Li2ZrO3-Coated Monocrystalline LiAl0.06Mn1.94O4 Particles as Cathode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
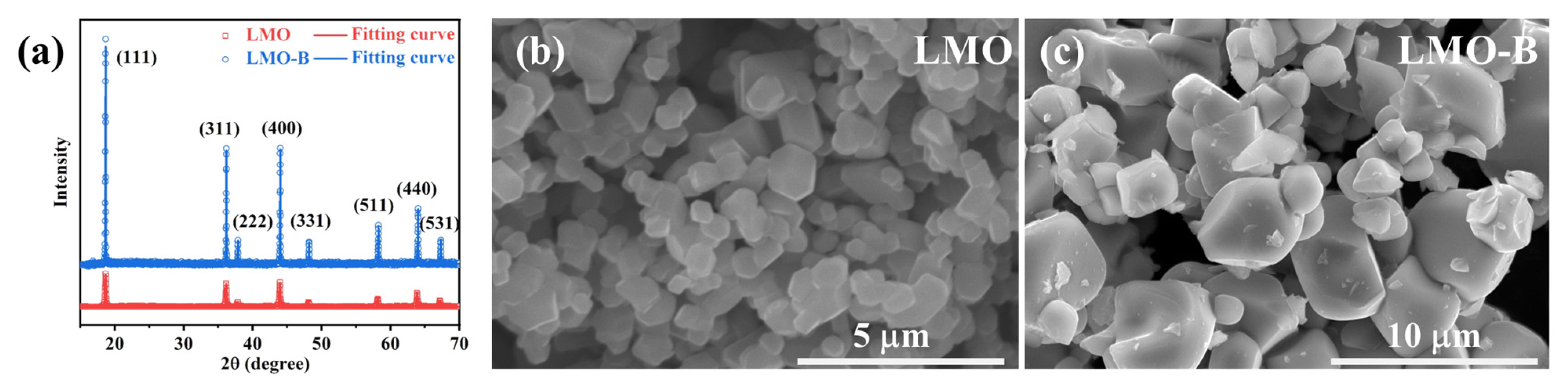
3.1. Li3BO3 Additive Promote the Grain Growth
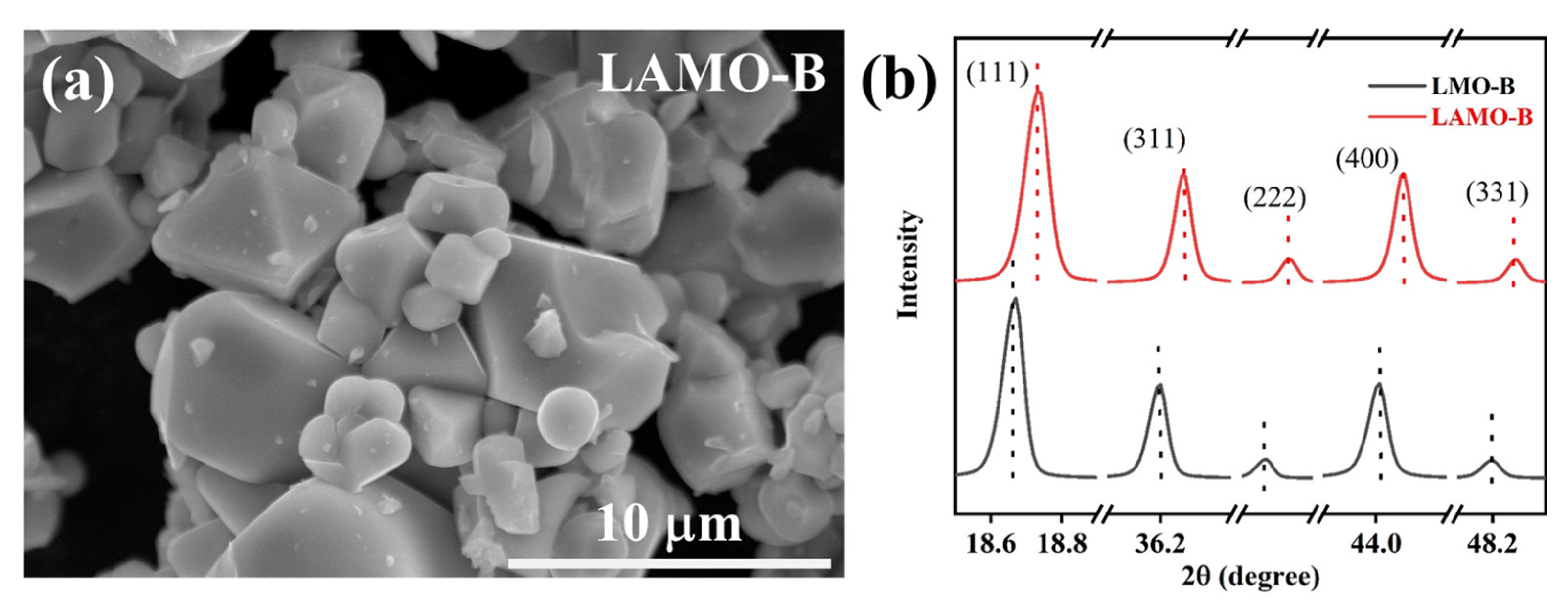
3.2. Effect of the Al-Doping
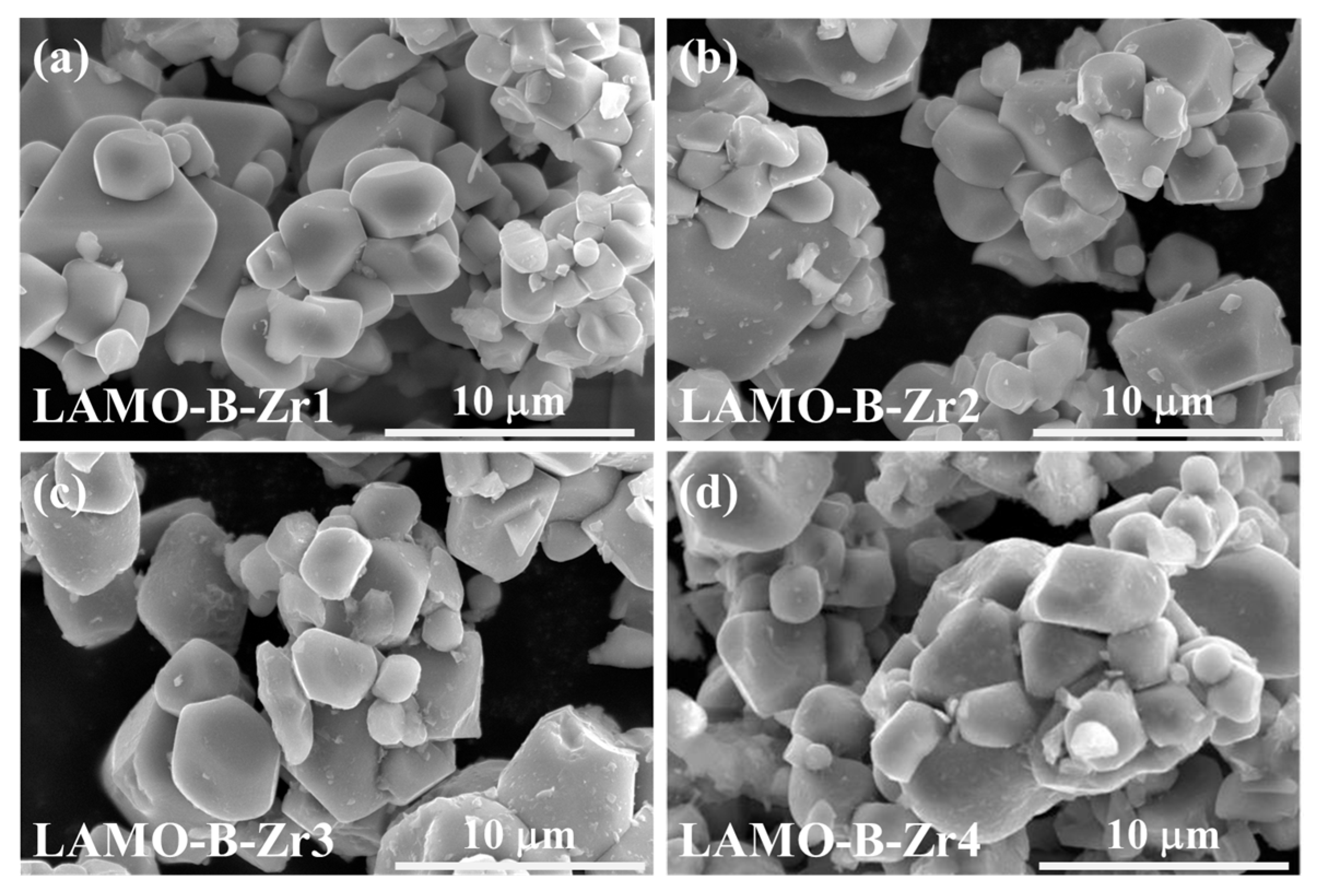
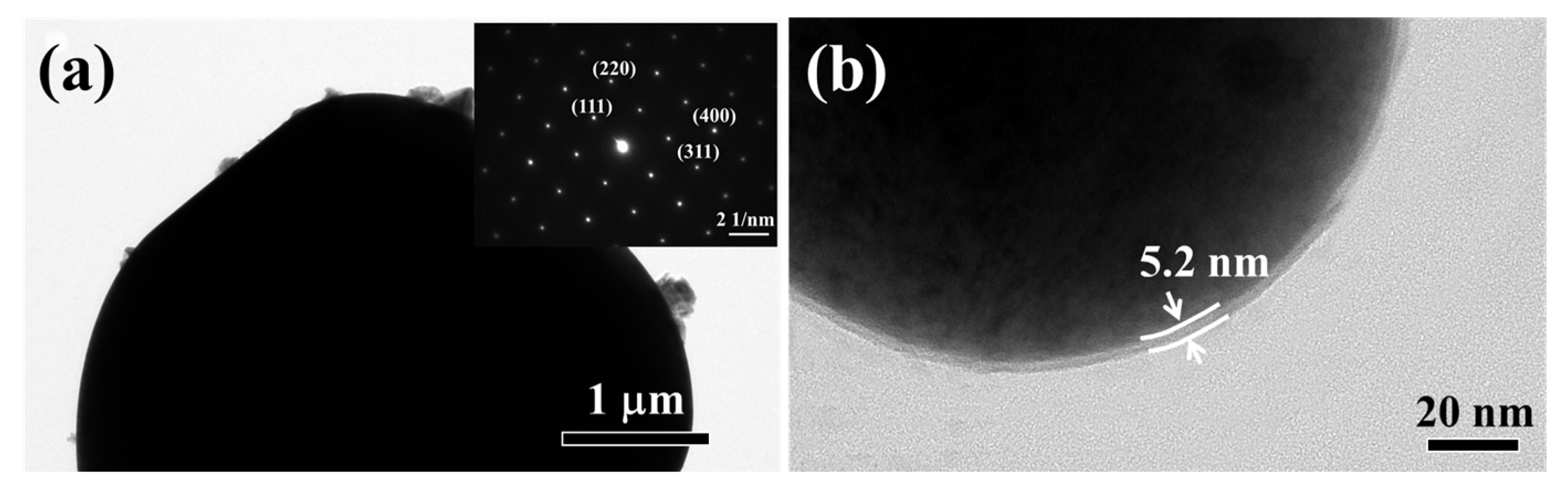
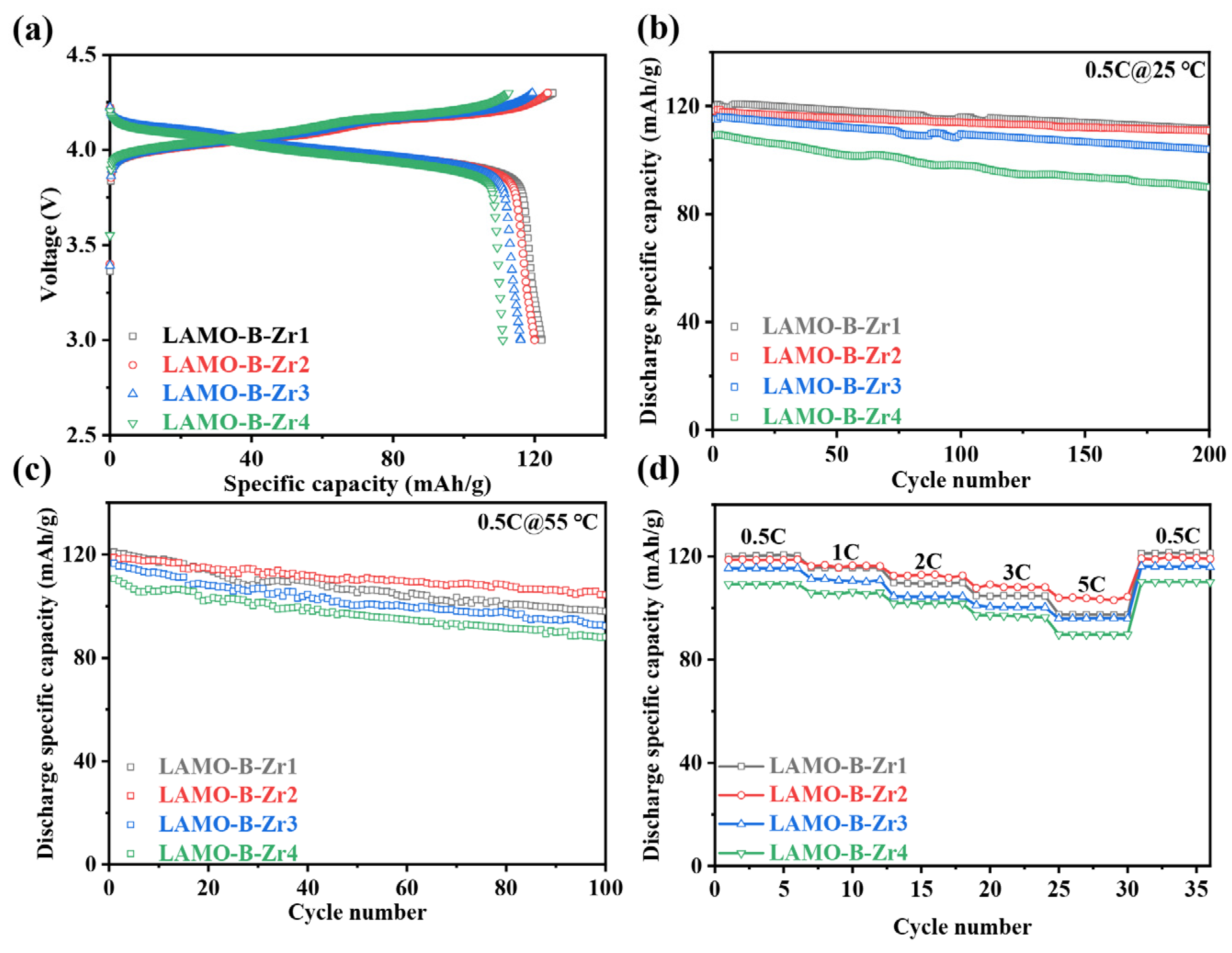
3.3. Li2ZrO3-Coating on the Monocrystal LiMn2O4
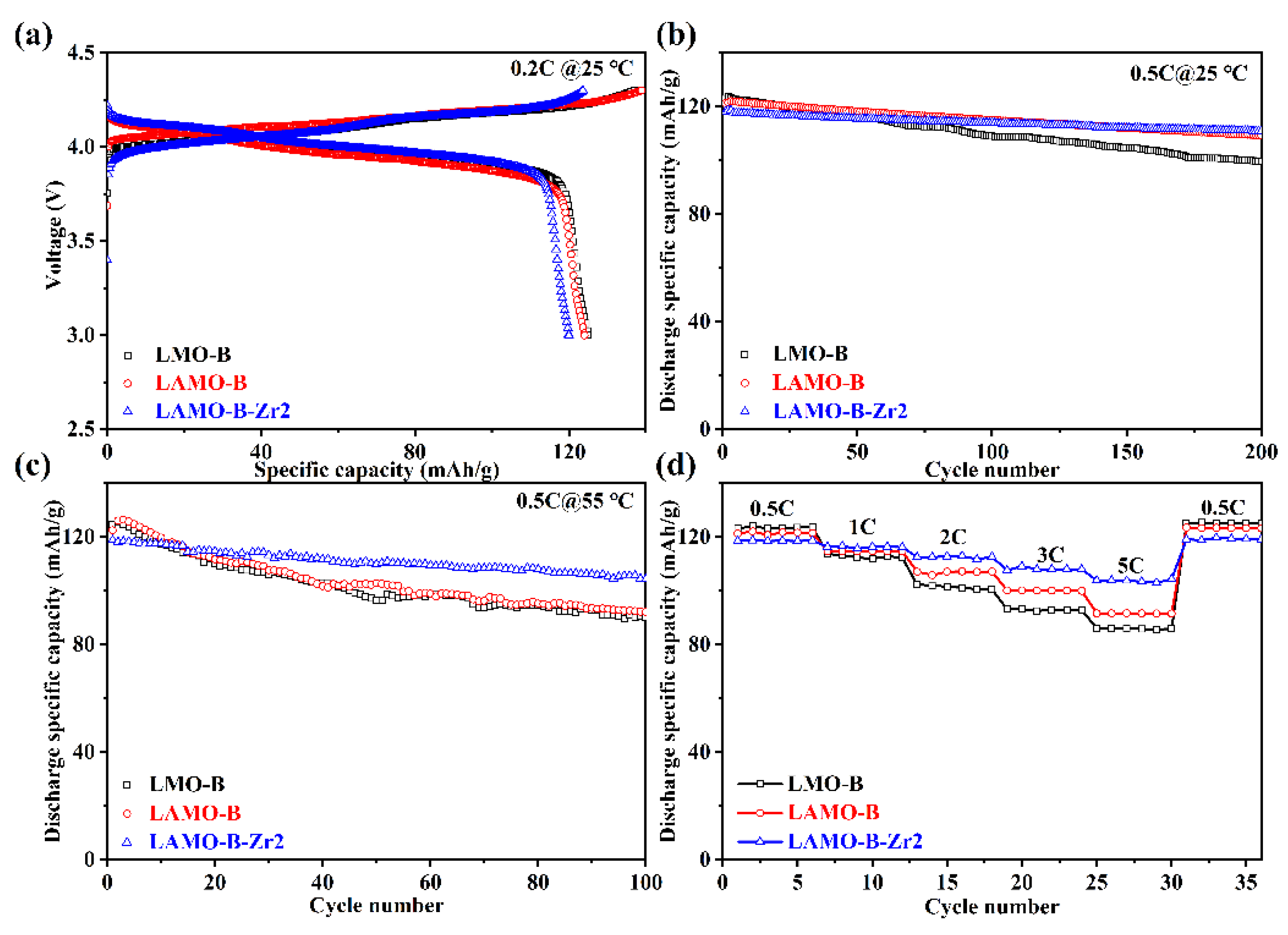
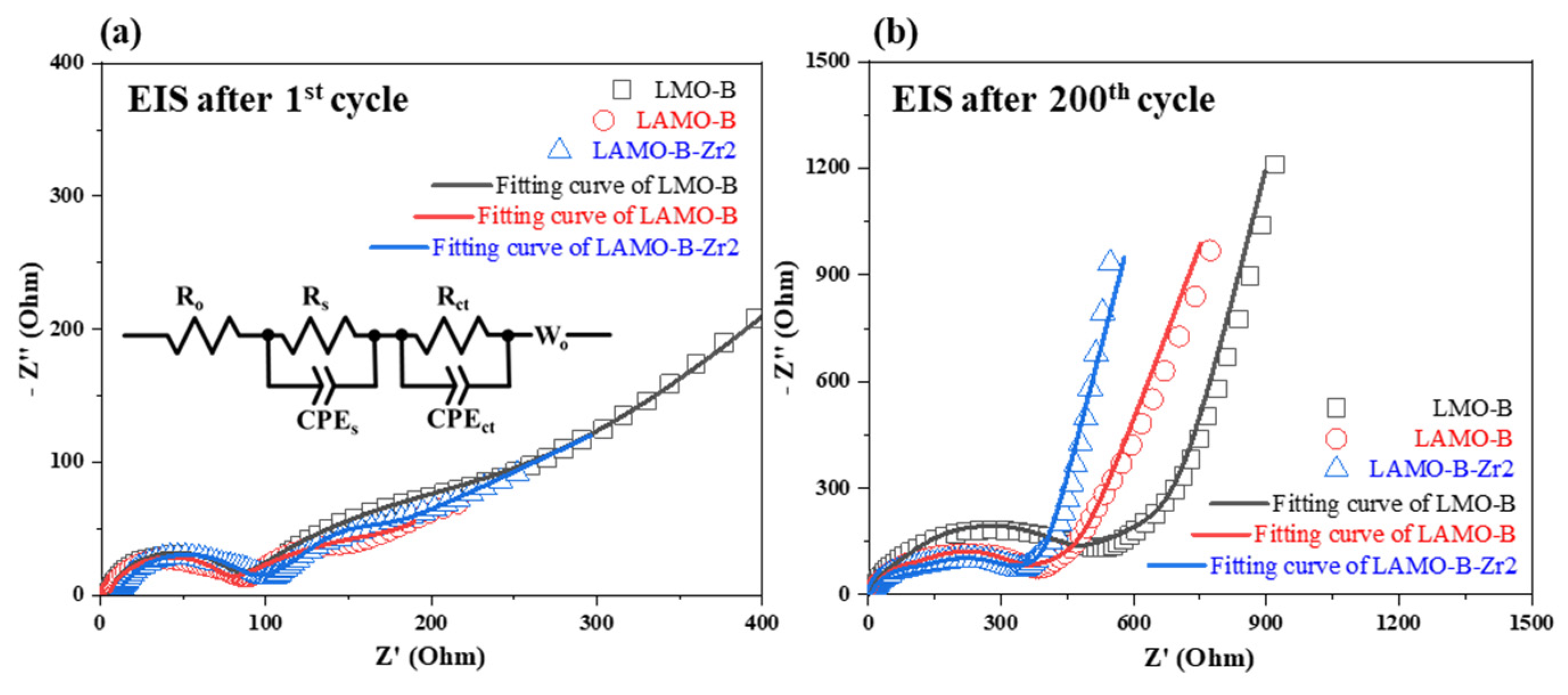
3.4. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulsen, J.M.; Thomas, C.L.; Dahn, J.R. Layered Li-Mn-Oxide with the O2 Structure: A Cathode Material for Li-Ion Cells Which Does Not Convert to Spinel. J. Electrochem. Soc. 1999, 146, 3560–3565. [Google Scholar] [CrossRef]
- Potapenko, A.V.; Kirillov, S.A. Enhancing high-rate electrochemical properties of LiMn2O4 in a LiMn2O4/LiNi0.5Mn1.5O4 core/shell composite. Electrochim. Acta 2017, 258, 9–16. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, K.; Cho, W.; Shin, W.H.; Kanno, R.; Choi, J.W. A Truncated Manganese Spinel Cathode for Excellent Power and Lifetime in Lithium-Ion Batteries. Nano Lett. 2012, 12, 6358–6365. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Ziółkowska, D.A.; Jasiński, J.B.; Lee, P.H.; Ławniczak, P.; Andrzejewski, B.; Ostrowski, A.; Bednarski, W.; Wu, S.H.; Lin, J.Y. Improved electrochemical performance of LiMn2O4 cathode material by Ce doping. Electrochim. Acta 2018, 276, 37–46. [Google Scholar] [CrossRef]
- Jiang, C.; Tang, Z.; Wang, S.; Zhang, Z. A truncated octahedral spinel LiMn2O4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries. J. Power Source 2017, 357, 144–148. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, X.; Doan, T.N.L.; Tian, Y.; Zhao, H.; Chen, P. Binder-free flexible LiMn2O4/carbon nanotube network as high power cathode for rechargeable hybrid aqueous battery. J. Power Source 2016, 326, 498–504. [Google Scholar] [CrossRef]
- Cao, J.; Guo, S.; Yan, R.; Zhang, C.; Guo, J.; Zheng, P. Carbon-coated single-crystalline LiMn2O4 nanowires synthesized by high-temperature solid-state reaction with high capacity for Li-ion battery. J. Alloys Compd. 2018, 741, 1–6. [Google Scholar] [CrossRef]
- Yi, T.-F.; Xie, Y.; Wu, Q.; Liu, H.; Jiang, L.; Ye, M.; Zhu, R. High rate cycling performance of lanthanum-modified Li4Ti5O12 anode materials for lithium-ion batteries. J. Power Source 2012, 214, 220–226. [Google Scholar] [CrossRef]
- Chen, M.; Chen, P.; Yang, F.; Song, H.; Liao, S. Ni, Mo Co-doped Lithium Manganate with Significantly Enhanced Discharge Capacity and Cycling Stability. Electrochim. Acta 2016, 206, 356–365. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, H.; Hu, Y.; Dai, Y.; Zhang, L.; Li, C. Synergistic Enhancement Effect of Al Doping and Highly Active Facets of LiMn2O4 Cathode Materials for Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2015, 54, 3800–3805. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Li, X.; Wang, Z.; Guo, H.; Xu, D.; Zhang, K. Sputtering graphite coating to improve the elevated-temperature cycling ability of the LiMn2O4 electrode. Phys. Chem. Chem. Phys. 2014, 16, 16021–16029. [Google Scholar] [CrossRef]
- Tron, A.; Park, Y.D.; Mun, J. AlF3-coated LiMn2O4 as cathode material for aqueous rechargeable lithium battery with improved cycling stability. J. Power Source 2016, 325, 360–364. [Google Scholar] [CrossRef]
- Waller, G.H.; Brooke, P.D.; Rainwater, B.H.; Lai, S.Y.; Hu, R.; Ding, Y.; Alamgir, F.M.; Sandhage, K.H.; Liu, M.L. Structure and surface chemistry of Al2O3 coated LiMn2O4 nanostructured electrodes with improved lifetime. J. Power Source 2016, 306, 162–170. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Mu, D.; Liu, X.; Peng, Y.; Xu, H.; Liu, Q.; Gai, L.; Wu, F. Compounds, Single-crystal LiNi0.6Co0.2Mn0.2O2 as high performance cathode materials for Li-ion batteries. J. Alloys Compd. 2016, 674, 360–367. [Google Scholar] [CrossRef]
- Li, J.; Cameron, A.R.; Li, H.; Glazier, S.; Xiong, D.; Chatzidakis, M.; Allen, J.; Botton, G.; Dahn, J. Comparison of single crystal and polycrystalline LiNi0.5Mn0.3Co0.2O2 positive electrode materials for high voltage Li-ion cells. J. Electrochem. Soc. 2017, 164, A1534. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, D.; Zhang, H.; Wang, S. Plasma-Assisted Sulfur Doping of LiMn2O4 for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 28776–28782. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Yu, Y.; Bai, H.; Shao, M.; Guo, J.; Su, C.; Liu, X.; Bai, W. Synthesis and electrochemical performance evaluations of polyhedra spinel LiAlxMn2-xO4 (x≤0.20) cathode materials prepared by a solution combustion technique. J. Alloys Compd. 2017, 728, 1315–1328. [Google Scholar] [CrossRef]
- Taniguchi, I.; Song, D.; Wakihara, M. Electrochemical properties of LiM1/6Mn11/6O4 (M = Mn, Co, Al and Ni) as cathode materials for Li-ion batteries prepared by ultrasonic spray pyrolysis method. J. Power Source 2002, 109, 333–339. [Google Scholar] [CrossRef]
- Yang, C.; Yu, S.; Lin, C.; Lv, F.; Wu, S.; Yang, Y.; Wang, W.; Zhu, Z.-Z.; Li, J.; Wang, N.; et al. Cr0.5Nb24.5O62 Nanowires with High Electronic Conductivity for High-Rate and Long-Life Lithium-Ion Storage. ACS Nano 2017, 11, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhao, H.; Wu, C.; Xie, H.; Cui, P.; Xiang, T.; Chen, S.; Zhang, L.; Fang, Y.; Wang, Z.; et al. Enhanced electrochemical performance of MoO3-coated LiMn2O4 cathode for rechargeable lithium-ion batteries. J. Mater. Chem. A 2017, 199, 203–208. [Google Scholar] [CrossRef]

| Samples | Composition | ||
|---|---|---|---|
| LiMn2O4 | Li3BO3 | Li2ZrO3 | |
| LMO | LiMn2O4 | * | * |
| LMO-B | LiMn2O4 | 1 mol% | * |
| LAMO-B | Li Al0.06Mn1.94O4 | 1 mol% | * |
| LAMO-B-Zr1 | Li Al0.06Mn1.94O4 | 1 mol% | 1 mol% |
| LAMO-B-Zr2 | Li Al0.06Mn1.94O4 | 1 mol% | 2 mol% |
| LAMO-B-Zr3 | Li Al0.06Mn1.94O4 | 1 mol% | 3 mol% |
| LAMO-B-Zr4 | Li Al0.06Mn1.94O4 | 1 mol% | 4 mol% |
| Samples | Capacity Retention Ratios | |
|---|---|---|
| After 200 Cycles at 25 °C | After 100 Cycles at 55 °C | |
| LMO-B | 81.0% | 72.2% |
| LAMO-B | 90.0% | 75.2% |
| LAMO-B-Zr2 | 93.9% | 87.8% |
| Sample | Rt (Ω) after 1st Cycle | Rt (Ω) after 200th Cycle |
|---|---|---|
| LMO-B | 248.1 | 377.8 |
| LAMO-B | 235.8 | 293.1 |
| LAMO-B-Zr2 | 101.1 | 270.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhao, B.; Yang, J.; Zhang, L.; Fang, Q.; Wang, X. Li2ZrO3-Coated Monocrystalline LiAl0.06Mn1.94O4 Particles as Cathode Materials for Lithium-Ion Batteries. Nanomaterials 2021, 11, 3223. https://doi.org/10.3390/nano11123223
Li C, Zhao B, Yang J, Zhang L, Fang Q, Wang X. Li2ZrO3-Coated Monocrystalline LiAl0.06Mn1.94O4 Particles as Cathode Materials for Lithium-Ion Batteries. Nanomaterials. 2021; 11(12):3223. https://doi.org/10.3390/nano11123223
Chicago/Turabian StyleLi, Chunliu, Banglei Zhao, Junfeng Yang, Linchao Zhang, Qianfeng Fang, and Xianping Wang. 2021. "Li2ZrO3-Coated Monocrystalline LiAl0.06Mn1.94O4 Particles as Cathode Materials for Lithium-Ion Batteries" Nanomaterials 11, no. 12: 3223. https://doi.org/10.3390/nano11123223
APA StyleLi, C., Zhao, B., Yang, J., Zhang, L., Fang, Q., & Wang, X. (2021). Li2ZrO3-Coated Monocrystalline LiAl0.06Mn1.94O4 Particles as Cathode Materials for Lithium-Ion Batteries. Nanomaterials, 11(12), 3223. https://doi.org/10.3390/nano11123223








