Nanostructure Engineering via Intramolecular Construction of Carbon Nitride as Efficient Photocatalyst for CO2 Reduction
Abstract
:1. Introduction
2. Experimental
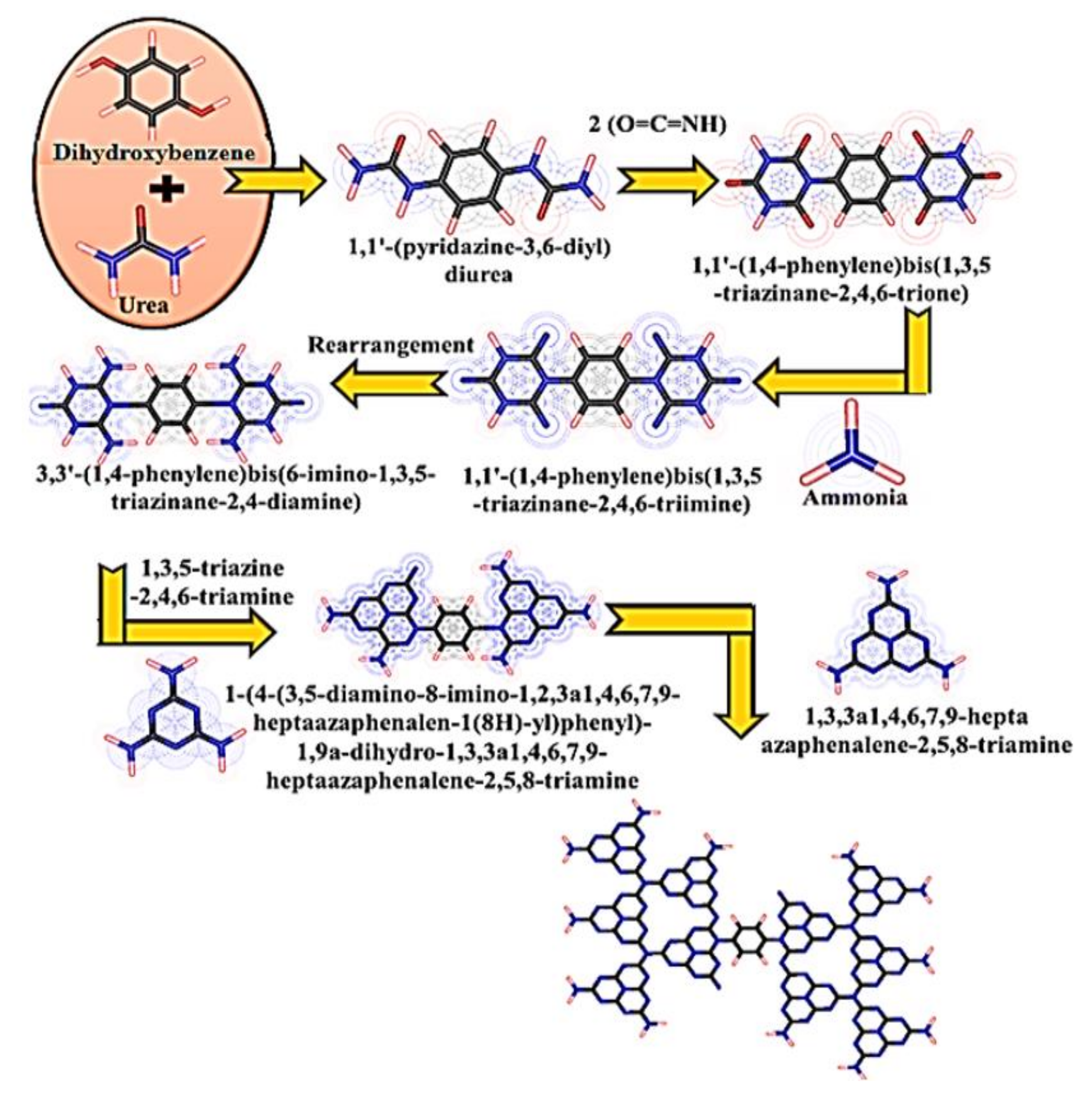
Synthesis of CN and Copolymerized CN Photocatalysts
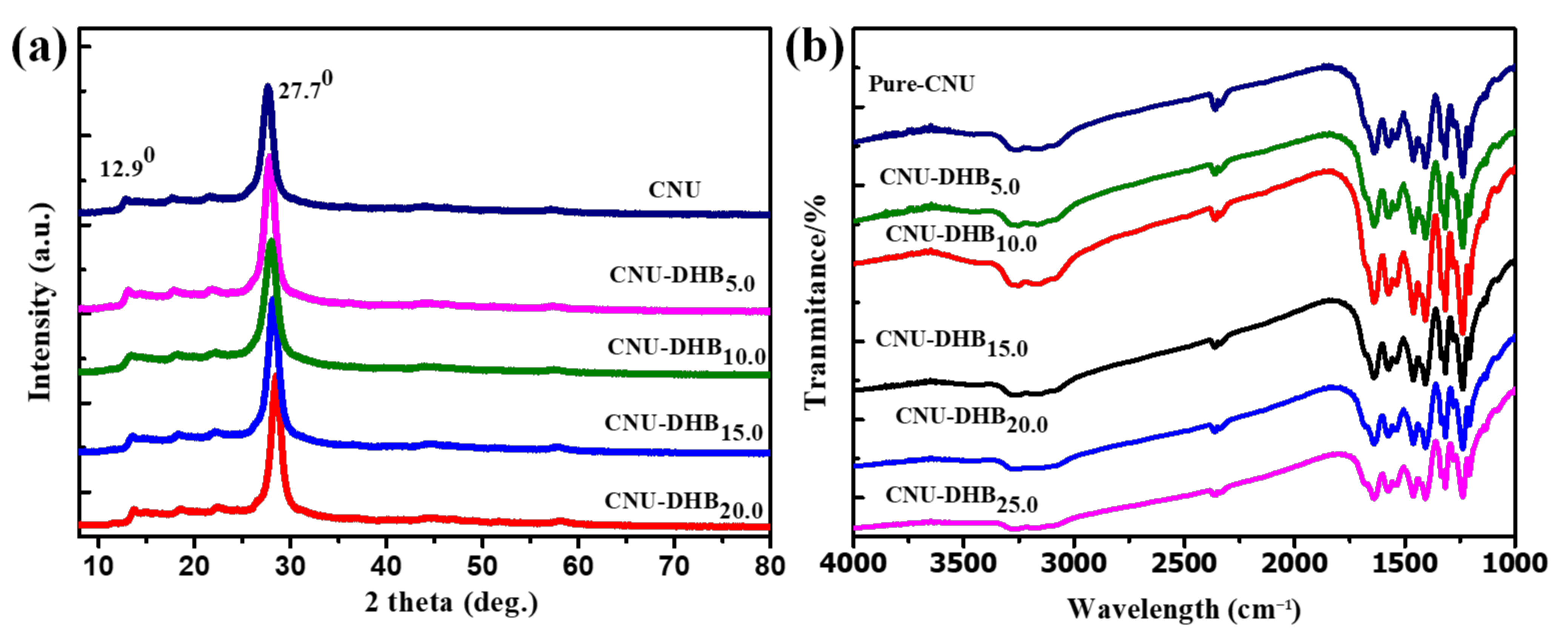
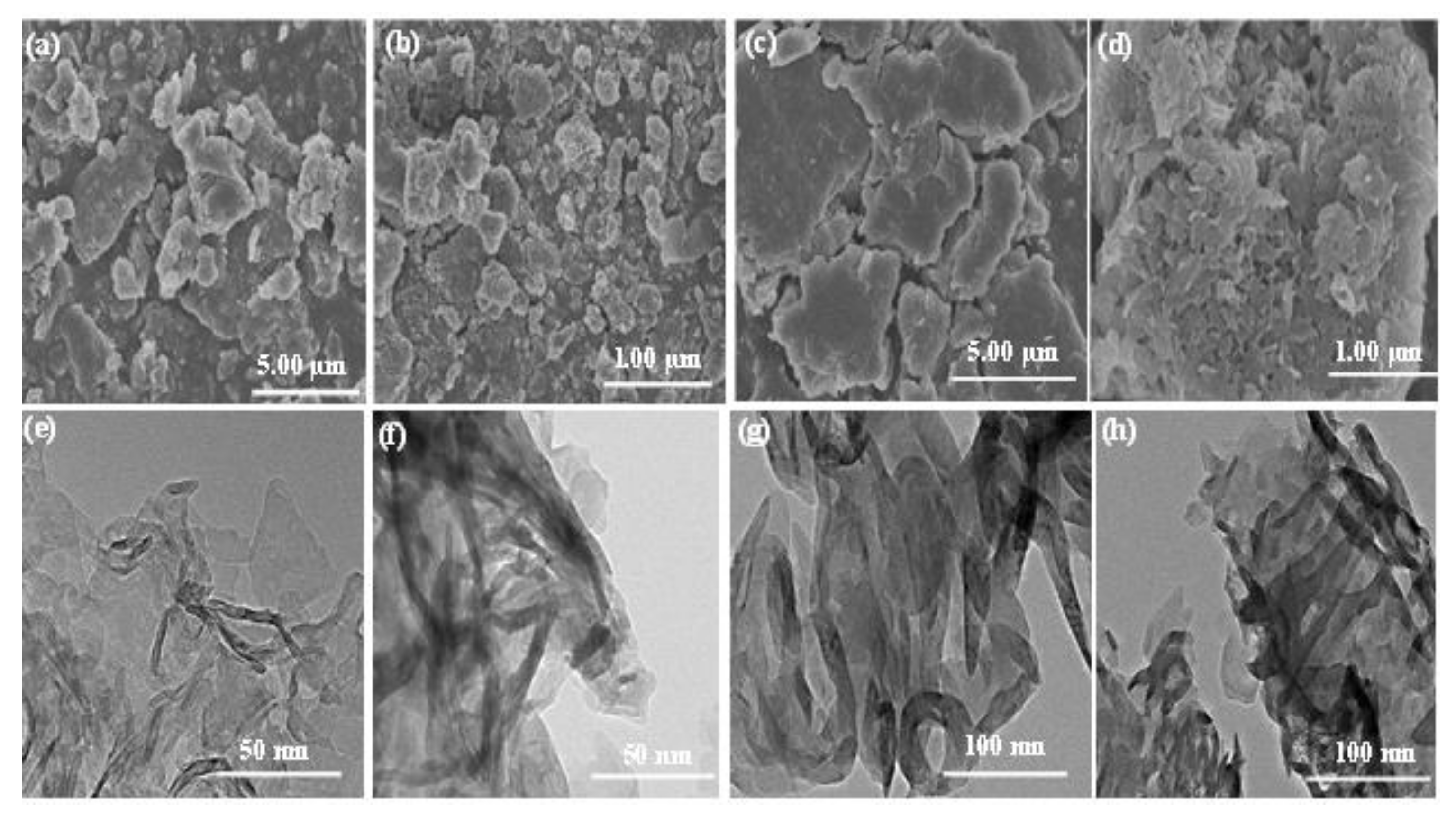
3. Result and Discussion
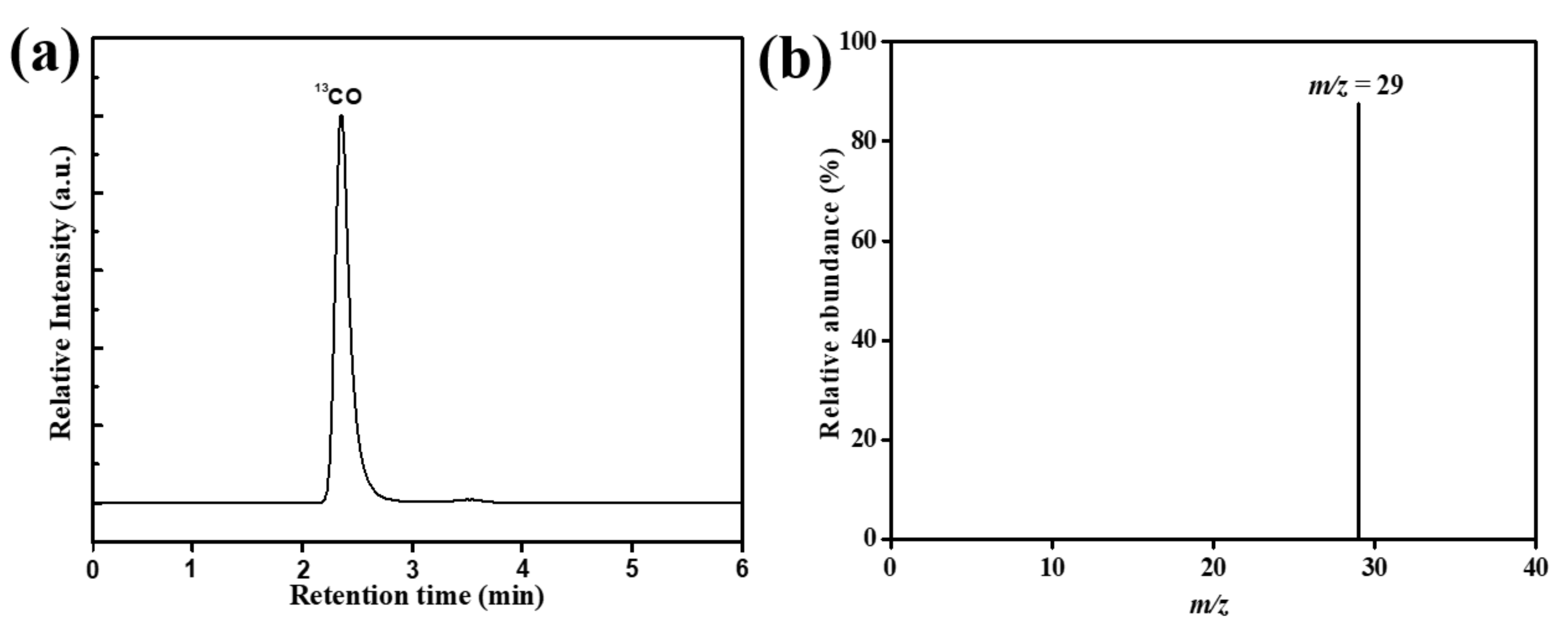
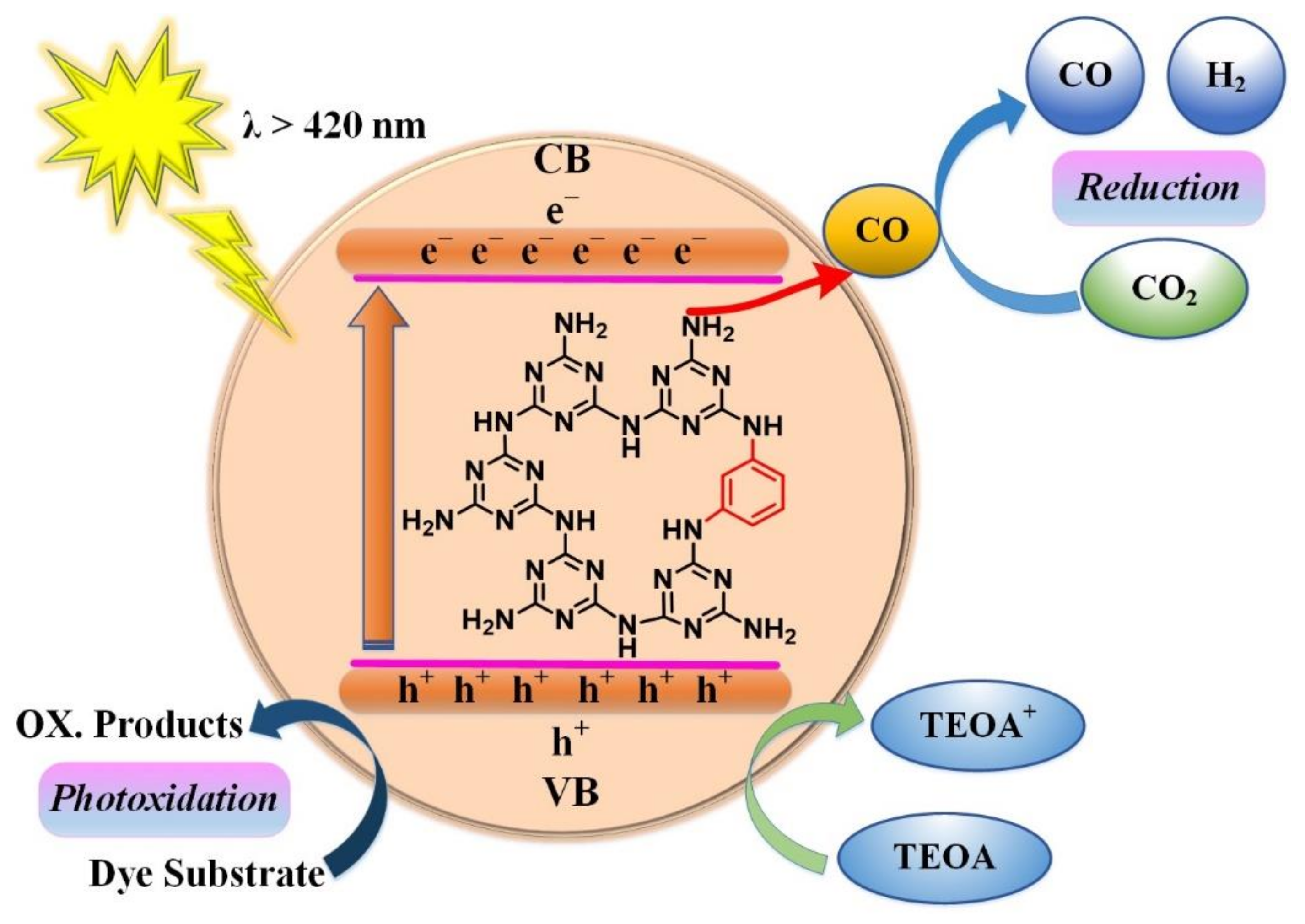
4. Photocatalytic Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Listorti, A.; Durrant, J.; Barber, J. Artificial photosynthesis: Solar to fuel. Nat. Mater. 2009, 8, 929–930. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes; Springer: New York, NY, USA, 2008. [Google Scholar]
- Hamid, A.; Khan, M.; Hayat, A.; Raza, J.; Hussain, F. robing the physio-chemical appraisal of green synthesized PbO nanoparticles in PbO-PVC nanocomposite polymer membranes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118303. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Perspectives and State of the Art in Producing Solar Fuels and Chemicals from CO2. Green Carbon Dioxide: Advances in CO2 Utilization; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 1–24. [Google Scholar]
- Hayat, A.; Khan, J.K.; Rahman, M.U.; Mane, S.B.; Khan, W.U.; Sohail, M.; Rahman, N.U.; Shaishta, N.; Chi, Z.; Wu, M. Synthesis and Optimization of the Trimesic Acid Modified Polymeric Carbon Nitride for Enhanced Photocatalytic Reduction of CO2. J. Colloid Interface Sci. 2019, 548, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Morikawa, T.; Saeki, S.; Kajino, T.; Motohiro, T. Visible-light-induced selective CO2 reduction utilizing a ruthenium complex electrocatalyst linked to a p-type nitrogen-doped Ta2O5 semiconductor. Angew. Chem. 2010, 122, 5227–5231. [Google Scholar] [CrossRef]
- Kuriki, R.; Matsunaga, H.; Nakashima, T.; Wada, K.; Yamakata, A.; Ishitani, O.; Maeda, K. Nature-Inspired, Highly Durable CO2 Reduction System Consisting of a Binuclear Ruthenium(II) Complex and an Organic Semiconductor Using Visible Light. J. Am. Chem. Soc. 2016, 138, 5159–5170. [Google Scholar] [CrossRef]
- Ullah, I.; Taha, T.A.; Alenad, A.M.; Uddin, I.; Hayat, A.; Hayat, A.; Sohail, M.; Irfan, A.; Khan, J.; Palamanit, A. Platinum-Alumina Modified SO42−-ZrO2/Al2O3 Based Bifunctional Catalyst for Significantly Improved n -butane Isomerization Performance. Surf. Interfaces 2021, 25, 101227. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2014, 5, 1360–1384. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Liu, Y.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. An anion exchange approach to Bi2WO6 hollow microspheres with efficient visible light photocatalytic reduction of CO2 to methanol. Chem. Commun. 2012, 48, 9729–9731. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Kou, J.; Chen, X.; Tian, Z.; Gao, J.; Yan, S.; Zou, Z. High-Yield Synthesis of Ultralong and Ultrathin Zn2GeO4 Nanoribbons toward Improved Photocatalytic Reduction of CO2 into Renewable Hydrocarbon Fuel. J. Am. Chem. Soc. 2010, 132, 14385–14387. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J. Effect of F-Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Nanostruct. Photocatal. 2016, 187–200. [Google Scholar] [CrossRef]
- Ah, A.; Ns, B.; Skbm, C.; Ah, D.; Jk, E.; Aur, F.; Tl, G. Molecular engineering of polymeric carbon nitride based Donor-Acceptor conjugated copolymers for enhanced photocatalytic full water splitting. J. Colloid Interface Sci. 2020, 560, 743–754. [Google Scholar]
- Lopez-Tenllado, F.J.; Murcia-López, S.; Gómez, D.M.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navío, J.A.; Hidalgo, M.C.; Gatica, J.M. A comparative study of Bi2WO6, CeO2, and TiO2 as catalysts for selective photo-oxidation of alcohols to carbonyl compounds. Appl. Catal. Gen. Int. J. Devoted Catal. Sci. Its Appl. 2015, 505, 375–381. [Google Scholar]
- Pare, B.; Singh, P.; Jonnalgadda, S. Degradation and mineralization of victoria blue B dye in a slurry photoreactor using advanced oxidation process. J. Sci. Ind. Res. 2009, 68, 724–729. [Google Scholar]
- Khan, M.; Hayat, A.; Mane, S.; Li, T.; Khan, W.U. Functionalized nano diamond composites for photocatalytic hydrogen evolution and effective pollutant degradation. Int. J. Hydrog. Energy 2020, 45, 29070–29081. [Google Scholar] [CrossRef]
- Sohail, M.; Xue, H.; Jiao, Q.; Li, H.; Khan, K.; Wang, S.; Zhao, Y. Synthesis of well-dispersed TiO2@reduced graphene oxide (rGO) nanocomposites and their photocatalytic properties. Mater. Res. Bull. 2017, 90, 125–130. [Google Scholar] [CrossRef]
- Hayat, A.; Rahman, M.U.; Khan, I.; Khan, J.; Sohail, M.; Yasmeen, H.; Liu, S.Y.; Qi, K.; Lv, W. Conjugated Electron Donor–Acceptor Hybrid Polymeric Carbon Nitride as a Photocatalyst for CO2 Reduction. Molecules 2019, 24, 1779. [Google Scholar] [CrossRef] [Green Version]
- Hayat, A.; Chen, Z.; Luo, Z.; Fang, Y.; Wang, X. π-deficient pyridine ring-incorporated carbon nitride polymers for photocatalytic H2 evolution and CO2 fixation. Res. Chem. Intermed. 2021, 47, 15–27. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Thakur, V.K.; Saini, V.; Khan, A.A.P.; Singh, N.; Singh, P. An overview on polymeric carbon nitride assisted photocatalytic CO2 reduction: Strategically manoeuvring solar to fuel conversion efficiency. Chem. Eng. Sci. 2021, 230, 116219. [Google Scholar] [CrossRef]
- Hasija, V.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P. Carbon quantum dots supported AgI/ZnO/phosphorus doped graphitic carbon nitride as Z-scheme photocatalyst for efficient photodegradation of 2,4-dinitrophenol. J. Environ. Chem. Eng. 2019, 7, 103272. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Thakur, S.; Saini, R.V.; Saini, A.K.; Singh, P.; Thakur, V.K.; Nguyen, V.-H.; Khan, A.A.P.; Asiri, A.M. Synergistic photocatalytic mitigation of imidacloprid pesticide and antibacterial activity using carbon nanotube decorated phosphorus doped graphitic carbon nitride photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 113, 142–154. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. A Chem. 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Gómez-Avilés, A.; Peñas-Garzón, M.; Rodriguez, J.J. Semiconductor Photocatalysis for Water Purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 581–651. [Google Scholar]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ran, J.; Qiao, S.-Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Hayat, A.; Taha, T.A.; Alenad, A.M.; Ullah, I.; Shah, S.; Uddin, I.; Ullah, I.; Hayat, A.; Khan, W.U. A simplistic molecular agglomeration of carbon nitride for optimized photocatalytic performance. Surf. Interfaces 2021, 25, 101166. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Taha, T.A.; Alenad, A.M.; Uddin, I.; Hayat, A.; Ali, T.; Shah, R.; Irfan, A.; Khan, W.U.; et al. A Superficial Intramolecular Alignment of Carbon Nitride through Conjugated Monomer for Optimized Photocatalytic CO2 Reduction. Catalysts 2021, 11, 935. [Google Scholar] [CrossRef]
- Raziq, F.; Hayat, A.; Humayun, M.; Mane, S.; Liang, Q. Photocatalytic solar fuel production and environmental remediation through experimental and DFT based research on CdSe-QDs-coupled P-doped-g-C3N4 composites. Appl. Catal. B Environ. 2020, 270, 118867. [Google Scholar] [CrossRef]
- Hayat, A.; Alrowaili, Z.A.; Taha, T.A.; Khan, J.; Khan, W.U. Organic heterostructure modified carbon nitride as apprehension for Quercetin Biosensor. Synth. Met. 2021. [Google Scholar] [CrossRef]
- Hayat, A.; Raziq, F.; Khan, M.; Khan, J.; Khan, W.U. Fusion of conjugated bicyclic co-polymer within polymeric carbon nitride for high photocatalytic performance. J. Colloid Interface Sci. 2019, 554, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Raziq, F.; Khan, M.; Ullah, I.; Khan, W.U. Visible-light enhanced photocatalytic performance of Polypyrrole/g-C3N4 composites for water splitting to evolve H2 and pollutants degradation. J. Photochem. Photobiol. A Chem. 2019, 379, 88–98. [Google Scholar] [CrossRef]
- Huang, C.; Wen, Y.; Ma, J.; Dong, D.; Zhang, Y. Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 2021, 12, 320. [Google Scholar] [CrossRef]
- Hayat, A.; Taha, T.A.; Alenad, A.M.; Ali, T.; Bashir, T.; Rehman, A.U.; Ullah, I.; Hayat, A.; Irfan, A.; Khan, W.U. A molecular amalgamation of carbon nitride polymer as emphasized photocatalytic performance. Int. J. Energy Res. 2021, 45, 19921–19928. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, J.; Sohail, M.; Hayat, A.; Khan, W.U. Fabrication of Polymer Carbon Nitride with Organic Monomer for Effective Photocatalytic Hydrogen Evolution. J. Photochem. Photobiol. A Chem. 2020, 401, 112764. [Google Scholar] [CrossRef]
- Khan, M.; Li, T.; Hayat, A.; Zada, A. A concise review on the elastomeric behavior of electroactive polymer materials. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Ah, A.; Ns, B.; Iu, C.; Mk, D.; Skbmb, E.; Ah, F.; Iu, G.; Aur, H.; Ta, I.; Gm, J. Molecular engineering of carbon nitride towards photocatalytic H2 evolution and dye degradation. J. Colloid Interface Sci. 2021, 597, 39–47. [Google Scholar]
- Xu, J.; Gao, J.; Chao, W.; Yu, Y.; Lei, W. NH2 -MIL-125(Ti)/graphitic carbon nitride heterostructure decorated with NiPd co-catalysts for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 219, 101–108. [Google Scholar] [CrossRef]
- Arif, N.; Uddin, I.; Hayat, A.; Khan, W.U.; Ullah, S.; Hussain, M. Homogeneous Iron-Doped Carbon Nitride-Based Organo-catalysts for sensational photocatalytic performance under Visible Light-Driven. Polym. Int. 2021, 70, 1273–1281. [Google Scholar] [CrossRef]
- Hayat, A.; Shaishta, N.; Mane, S.K.B.; Khan, J.; Hayat, A. Rational Ionothermal Copolymerization of TCNQ with PCN Semiconductor for Enhanced Photocatalytic Full Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 46756–46766. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.U.; Hayat, A. Green synthesis, properties, and catalytic application of zeolite (P) in production of biofuels from bagasse. Int. J. Energy Res. 2019, 43, 4820–4827. [Google Scholar] [CrossRef]
- Hayat, A.; Li, T. A facile supramolecular aggregation of trithiocyanuric acid with PCN for high photocatalytic hydrogen evolution from water splitting. Int. J. Energy Res. 2019, 43, 5479–5492. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2018, 441, 12–22. [Google Scholar]
- Zhu, W.; Ke, J.; Wang, S.B.; Ren, J.; Wang, H.H.; Zhou, Z.Y.; Si, R.; Zhang, Y.W.; Yan, C.H. Shaping Single-Crystalline Trimetallic Pt-Pd-Rh Nanocrystals toward High-Efficiency C–C Splitting of Ethanol in Conversion to CO2. ACS Catal. 2015, 5, 1995–2008. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. ChemInform Abstract: Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. ChemInform 2012, 43. [Google Scholar] [CrossRef]
- Lin, J.; Pan, Z.; Wang, X. Photochemical Reduction of CO2 by Graphitic Carbon Nitride Polymers. ACS Sustain. Chem. Eng. 2014, 2, 353–358. [Google Scholar] [CrossRef]
- Di, W.; Yang, J.; Zhang, Y.; Li, J.; Yu, Y.; Zhang, Y.; Zhu, Z.; Li, W.; Wu, C.; Luo, L. Device structure-dependent field-effect and photoresponse performances of p-type ZnTe:Sb nanoribbon. J. Mater. Chem. 2012, 22, 6206–6212. [Google Scholar]
- Kibria, M.G.; Nguyen, H.; Cui, K.; Zhao, S.; Mi, Z. One-Step Overall Water Splitting under Visible Light Using Multiband InGaN/GaN Nanowire Heterostructures. ACS Nano 2013, 7, 7886–7893. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Khan, M.; Zheng, M.; Khan, A.R.; Hayat, A. Thermochemical heat storage behavior of ZnSO4·7H2O under low-temperature. Heat Mass Transf. 2020, 57, 765–775. [Google Scholar] [CrossRef]
- Nickel Oxide Nano-Particles on 3D Nickel Foam Substrate as a Non-Enzymatic Glucose Sensor. J. Electrochem. Soc. 2019, 166, B1602–B1611. [CrossRef]
- Rehman, A.U.; Shah, M.Z.; Zhao, T.; Shah, R.; Zheng, M. Thermochemical heat storage ability of ZnSO4 7H2O as potential long-term heat storage material. Int. J. Energy Res. 2020, 45, 4746–4754. [Google Scholar] [CrossRef]
- Shaishta, N.; Khan, W.U.; Mane, S.; Hayat, A.; Manjunatha, G. Red-emitting CaSc2O4 : Eu3+ phosphor for NUV-based warm white LEDs: Structural elucidation and Hirshfeld surface analysis. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- Uddin, I.; Wang, G.; Gao, D.; Hussain, Z.; Hayat, A. Conventional and cement-catalyzed co-pyrolysis of rice straw and waste polyethylene into liquid and gaseous fuels by using a fixed bed reactor. Biomass-Convers. Biorefinery 2021, 1–10. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, Y.; Yang, X. High efficiency photocatalytic CO2 reduction realized by Ca2+ and HDMP group Co-modified graphitic carbon nitride. Int. J. Hydrog. Energy 2021, 46, 32893–32903. [Google Scholar] [CrossRef]
- Fang, Y.; Fu, X.; Wang, X. Diverse polymeric carbon nitride-based semiconductors for photocatalysis and variations. ACS Mater. Lett. 2020, 2, 975–980. [Google Scholar] [CrossRef]
- Sohail, M.; Xue, H.; Jiao, Q.; Li, H.; Khan, K.; Wang, S.; Feng, C.; Zhao, Y. Synthesis of well-dispersed TiO2/CNTs@CoFe2O4 nanocomposites and their photocatalytic properties. Mater. Res. Bull. 2018, 101, 83–89. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, M.; Altalhi, T.; Al-Sehemi, A.G.; Taha, T.A.M.; S. El-Nasser, K.; Al-Ghamdi, A.A.; Boukhari, M.; Palamanit, A.; Hayat, A.; A. Amin, M.; et al. Nanostructure Engineering via Intramolecular Construction of Carbon Nitride as Efficient Photocatalyst for CO2 Reduction. Nanomaterials 2021, 11, 3245. https://doi.org/10.3390/nano11123245
Sohail M, Altalhi T, Al-Sehemi AG, Taha TAM, S. El-Nasser K, Al-Ghamdi AA, Boukhari M, Palamanit A, Hayat A, A. Amin M, et al. Nanostructure Engineering via Intramolecular Construction of Carbon Nitride as Efficient Photocatalyst for CO2 Reduction. Nanomaterials. 2021; 11(12):3245. https://doi.org/10.3390/nano11123245
Chicago/Turabian StyleSohail, Muhammad, Tariq Altalhi, Abdullah G. Al-Sehemi, Taha Abdel Mohaymen Taha, Karam S. El-Nasser, Ahmed A. Al-Ghamdi, Mahnoor Boukhari, Arkom Palamanit, Asif Hayat, Mohammed A. Amin, and et al. 2021. "Nanostructure Engineering via Intramolecular Construction of Carbon Nitride as Efficient Photocatalyst for CO2 Reduction" Nanomaterials 11, no. 12: 3245. https://doi.org/10.3390/nano11123245
APA StyleSohail, M., Altalhi, T., Al-Sehemi, A. G., Taha, T. A. M., S. El-Nasser, K., Al-Ghamdi, A. A., Boukhari, M., Palamanit, A., Hayat, A., A. Amin, M., & Nawawi Bin Wan Ismail, W. I. (2021). Nanostructure Engineering via Intramolecular Construction of Carbon Nitride as Efficient Photocatalyst for CO2 Reduction. Nanomaterials, 11(12), 3245. https://doi.org/10.3390/nano11123245






