] [CrossRef]
- Duan, Z.; Huang, Y.; Zhang, D.; Chen, S. Electrospinning fabricating Au/TiO2 network-like nanofibers as visible light activated Photocatalyst. Sci. Rep. 2019, 9, 8008. [Google Scholar] [CrossRef]
- Saha, D.; Gismondi, P.; Kolasinski, K.W.; Shumlas, S.L.; Rangan, S.; Eslami, B.; McConnell, A.; Bui, T.; Cunfer, K. Fabrication of electrospun nanofiber composite of g-C3N4 and Au nanoparticles as plasmonic photocatalyst. Surf. Interfaces 2021, 26, 101367. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Pan, W.; Agrios, A. Enhanced Photocatalytic Activity of Electrospun TiO2 Nanofibers with Optimal Anatase/Rutile Ratio. J. Am. Ceram. Soc. 2011, 94, 3184–3187. [Google Scholar] [CrossRef]
- Cheng, J.; Feng, J.; Pan, W. Enhanced photocatalytic activity in electrospun bismuth vanadate nanofibers with phase junction. ACS Appl. Mater. Inter. 2015, 7, 9638–9644. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.G.; He, H.W.; Wang, X.X.; Zhang, J.; Zhang, Q.Q.; Tong, Y.F.; Liu, H.L.; Ramakrishna, S.; Yan, S.Y.; et al. One-Step Synthesis Heterostructured g-C3N4/TiO2 Composite for Rapid Degradation of Pollutants in Utilizing Visible Light. Nanomaterials 2018, 8, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, B.; Ojha, G.P.; Kuk, Y.S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Y.; Xing, Y.; Muhammad, S.; Pan, W. Synthesis and Photocatalytic Performance of Ag/BiVO4 Composite Nanofiber. Rare Metal Mat. Eng. 2018, 47, 356–360. [Google Scholar]
- Zhang, R.; Wu, H.; Lin, D.; Pan, W. Preparation of Necklace-Structured TiO2/SnO2 Hybrid Nanofibers and Their Photocatalytic Activity. J. Am. Ceram. Soc. 2009, 92, 2463–2466. [Google Scholar] [CrossRef]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon nanofiber Composite: An efficient Visible-light photocatalyst obtained from eelectrospinning and hydrothermal methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Pant, B.; Barakat, N.A.M.; Pant, H.R.; Park, M.; Saud, P.S.; Kim, J.W.; Kim, H.Y. Synthesis and photocatalytic activities of CdS/TiO2 nanoparticles supported on carbon nanofibers for high efficient adsorption and simultaneous decomposition of organic dyes. J. Colloid Interface Sci. 2014, 434, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, Q.; Zheng, S.; Yu, L.; Huang, F.; Zhang, C.; Sheng, J.; Yang, H. Direct electrospinning preparation of Z-scheme mixed-crystal Bi2O3/g-C3N4 composite photocatalysts with enhanced visible-light photocatalytic activity. New J. Chem. 2021, 45, 14522–14531. [Google Scholar] [CrossRef]
- Panthi, G.; Kwon, O.H.; Kuk, Y.S.; Gyawali, K.R.; Park, Y.W.; Park, M. Ternary Composite of Co-Doped CdSe@electrospun Carbon Nanofibers: A Novel Reusable Visible Light-Driven Photocatalyst with Enhanced Performance. Catalysts 2020, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Hollow SnO2 nanotubes decorated with ZnIn2S4 nanosheets for enhanced visible-light photocatalytic activity. J. Alloys Compd. 2020, 843, 155772. [Google Scholar] [CrossRef]
- Li, X.; Raza, S.; Liu, C. Directly electrospinning synthesized Z-scheme heterojunction TiO2@Ag@Cu2O nanofibers with enhanced photocatalytic degradation activity under solar light irradiation. J. Environ. Chem. Eng. 2021, 9, 106133. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M.; Avargani, V.M. Construction of efficient and stable ternary ZnFe2O4/Ag/AgBr Z-scheme photocatalyst based on ZnFe2O4 nanofibers under LED visible light. Mater. Res. Bull. 2021, 143, 111449. [Google Scholar] [CrossRef]
- Kessick, R.; Fenn, J.; Tepper, G. The use of AC potentials in electrospraying and electrospinning processes. Polymer 2004, 45, 2981–2984. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Electrospinning: A whipping fluid jet generates submicron polymer fibers. Appl. Phys. Lett. 2001, 78, 1149–1151. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J. Appl. Phys. 2001, 90, 4836–4846. [Google Scholar] [CrossRef] [Green Version]
- Shina, Y.M.; Hohmanb, M.M.; Brennerc, M.P.; Rutledge, G.C. Experimental characterization of electrospinning: The electrically forced jet and instabilities. Polymer 2001, 42, 9955–9967. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.J. The stretching of an electrified non-Newtonian jet: A model for electrospinning. Phys. Fluids 2002, 14, 3912–3926. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Dong, Y.; Feng, Y.; Xu, J. Compositing Two-Dimensional Materials with TiO2 for Photocatalysis. Catalysts 2018, 8, 590. [Google Scholar] [CrossRef] [Green Version]
- Marinho, B.A.; de Souza, S.M.A.G.U.; de Souza, A.A.U.; Hotza, D. Electrospun TiO2 nanofibers for water and wastewater treatment: A review. J. Mater. Sci. 2020, 56, 5428–5448. [Google Scholar] [CrossRef]
- Dudley, B. BP Statistical Review of World Energy; BP: London, UK, 2013. [Google Scholar]
- Yang, W.; Chen, Y.; Gao, S.; Sang, L.; Tao, R.; Sun, C.; Shang, J.K.; Li, Q. Post-illumination activity of Bi2WO6 in the dark from the photocatalytic “memory” effect. J. Adv. Ceram. 2021, 10, 355–367. [Google Scholar] [CrossRef]
- Gao, B.; Hu, D.; Xiao, C.; Li, D.; Zheng, Y. Enhanced visible-light-driven photocatalytic performance of AgNbO3 cubes with a high-energy (001) facet. J. Phys. Chem. Solids 2019, 135, 109083. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Min, X.; Xing, Y. A novel 2D graphene oxide modified α-AgVO3 nanorods: Design, fabrication and enhanced visible-light photocatalytic performance. J. Adv. Ceram. 2021. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Huang, S.; Xie, M.; He, M.; Xu, H.; Li, H.; Zhang, Q. Novel magnetic CoFe2O4 /Ag/Ag3VO4 composites: Highly efficient visible light photocatalytic and antibacterial activity. Appl. Catal. B Environ. 2016, 199, 11–22. [Google Scholar] [CrossRef]
- Sajid, M.M.; Shad, N.A.; Javed, Y.; Khan, S.B.; Zhai, H. Preparation and characterization of Vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Sur. Interfaces 2020, 19, 100502. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yu, J.; Zhang, Y.; Cui, Z.; Sun, Y.; Hou, B. Fabrication of InVO4/AgVO3 heterojunctions with enhanced photocatalytic antifouling efficiency under visible-light. Appl. Catal. B Environ. 2018, 220, 57–66. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Ren, J.; Sun, S.; Wang, L.; Zhang, L. A practical visible-light-driven Bi2WO6 nanofibrous mat prepared by electrospinning. J. Mater. Chem. 2009, 19, 6213. [Google Scholar] [CrossRef]
- Sedghi, R.; Moazzami, H.R.; Hosseiny Davarani, S.S.; Nabid, M.R.; Keshtkar, A.R. A one step electrospinning process for the preparation of polyaniline modified TiO2/polyacrylonitile nanocomposite with enhanced photocatalytic activity. J. Alloys Compd. 2017, 695, 1073–1079. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.; Mao, S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh-Attar, A. Photocatalytic degradation evaluation of N-Fe codoped aligned TiO2 nanorods based on the effect of annealing temperature. J. Adv. Ceram. 2020, 9, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.M.; Wang, H.C.; Shen, Y.; Lin, Y.H.; Nan, C.W. Low-dimensional nanostructured photocatalysts. J. Adv. Ceram. 2015, 4, 159–182. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Chai, S.; Yang, H.; Gao, Z.; Zhang, R.; Wang, L.; Kang, L. Enhancing visible-light driven photocatalytic performance of BiOBr by self-doping and in-situ deposition strategy: A synergistic effect between Bi5+ and metallic Bi. Sep. Purif. Technol. 2020, 253, 117388. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Borgarello, E.; Kiwi, J.; Graetzel, M.; Pelizzetti, E.; Visca, M. Visible light induced water cleavage in colloidal solutions of chromium-doped titanium dioxide particles. J. Am. Chem. Soc. 1982, 104, 2996–3002. [Google Scholar] [CrossRef]
- Jing, P.; Wei, L.; Su, Q.; Xie, E. High photocatalytic activity of V-doped SrTiO3 porous nanofibers produced from a combined electrospinning and thermal diffusion process. Beilstein J. Nanotechnol. 2015, 6, 1281–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouypazadeh, H.; Farrokhpour, H.; Momeni, M.M. Theoretical Investigation of the water splitting photocatalytic properties of pristine, Nb and V doped, and Nb-V co-doped (111) TaON nanosheets. Appl. Surf. Sci. 2021, 541, 148572. [Google Scholar] [CrossRef]
- Shao, Y.; Feng, K.; Guo, J.; Zhang, R.; He, S.; Wei, X.; Lin, Y.; Ye, Z.; Chen, K. Electronic structure and enhanced photoelectrocatalytic performance of RuxZn1−xO/Ti electrodes. J. Adv. Ceram. 2021, 10, 1025–1041. [Google Scholar] [CrossRef]
- Shen, J.; Li, F.; Wu, Y.; Fu, L.; Zhang, B. Preparation of doped TiO2 nanofiber membranes through electrospinning and their application for photocatalytic degradation of malachite green. J. Mater. Sci. 2014, 49, 2303–2314. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S. Gold Nanoparticles Located at the Interface of Anatase/Rutile TiO2 Particles as Active Plasmonic Photocatalysts for Aerobic Oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Cheminform 2010, 40, 1578–1586. [Google Scholar]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Q.; Chen, X.; Wei, L.; Yang, K.; Yu, C. The role of photoluminescence induced by Yb3+/Yb2+ transformation in promoting the SPR effect in Pd/Ybn+/BiOBr photocatalyst system. Opt. Mater. 2020, 109, 110316. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, P.; Zhao, J.; Zhang, Y.; Hu, J.; Shao, G. Plasmon enhancement on photocatalytic hydrogen production over the Z-scheme photosynthetic heterojunction system. Appl. Catal. B Environ. 2017, 210, 297–305. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.T. Upconversion and Downconversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Batentschuk, M.; Osvet, A.; Pinna, L.; Brabec, C.J. Rare-Earth Ion Doped Up-Conversion Materials for Photovoltaic Applications. Adv. Mater. 2011, 23, 2675–2680. [Google Scholar] [CrossRef]
- Gao, W.; Wu, Y.; Lu, G. 980 nm NIR light driven overall water splitting over a combined CdS-RGO-NaYF4-Yb3+/Er3+ photocatalyst. Catal. Sci. Technol. 2020, 10, 2389–2397. [Google Scholar] [CrossRef]
- Mao, Z.; Xie, R.; Fu, D.; Zhang, L.; Xu, H.; Zhong, Y.; Sui, X. PAN supported Ag-AgBr@Bi20TiO32 electrospun fiber mats with efficient visible light photocatalytic activity and antibacterial capability. Sep. Purif. Technol. 2017, 176, 277–286. [Google Scholar] [CrossRef]
- Chang, W.; Xu, F.; Mu, X.; Ji, L.; Ma, G.; Nie, J. Fabrication of nanostructured hollow TiO2 nanofibers with enhanced photocatalytic activity by coaxial electrospinning. Mater. Res. Bull. 2013, 48, 2661–2668. [Google Scholar] [CrossRef]
- Khore, S.K.; Kadam, S.R.; Kale, B.B.; Sonawane, R.S. A green approach: Scalable dry media synthesis of a γ-TaON photocatalyst for solar H2 production and rhodamine B degradation. Catal. Sci. Technol. 2020, 4, 4671–4678. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, M.H.; Kim, J.; Kim, C.W.; Youn, D.H. Facile nanocrystalline Ta3N5 synthesis for photocatalytic dye degradation under visible light. Chem. Phys. Lett. 2020, 738, 136900. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A. Novel Cu2ZnSnS4/Pt/g-C3N4 heterojunction photocatalyst with straddling band configuration for enhanced solar to fuel conversion. Appl. Catal. B Environ. 2020, 277, 119239. [Google Scholar] [CrossRef]
- Xu, J.; Sun, X.; Shan, Y.; Xu, J.; Wang, G.; Wang, L. Enhanced UV-light driven photocatalytic performances and recycling properties of TiO2/AlON composite photocatalyst. Ceram. Int. 2019, 45, 6767–6773. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Li, B.; Dai, W.; Si, Y.; Yang, L.; Luo, S. Hierarchical Ag3PO4@ZnIn2S4 nanoscoparium: An innovative Z-scheme photocatalyst for highly efficient and predictable tetracycline degradation. J. Colloid Interface Sci. 2020, 586, 708–718. [Google Scholar] [CrossRef]
- Hua, H.; Feng, F.; Du, M.; Ma, Y.; Pu, Y.; Zhang, J.; Li, X.A. 0D-2D Z-Scheme photocatalyst Cd0.5Zn0.5S@Bi2Fe4O9 for effective hydrogen evolution from water. Appl. Surf. Sci. 2021, 541, 148428. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Li, Y.; Wu, X.; Zhang, G. Promoted charge separation from nickel intervening in [Bi2O2]2+ layers of Bi2O2S crystals for enhanced photocatalytic CO2 conversion. Appl. Catal. B Environ. 2021, 294, 120249. [Google Scholar] [CrossRef]
- Xiang, H.; Xing, Y.; Dai, F.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Li, T.; Yao, Y.; Ko, B.H.; Huang, Z.; Dong, Q.; Gao, J.; Chen, W.; Li, J.; Li, S.; Wang, X.; et al. Carbon-Supported High-Entropy Oxide Nanoparticles as Stable Electrocatalysts for Oxygen Reduction Reactions. Adv. Funct. Mater. 2021, 31, 2010561. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Rhoderick, E.H. Metal-semiconductor contacts. IEEE Proc. 1982, 129, 1–14. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.P.; Li, S.; Huang, Z.; Yang, C.; Wang, H. Enhanced photocatalytic activity in Ag-nanoparticle-dispersed BaTiO3 composite thin films: Role of charge transfer. J. Adv. Ceram. 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Bian, Z.; Zhu, J.; Huo, Y.; Lu, Y. Mesoporous Au/TiO2 Nanocomposites with Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 4538–4539. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.A.; Dan, L.A.; Tl, A.; Wei, Z.A.; Xw, A.; Xh, B.; Hya, C. Plasmonic Z-scheme Pt-Au/BiVO4 photocatalyst: Synergistic effect of crystal-facet engineering and selective loading of Pt-Au cocatalyst for improved photocatalytic performance. J. Colloid Interface Sci. 2020, 570, 232–241. [Google Scholar]
- Luo, J.; Li, R.; Chen, Y.; Zhou, X.; Ning, X.; Zhan, L.; Ma, L.; Xu, X.; Xu, L.; Zhang, L. Rational design of Z-scheme LaFeO3/SnS2 hybrid with boosted visible light photocatalytic activity towards tetracycline degradation. Sep. Purif. Technol. 2019, 210, 417–430. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 Nanocomposite as a Direct Z-Scheme Photocatalyst for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2017, 6, 965–973. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Ming, K.; Li, Y.; Chen, X.; Tian, T.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414. [Google Scholar]
- Jiang, L.; Yang, J.; Yuan, X.; Guo, J.; Liang, J.; Tang, W.; Chen, Y.; Li, X.; Wang, H.; Chu, W. Defect engineering in polymeric carbon nitride photocatalyst: Synthesis, properties and characterizations. Adv. Colloid Interface Sci. 2021, 296, 102523. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, P.; Gao, Y.; Huang, H.; Tong, F.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Dai, Y.; et al. Design and synthesis of porous M-ZnO/CeO2 microspheres as efficient plasmonic photocatalysts for nonpolar gaseous molecules oxidation: Insight into the role of oxygen vacancy defects and M = Ag, Au nanoparticles. Appl. Catal. B Environ. 2020, 260, 118151. [Google Scholar] [CrossRef]


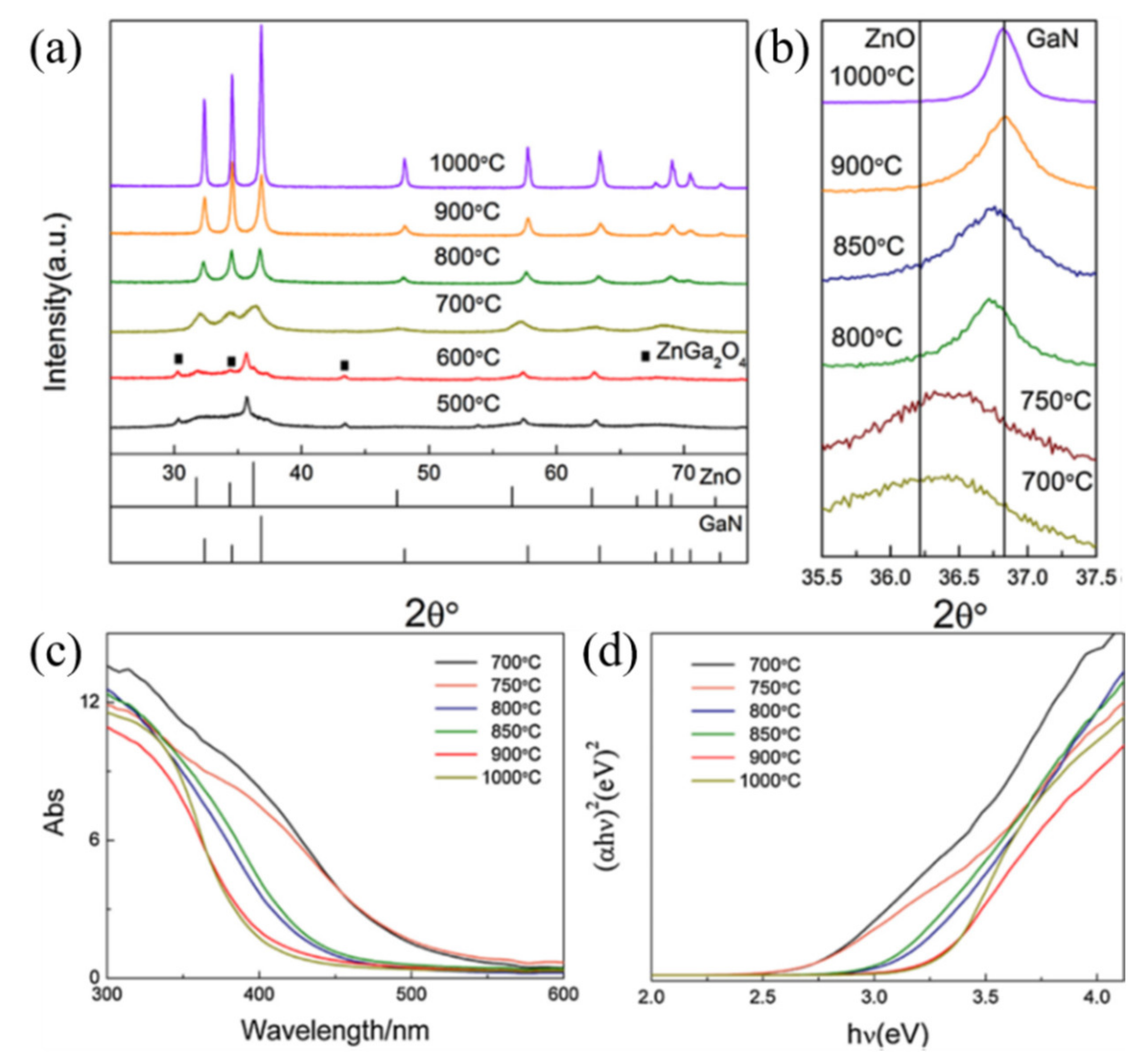

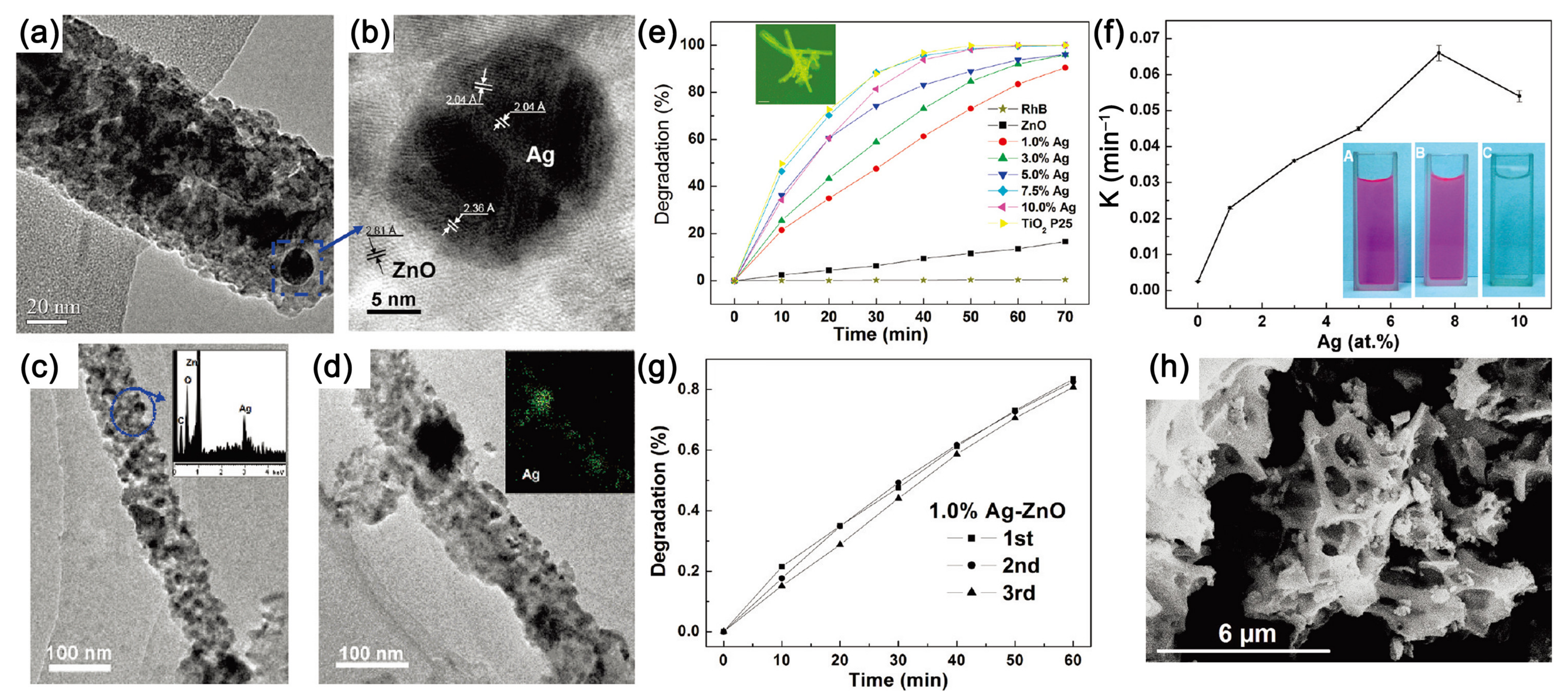
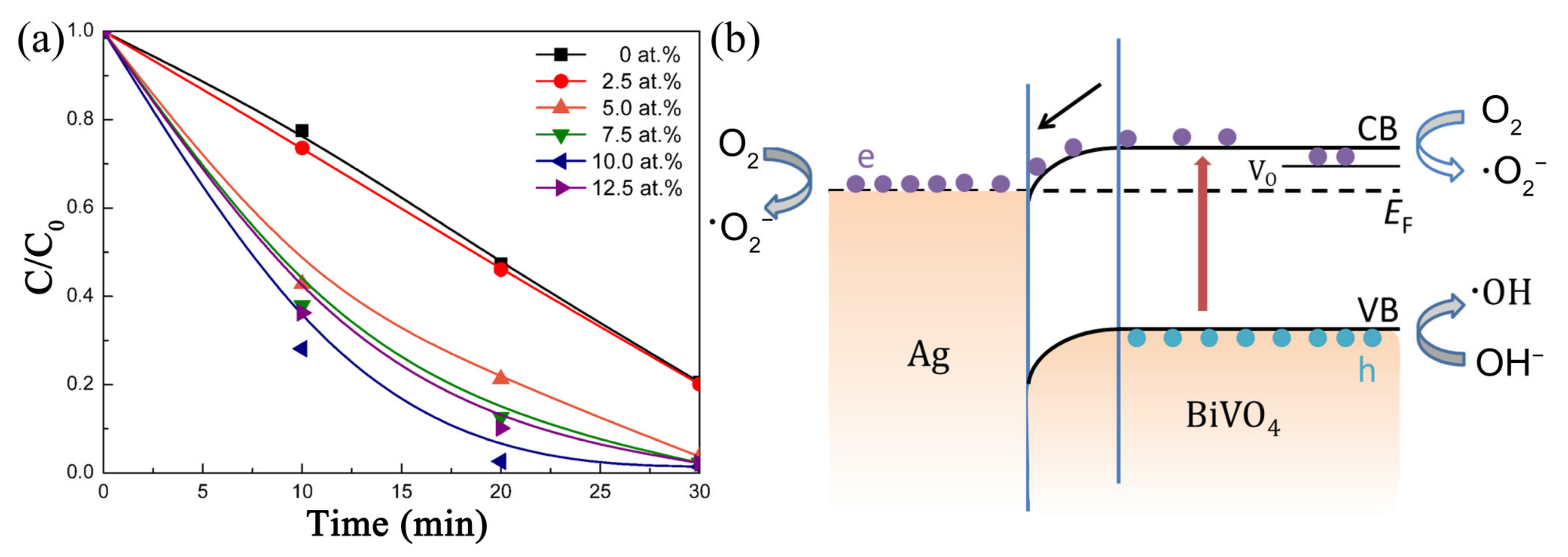
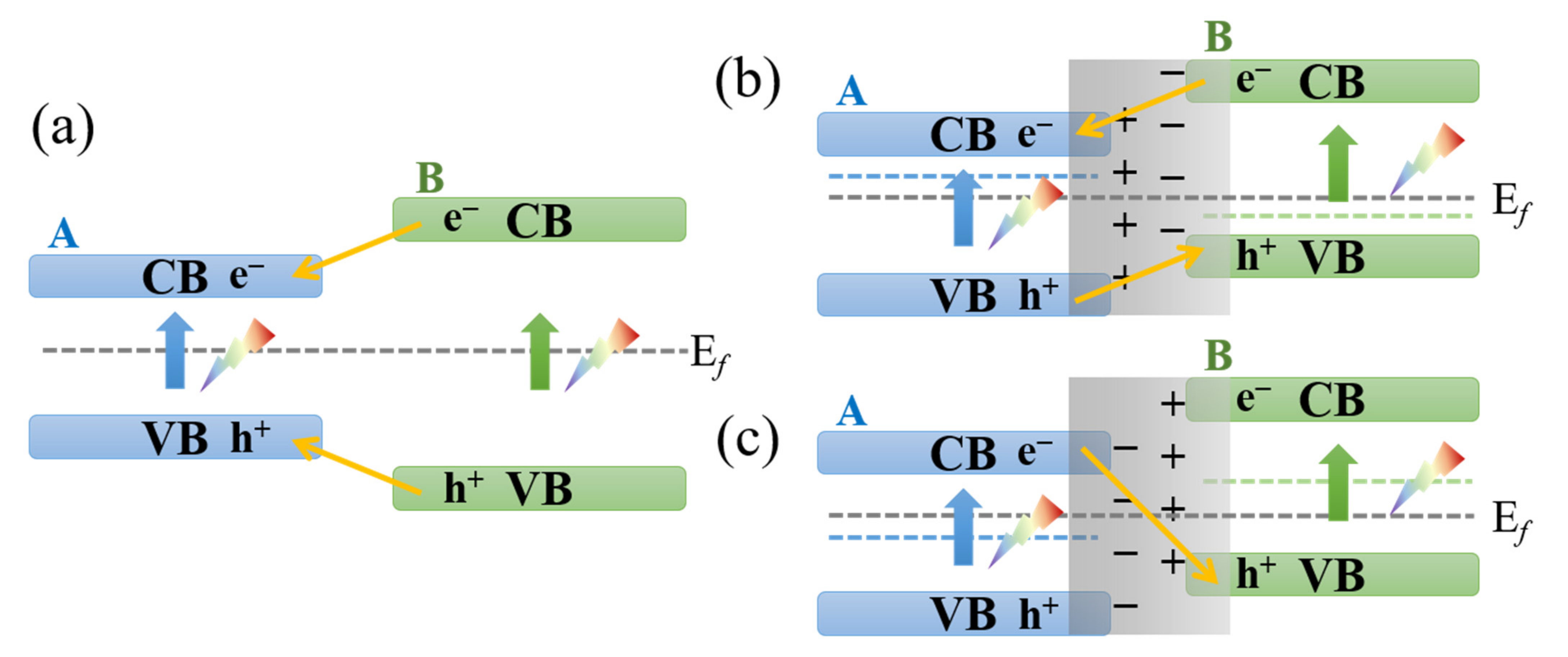

| Photocatalyst | Strategy | Light Source | Experimental Conditions | Performance Efficiency/Time | Reference |
|---|---|---|---|---|---|
| TiO2 | Diameter control | UV lamp | Catalyst: 0.1 wt.% RhB: 2.5 × 10−5 mol/L | ~90%/20 min | [48] |
| Bimetal-PANNM | Surface area | UV lamp | Catalyst: 4 g/L Reactive blue 19: 25 mg/L | 100%/80 min | [49] |
| CuWO4 | Morphology | Visible light | Catalyst: 1 g/L MO: 5 mg/L | 90%/180 min | [50] |
| BiVO4 | Microstructure (Hollow fibers) | Visible light | Catalyst: 0.5 g/L Cr(VI): 10 mg/L | 95.3%/80 min | [51] |
| ZnO | Defect Microstructure | UV light | Catalyst: 1 g/L MB: 1.5 × 10−5 mol/L | 90%/85 min | [52] |
| Mn2+/ZnO | Doping | Visible light | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | ~80%/260 min | [53] |
| N-Mn/ZnO | Doping Defect | Visible light | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | ~95%/100 min | [54] |
| N-TiO2 | Doping Defect | Visible light | Catalyst: 0.1 wt.% RhB: 2.5 × 10−5 mol/L | 25%/60 min | [55] |
| (ZnO)x(GaN)1−x | Doping Defect | Visible light | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | ~100%/80 min | [56] |
| Fe-TiO2/SnO2 | Doping Heterostructure | Visible light | Catalyst: 0.05 wt.% RhB: 2.5 × 10−5 mol/L | 10%/60 min | [57] |
| Au/TiO2 | Surface plasmon resonance | Visible light | Catalyst: 0.4 g/L RhB: 10 mg/L | 42%/120 min | [58] |
| Au/g-C3N4 | Surface plasmon resonance | LED light | Catalyst: 2 cassettes MB: 5 ppm | ~90%/50 min | [59] |
| TiO2 | Phase junction | UV lamp | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | ~100%/40 min | [60] |
| BiVO4 | Phase junction | Visible light | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | 100%/40 min | [61] |
| ZnO-ZnS | Heterostructure | UV lamp | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | 73%/60 min | [39] |
| g-C3N4/TiO2 | Heterostructure | Visible light | Catalyst: 1 g/L RhB: 5 mg/L | ~75%/120 min | [62] |
| ZnO-TiO2-CNFs | Heterostructure | UV lamp | Catalyst: 0.8 g/L MB: 10 ppm | ~95%/120 min | [63] |
| Ag-ZnO | Heterostructure | UV lamp | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | 95%/40 min | [17] |
| Ag/BiVO4 | Heterostructure | Visible light | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | ~100%/20 min | [64] |
| TiO2/SnO2 | Heterostructure | UV lamp | Catalyst: 0.1 wt.% RhB: 2.5 × 10−5 mol/L | 90%/60 min | [65] |
| Ag3PO4-TiO2-CNFs | Heterostructure | Visible light | Catalyst: 0.5 g/L MB: 10 ppm | 100%/10 min | [66] |
| Carbon-CdS/TiO2 | Heterostructure | Visible light | Catalyst: 0.5 g/L MB: 10 ppm | 100%/30 min | [67] |
| Bi2O3/g-C3N4 | Heterostructure | LED light | Catalyst: 0.25 g/L TC: 20 mg/L | ~60%/3 h | [68] |
| Co-CdSe@ECNFs | Heterostructure Doping | Visible light | Catalyst: 1 g/L MB: 10 mg/L | 87%/90 min | [69] |
| ZnIn2S4/SnO2 | Heterostructure Microstructure | Visible light | Catalyst: 0.6 g/L Cr(VI): 50 ppm | 100%/80 min | [70] |
| TiO2@Ag@Cu2O | Heterostructure Surface plasmon resonance | Visible light | Catalyst: 0.5 g/L MB: 10 mg/L | 99%/90 min | [71] |
| ZnFe2O4/Ag/AgBr | Heterostructure Surface plasmon resonance | LED light | Catalyst: 1 g/L RhB: 100 mg/L | 86%/100 min | [72] |
| CQDs-TiO2 | Up-conversion luminescence Heterostructure | Visible light | Catalyst: 1 g/L RhB: 2.5 × 10−5 mol/L | 100%/20 min | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Cheng, J.; Li, H.; Lin, D.; Wang, Y.; Wu, H.; Pan, W. Electrospun Ceramic Nanofibers for Photocatalysis. Nanomaterials 2021, 11, 3221. https://doi.org/10.3390/nano11123221
Xing Y, Cheng J, Li H, Lin D, Wang Y, Wu H, Pan W. Electrospun Ceramic Nanofibers for Photocatalysis. Nanomaterials. 2021; 11(12):3221. https://doi.org/10.3390/nano11123221
Chicago/Turabian StyleXing, Yan, Jing Cheng, Heping Li, Dandan Lin, Yuting Wang, Hui Wu, and Wei Pan. 2021. "Electrospun Ceramic Nanofibers for Photocatalysis" Nanomaterials 11, no. 12: 3221. https://doi.org/10.3390/nano11123221
APA StyleXing, Y., Cheng, J., Li, H., Lin, D., Wang, Y., Wu, H., & Pan, W. (2021). Electrospun Ceramic Nanofibers for Photocatalysis. Nanomaterials, 11(12), 3221. https://doi.org/10.3390/nano11123221









