Photocatalytic Activity of Revolutionary Galaxaura elongata, Turbinaria ornata, and Enteromorpha flexuosa’s Bio-Capped Silver Nanoparticles for Industrial Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Collection and Preparation of Macroalgae Samples
2.2.2. Preparation of Aqueous Seaweed Extract
Preparation of Galaxaura elongata Extract
Preparation of Turbinaria ornata Extract
Preparation of Enteromorpha flexuosa Extract
2.2.3. Biosynthesis of Ag-NPs
Biosynthesis of Ag-NPs by Galaxaura elongata
Biosynthesis of Ag-NPs by Turbinaria ornata
Biosynthesis of Ag-NPs by Enteromorpha flexuosa
2.2.4. Purification and Drying the Biosynthesized Ag-NPs
2.2.5. Characterization of Biosynthesized Ag-NPs
2.2.6. Catalytic Experiments
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Samples Characterization
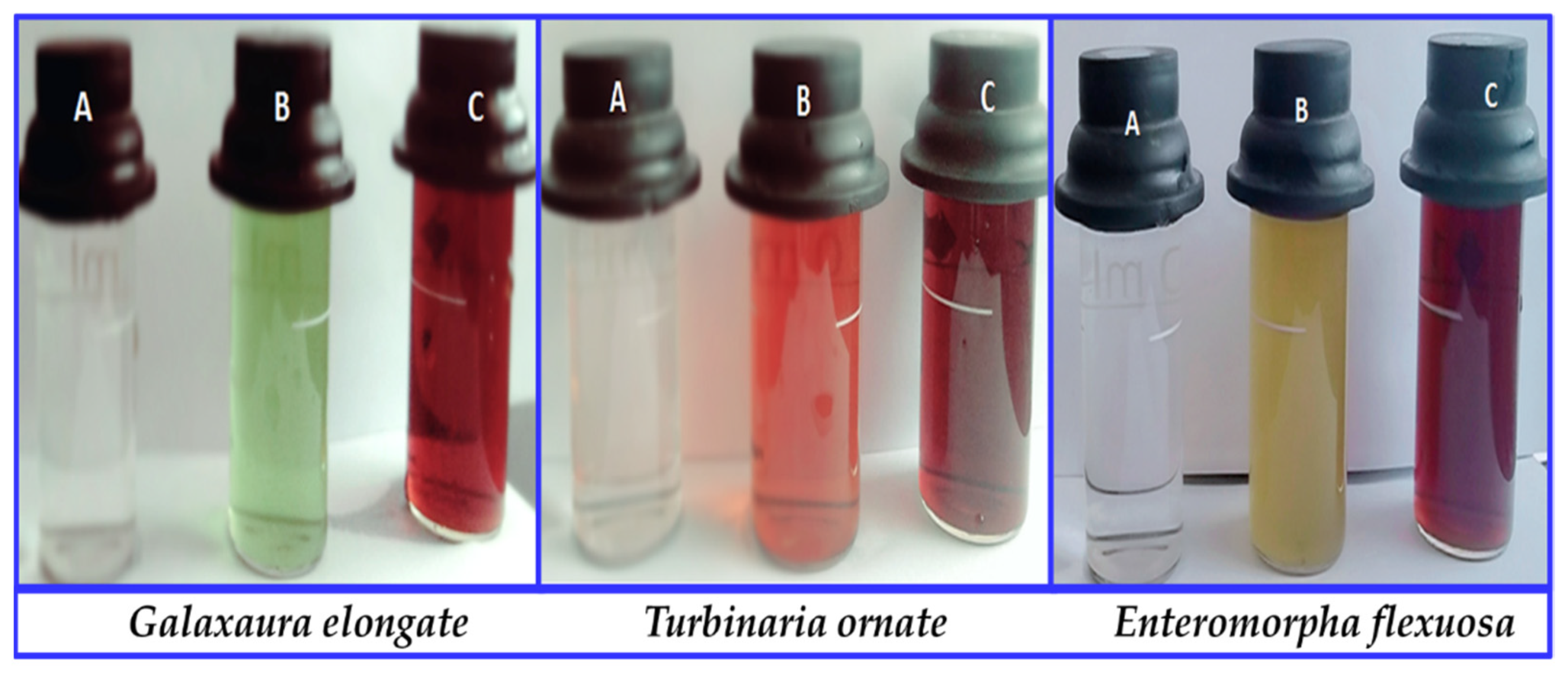
3.1.1. Optical Properties
3.1.2. Mechanism of Silver Nanoparticle Biosynthesis
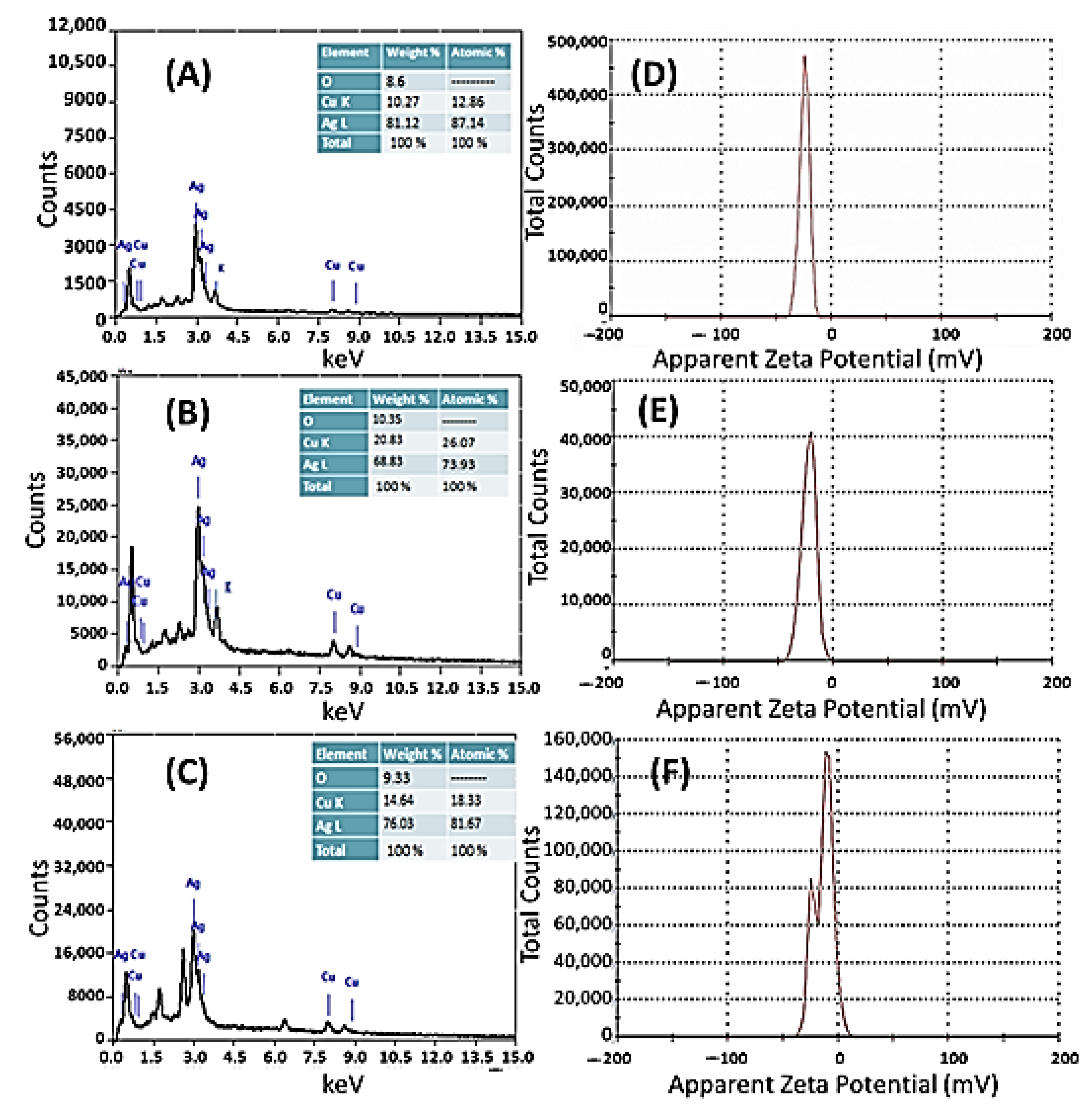
3.1.3. Energy-Dispersive X-ray Spectroscopy and Zeta Potential Analyses
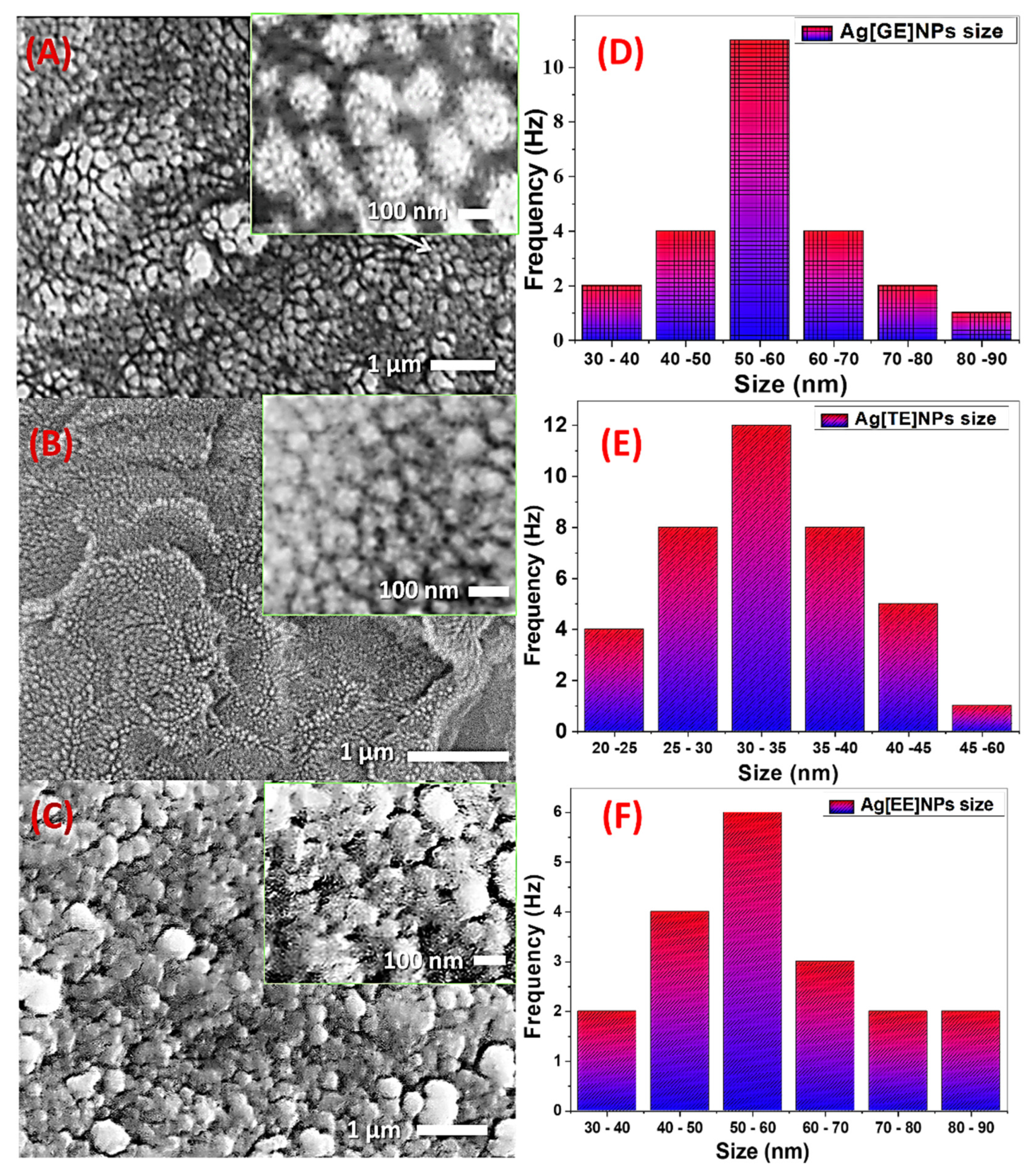
3.1.4. Scanning Electron Microscope (SEM)
3.1.5. X-ray Diffraction Analysis
3.2. Dye Degradation
3.2.1. Optimization of Variables in the Photocatalytic Degradation Process
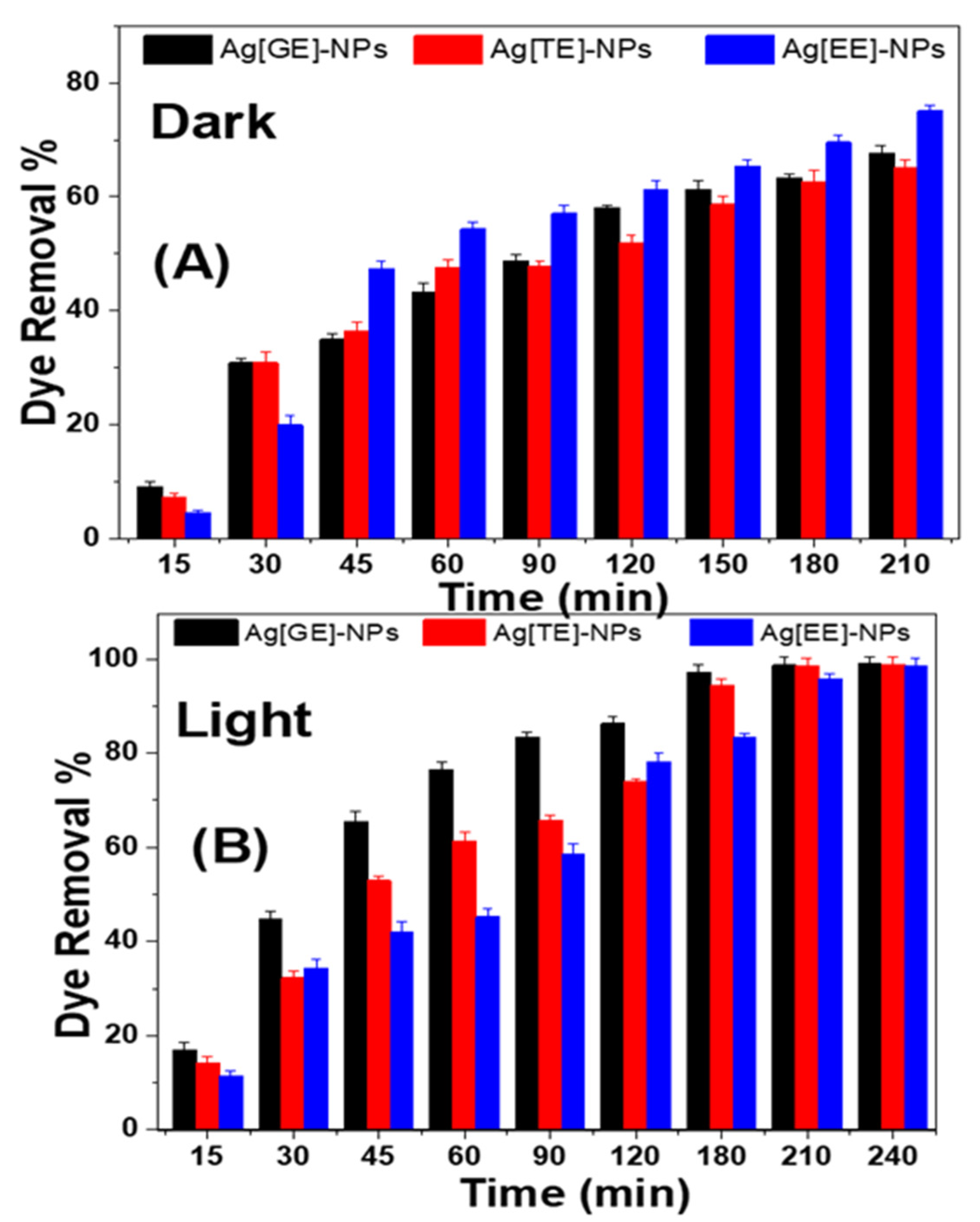
3.2.2. Impacts of Ag-NPs on Methylene Blue in Dark and Solar Light
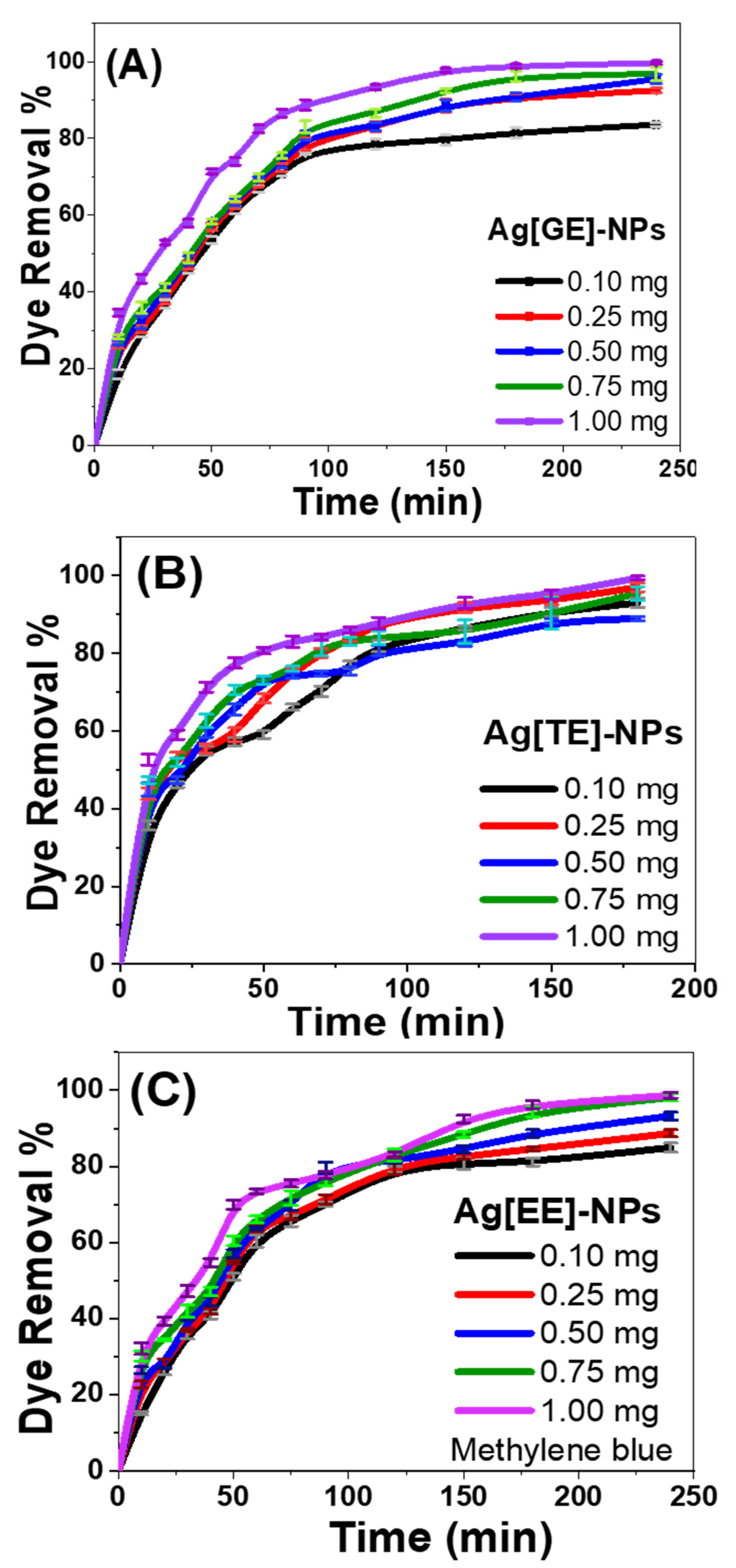
3.2.3. Effect of Catalyst Dose
3.2.4. Effects of Initial Methylene Blue Dye Concentrations
3.2.5. Effect of Temperature on Photodegradation and Calculations of Activation Energy, Enthalpy, and Entropy
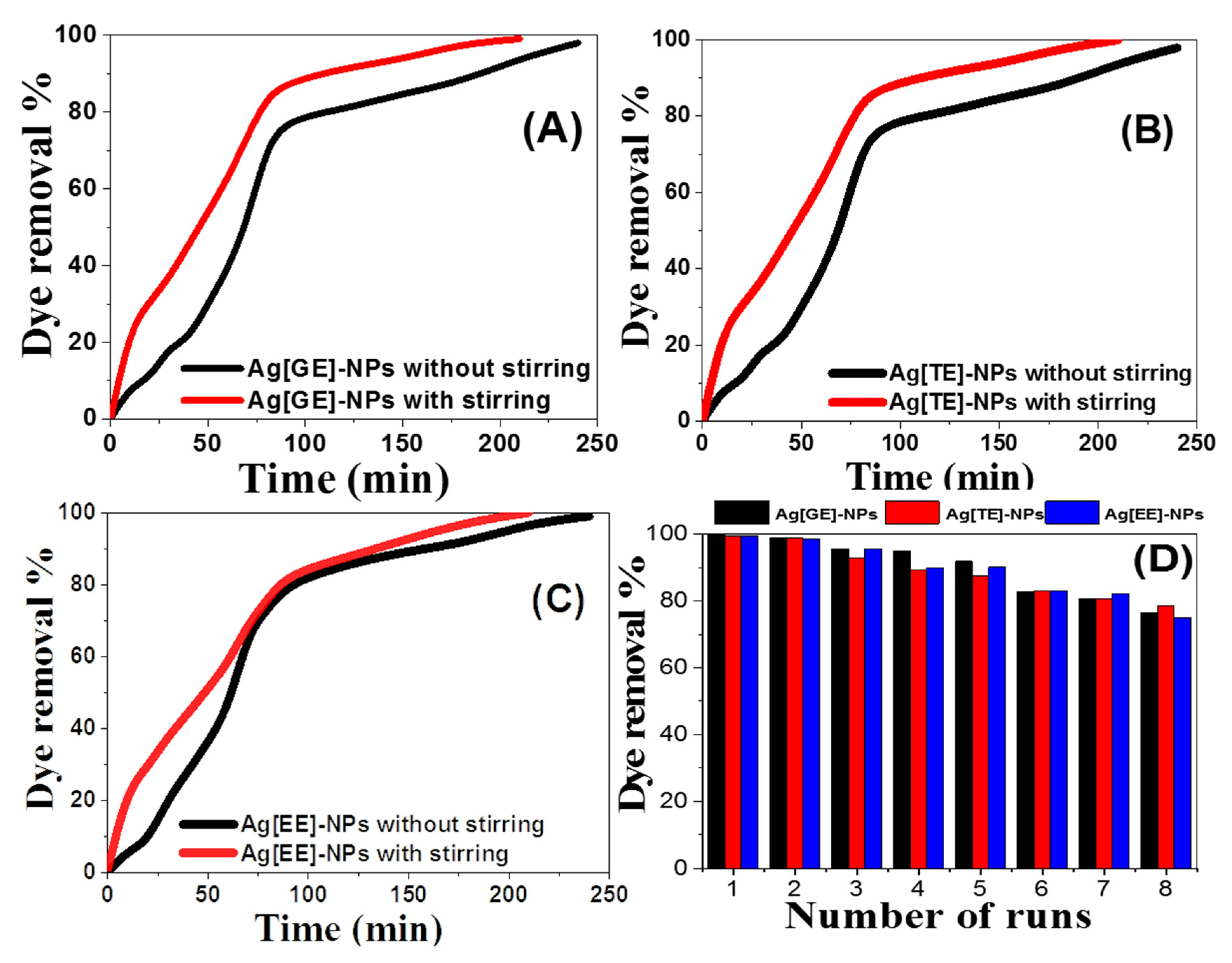
3.2.6. Effect of Stirring
3.2.7. Reusability of Ag-NPs for Methylene Blue Dye Degradation
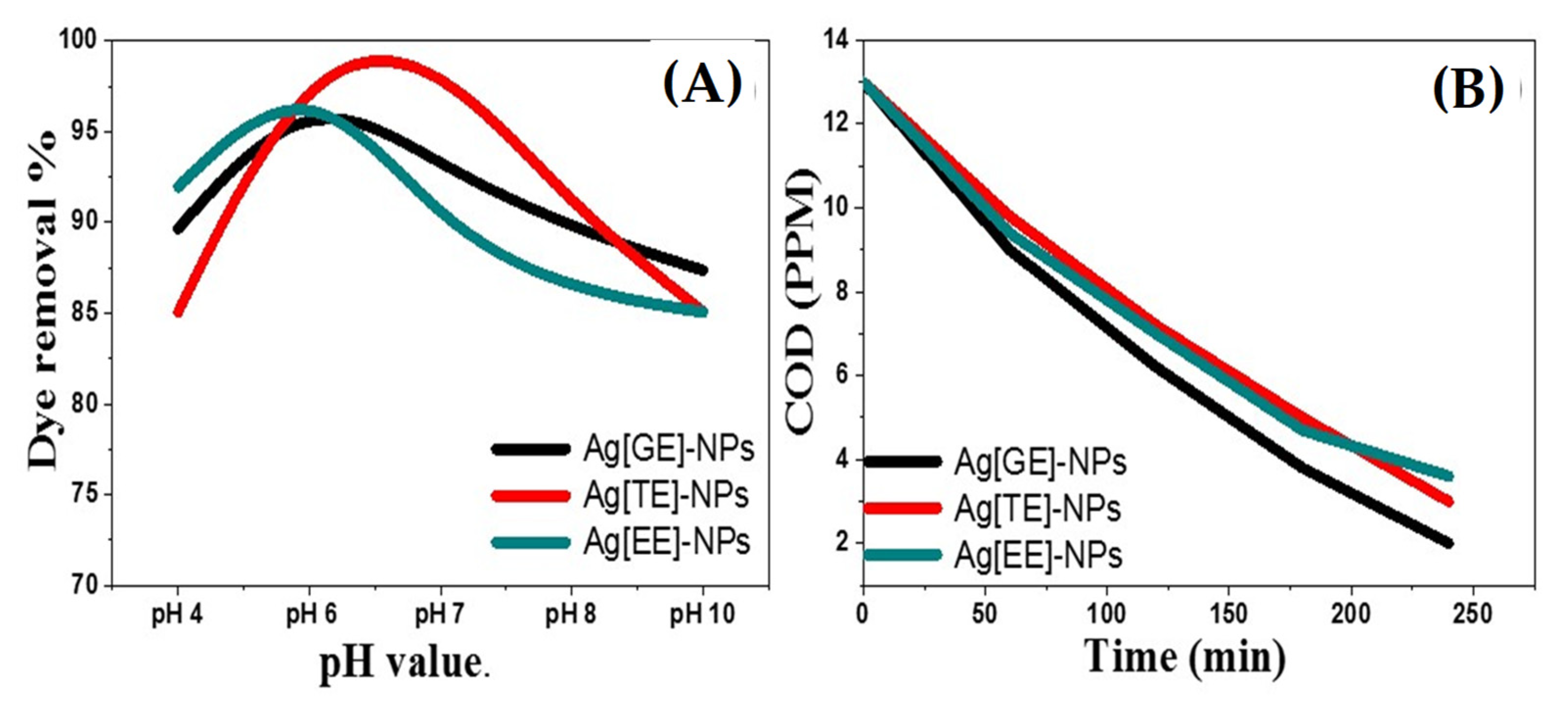
3.2.8. The Effect of pH Value on Photocatalytic Activities of Biosynthesized Ag-NPs
3.2.9. Chemical Oxygen Demand (COD) of Biodegraded Methylene Blue Dye by Ag-NPs
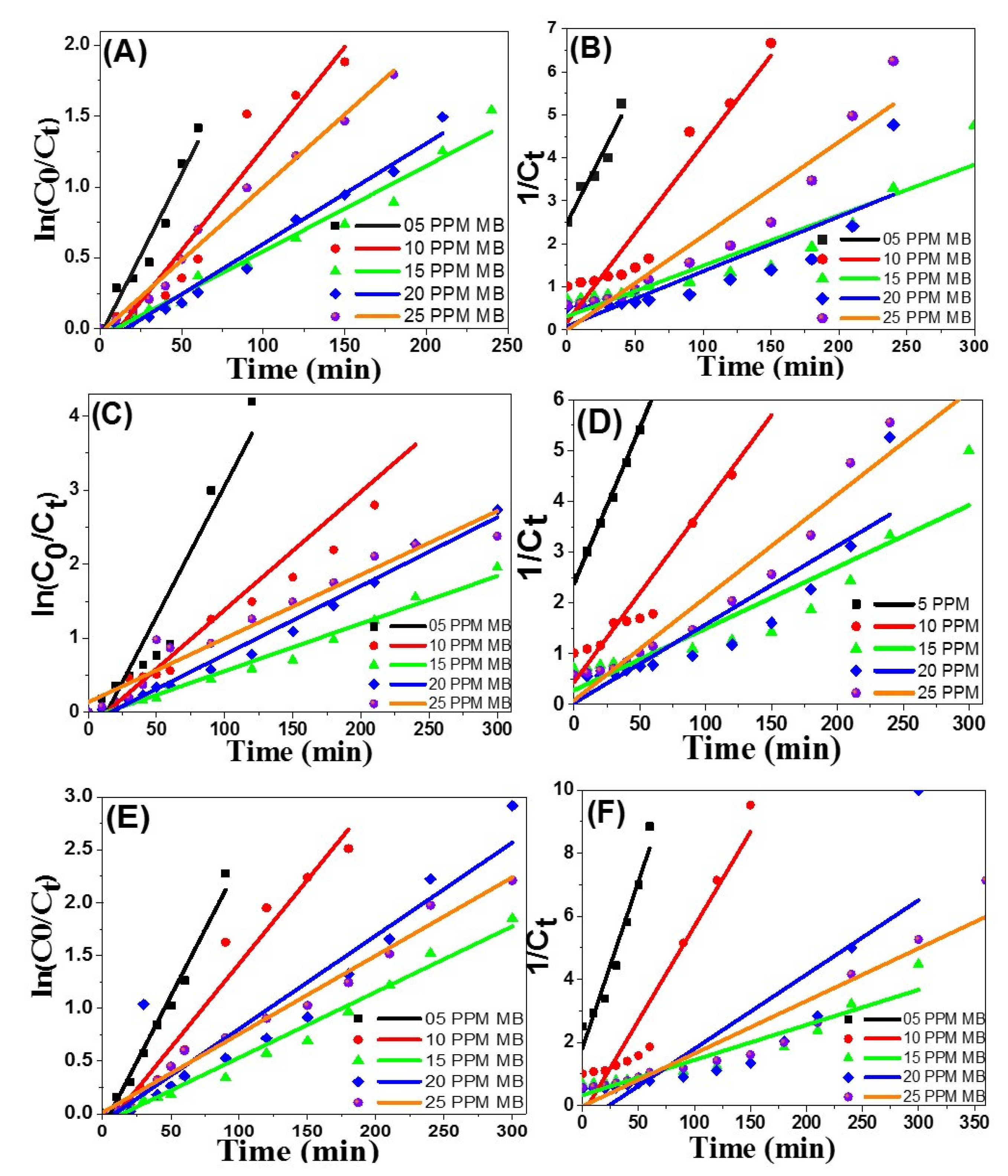
3.2.10. The Kinetic Reactions
3.2.11. Degradation Mechanism and Effect of Scavengers on the Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosa, L. Global Water Challenges of Food and Energy Systems in the 21st Century. Doctoral Dissertation, University of California, Berkeley, CA, USA, 2020. [Google Scholar]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of industries in water scarcity and its adverse effects on environment and human health. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 235–256. [Google Scholar]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of color from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Talarposhti, A.M.; Donnelly, T.; Anderson, G.K. Color removal from a simulated dye wastewater using a two-phase anaerobic packed bed reactor. Water Res. 2001, 35, 425–432. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mangwani, N.; Rao, T.S. Bioremediation approaches for persistent organic pollutants using microbial biofilms. Microb. Biofilms Bioremed. Wastewater Treat. 2019, 179, 179–206. [Google Scholar]
- Singh, A.; Mittal, A.; Jangid, N.K. Toxicology of dyes. In Impact of Textile Dyes on Public Health and the Environment; IGI Global: Bijbehara, India, 2020; pp. 50–69. [Google Scholar]
- Prado, A.G.S.; Costa, L.L. Photocatalytic decoloration of malachite green dye by application of TiO2 nanotubes. J. Hazard. Mater. 2009, 169, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; Elharfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Maji, S.K.; Adhikary, B. γ-Fe2O3 nanoparticles: An easily recoverable effective photo-catalyst for the degradation of rose bengal and methylene blue dyes in the waste-water treatment plant. Mater. Res. Bull. 2014, 49, 28–34. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2020, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Gonawala, K.H.; Mehta, M.J.; Eng, J. Removal of color from different dye wastewater by using ferric oxide as an adsorbent. Int. J. Eng. Res. Appl. 2014, 4, 102–109. [Google Scholar]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Macedo, T.R.; Airoldi, C. A useful organo-functionalized layered silicate for textile dye removal. J. Hazard. Mater. 2010, 181, 366–374. [Google Scholar] [CrossRef]
- Mani, A.; Hameed, S.A.S. Improved bacterial-fungal consortium as an alternative approach for enhanced decolorization and degradation of azo dyes: A Review. Nat. Environ. Pollut. Technol. 2019, 18, 49–64. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Gupta, S.; Tejavath, K.K. Phytosynthesized nanoparticle-directed catalytic reduction of synthetic dyes: Beast to beauty. Nanotechnol. Environ. Eng. 2021, 6, 1–36. [Google Scholar] [CrossRef]
- Alves de Lima, R.O.; Bazo, A.P.; Salvadori, D.F.; Rech, C.M.; de Palma Oliveira, D.; de Aragão Umbuzeiro, G. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat. Res. 2007, 626, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, H.S.; Cunningham, S.L.; Mermelstein, R.; Cunningham, A.R. The challenge of testing chemicals for potential carcinogenicity using multiple short-term assays: An analysis of a proposed test battery for hair dyes. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 633, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I. Influence of dyes, salts and auxiliary chemicals on the nanofiltration of reactive dye baths: Experimental observations and model verification. Desalination 2003, 154, 79–88. [Google Scholar] [CrossRef]
- Diamond, J.; Profili, J.; Hamdan, A. Characterization of Various Air Plasma Discharge Modes in Contact with Water and Their Effect on the Degradation of Reactive Dyes. Plasma Chem. Plasma Process. 2019, 39, 1483–1498. [Google Scholar] [CrossRef]
- Hamdan, A.; James, D.; Gagnon, C.; Aykul, M.; Profili, J. Characterization of a microwave plasma jet (TI-AGO) in-contact with water: Application in degradation of methylene blue dye. Plasma Process. Polym. 2020, 17, 1900157. [Google Scholar] [CrossRef]
- Liu, J.-L.L.; Park, H.-W.; Hamdan, A.; Cha, M.S. In-Liquid arc plasma jet and its application to phenol degradation. J. Phys. D Appl. Phys. 2018, 51, 114005. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2020, 1224, 129195. [Google Scholar] [CrossRef]
- Prado, A.G.; Miranda, B.S.; Jacintho, G.V. Interaction of indigo carmine dye with silica modified with humic acids at solid/liquid interface. Surf. Sci. 2003, 542, 276–282. [Google Scholar] [CrossRef]
- Mittal, J.; Jain, R.; Sharma, M.M. Phyto-Fabrication of silver nanoparticles using aqueous leaf extract of Xanthium strumerium L. and their bactericidal efficacy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025011. [Google Scholar] [CrossRef] [Green Version]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int. J. Environ. Res. Public Health 2013, 10, 5221–5238. [Google Scholar] [CrossRef] [Green Version]
- Rao, B.; Boominathan, M. Antibacterial activity of silver nanoparticles of seaweeds. Am. J. Adv. Drug Deliv. 2015, 3, 296–307. [Google Scholar]
- Gülbağça, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon 2019, 5, e02980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottoni, C.A.; Neto, M.L.; Leo, P.; Ortolan, B.D.; Barbieri, E.; de Souza, A.O. Environmental impacts of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere 2020, 239, 124698. [Google Scholar] [CrossRef]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-Fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2017, 25, 10392–10406. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mahmoudi-Gom Yek, S.; Motahharifar, N.; Ghafori Goorab, M. Recent development in the plant-mediated green synthesis of Ag-based nanoparticles for environmental and catalytic applications. Chem. Rec. 2019, 19, 2436–2479. [Google Scholar] [CrossRef]
- Yu, C.; Tang, J.; Liu, X.; Ren, X.; Zhen, M.; Wang, L. Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis. Materials 2019, 12, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of Iron and Silver Nanoparticles at Room Temperature Using Aqueous Sorghum Bran Extracts. Langmuir 2010, 27, 264–271. [Google Scholar] [CrossRef]
- Shaban, M.; El Sayed, A.M. Influence of the spin deposition parameters and La/Sn double doping on the structural, optical, and photoelectrocatalytic properties of CoCo2O4 photoelectrodes. Sol. Energy Mater. Sol. Cells 2020, 217, 110705. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Arunachalam, R.; Dhanasingh, S.; Kalimuthu, B.; Uthirappan, M.; Rose, C.; Mandal, A.B. Phytosynthesis of silver nanoparticles using Coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surf. B Biointerfaces 2012, 94, 226–230. [Google Scholar] [CrossRef]
- Bulut, E.; Özacar, M. Rapid, Facile Synthesis of Silver Nanostructure Using Hydrolyzable Tannin. Ind. Eng. Chem. Res. 2009, 48, 5686–5690. [Google Scholar] [CrossRef]
- Abideen, S.; Sankar, M. In-Vitro screening of antidiabetic and antimicrobial activity against green synthesized AgNO3 using seaweeds. J. Nanomed. Nanotechnol. 2015, 10, 2157–7439. [Google Scholar]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2015, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Priya, K.; Nancy, F.T.; Noorlidah, A.; Ahmed, A.B.A. Biosynthesis, characterization and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind. Crop. Prod. 2013, 41, 235–240. [Google Scholar] [CrossRef]
- Chiavola, A. Textiles. Water Environ. Res. 2009, 81, 1696–1730. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient catalytic degradation of dyes using K-carrageenan stabilized Silver nanoparticles nanocatalyst. Carbohydr. Polym. 2020, 230, 115597. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Hamd, A. Recycling of glass in synthesis of MCM-48 mesoporous silica as catalyst support for Ni2O3 photocatalyst for Congo red dye removal. Clean Technol. Environ. Policy 2017, 20, 13–28. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, Y.F.; Chang, P.S.; Chen, C.Y. Comparative study of oxidation of dye-reactive black B by different advanced oxidation processes: Fenton, electro-Fenton and photo-Fenton. J. Hazard. Mater. 2008, 154, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Javid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmy, A.; Rabia, M.; Shaban, M.; Ashraf, A.M.; Ahmed, S.; Ahmed, A.M. Graphite/rolled graphene oxide/carbon nanotube photoelectrode for water splitting of exhaust car solution. Int. J. Energy Res. 2020, 44, 7687–7697. [Google Scholar] [CrossRef]
- Rabia, M.; Abolaila SH Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiO × NY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2019, 27, 133–139. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Jannesar, R.; Goudarzi, A. Design and construction of nanoscale material for ultrasonic assisted adsorption of dyes: Application of derivative spectrophotometry and experimental design methodology. Ultrason. Sonochem. 2017, 35, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Behnajady, M.A.; Eskandarloo, H. Preparation of TiO2 nanoparticles by the sol–gel method under different pH conditions and modeling of photocatalytic activity by artificial neural network. Res. Chem. Intermed. 2013, 41, 2001–2017. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Baek, S.R.; Kim, J.I.; Choi, J.W.; Hur, J.; Lee, T.U.; Lee, B.J. Characteristics and biodegradability of wastewater organic matter in municipal wastewater treatment plants collecting domestic wastewater and industrial dis-charge. Water 2017, 9, 409. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.P.; Li, C.J.; Sun, J.H.; Shi, S.H.; Fan, M.H.; Zhou, Q. Decolorization of an azo dye Orange G in aqueous so-lution by Fenton oxidation process: Effect of system parameters and kinetic study. J. Hazard. Mater. 2009, 161, 1052–1057. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. Am. Chem. Soc. Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Mahmoud, M.; Poncheri, A.; Badr, Y.; Abd El Wahed, M.A. Photocatalytic degradation of methyl red dye. S. Afr. J. Sci. 2010, 105, 299–303. [Google Scholar] [CrossRef] [Green Version]






| Samples | Peaks | FWHM (Radian) | Ds (nm) | Average Ds (nm) | DDm (10−3 nm−2) | |
|---|---|---|---|---|---|---|
| 2θ (Degree) | Planes | |||||
| Ag[GE]-NPs | 38.472 | (111) Ag | 0.451 | 18.65 | 21.33 | 2.88 |
| 44.558 | (200) Ag | 0.5418 | 15.84 | 3.99 | ||
| 64.476 | (220) Ag | 0.289 | 32.48 | 0.95 | ||
| 76.985 | (311) Ag | 0.5464 | 18.57 | 2.9 | ||
| 55.046 | (020) CuO | 0.2130 | 42.04 | 35.95 | 0.57 | |
| 67.91 | (022) CuO | 0.32 | 29.92 | 1.12 | ||
| Ag[TE]-NPs | 38.111 | (111) Ag | 0.4506 | 18.47 | 22.33 | 2.93 |
| 44.148 | (200) Ag | 0.541 | 15.84 | 3.99 | ||
| 64.323 | (220) Ag | 0.2874 | 32.64 | 0.94 | ||
| 76.817 | (311) Ag | 0.4534 | 22.35 | 2 | ||
| 54.241 | (020) CuO | 0.379 | 23.54 | 26.48 | 1.8 | |
| 67.531 | (022) CuO | 0.468 | 20.41 | 2.4 | ||
| Ag[EE]-NPs | 38.095 | (111) Ag | 0.315 | 26.67 | 25.61 | 1.41 |
| 44.059 | (200) Ag | 0.3337 | 25.67 | 1.52 | ||
| 64.222 | (220) Ag | 0.2867 | 32.7 | 0.94 | ||
| 76.846 | (311) Ag | 0.5482 | 17.41 | 3.3 | ||
| 54.939 | (020) CuO | 0.3653 | 24.5 | 24.54 | 1.67 | |
| 67.566 | (022) CuO | 0.3886 | 24.58 | 1.66 | ||
| Material | Ag[GE]-NPs | Ag[TE]-NPs | Ag[EE]-NPs |
|---|---|---|---|
| The activation energy kJ/mol | 21.10 ± 0.39 | 23.42 ± 1.28 | 17.96 ± 1.11 |
| Enthalpy(∆H) kJ·mol−1 | −23.82 ± 0.412 | −26.14 ± 1.27 | −20.68 ± 1.11 |
| Entropy (∆S) J·mol−1·K−1 | 224.36 ± 1.27 | 230.54 ± 3.91 | 213.78 ± 3.42 |
| Material | Kinetic Models | 5 mg/L | 10 mg/L | 15 mg/L | 20 mg/L | 25 mg/L | |
|---|---|---|---|---|---|---|---|
| Ag[GE] | First-order kinetic mode | R2 | 0.9524 | 0.9325 | 0.97563 | 0.98041 | 0.98964 |
| K1 (min−1) | 0.0228 | 0.01435 | 0.00603 | 0.00709 | 0.01028 | ||
| K1· R2 | 0.02171 | 0.01338 | 0.00588 | 0.00695 | 0.01017 | ||
| Second-order kinetic mode | R2 | 0.93117 | 0.91202 | 0.88416 | 0.73867 | 0.92093 | |
| K2 (L/mol·min) | 0.0619 | 0.04124 | 0.01177 | 0.0127 | 0.0218 | ||
| K2· R2 | 0.05767 | 0.03762 | 0.01041 | 0.0094 | 0.02014 | ||
| Ag[TE] | First-order kinetic mode | R2 | 0.90855 | 0.92507 | 0.98099 | 0.98415 | 0.94586 |
| K1 (min−1) | 0.0353 | 0.01593 | 0.00642 | 0.00927 | 0.0086 | ||
| K1· R2 | 0.03207 | 0.01474 | 0.0063 | 0.00912 | 0.00813 | ||
| Second-order kinetic mode | R2 | 0.99272 | 0.94107 | 0.86014 | 0.80818 | 0.96284 | |
| K2 (L/mol·min) | 0.06153 | 0.03506 | 0.01214 | 0.01547 | 0.02028 | ||
| K1· R2 | 0.06108 | 0.03299 | 0.01044 | 0.0125 | 0.01953 | ||
| Ag[EE] | First-order kinetic mode | R2 | 0.98167 | 0.96358 | 0.98073 | 0.8855 | 0.98633 |
| K1 (min−1) | 0.02503 | 0.01589 | 0.00622 | 0.00882 | 0.00742 | ||
| K1· R2 | 0.02457 | 0.01531 | 0.0061 | 0.00781 | 0.00732 | ||
| Second-order kinetic mode | R2 | 0.95132 | 0.91761 | 0.88492 | 0.72758 | 0.90685 | |
| K2 (L/mol·min) | 0.10569 | 0.05981 | 0.01112 | 0.02354 | 0.01667 | ||
| K2. R2 | 0.10055 | 0.05488 | 0.01713 | 0.00984 | 0.01512 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Azeem, M.N.; Hassaballa, S.; Ahmed, O.M.; Elsayed, K.N.M.; Shaban, M. Photocatalytic Activity of Revolutionary Galaxaura elongata, Turbinaria ornata, and Enteromorpha flexuosa’s Bio-Capped Silver Nanoparticles for Industrial Wastewater Treatment. Nanomaterials 2021, 11, 3241. https://doi.org/10.3390/nano11123241
Abdel Azeem MN, Hassaballa S, Ahmed OM, Elsayed KNM, Shaban M. Photocatalytic Activity of Revolutionary Galaxaura elongata, Turbinaria ornata, and Enteromorpha flexuosa’s Bio-Capped Silver Nanoparticles for Industrial Wastewater Treatment. Nanomaterials. 2021; 11(12):3241. https://doi.org/10.3390/nano11123241
Chicago/Turabian StyleAbdel Azeem, Manal N., Safwat Hassaballa, Osama M. Ahmed, Khaled N. M. Elsayed, and Mohamed Shaban. 2021. "Photocatalytic Activity of Revolutionary Galaxaura elongata, Turbinaria ornata, and Enteromorpha flexuosa’s Bio-Capped Silver Nanoparticles for Industrial Wastewater Treatment" Nanomaterials 11, no. 12: 3241. https://doi.org/10.3390/nano11123241








