Synthesis and Luminescent Properties of Carbon Nanodots Dispersed in Nanostructured Silicas
Abstract
:1. Introduction
2. Por-SiO2:C Thin Layers and a-SiOxC:H Thin Films
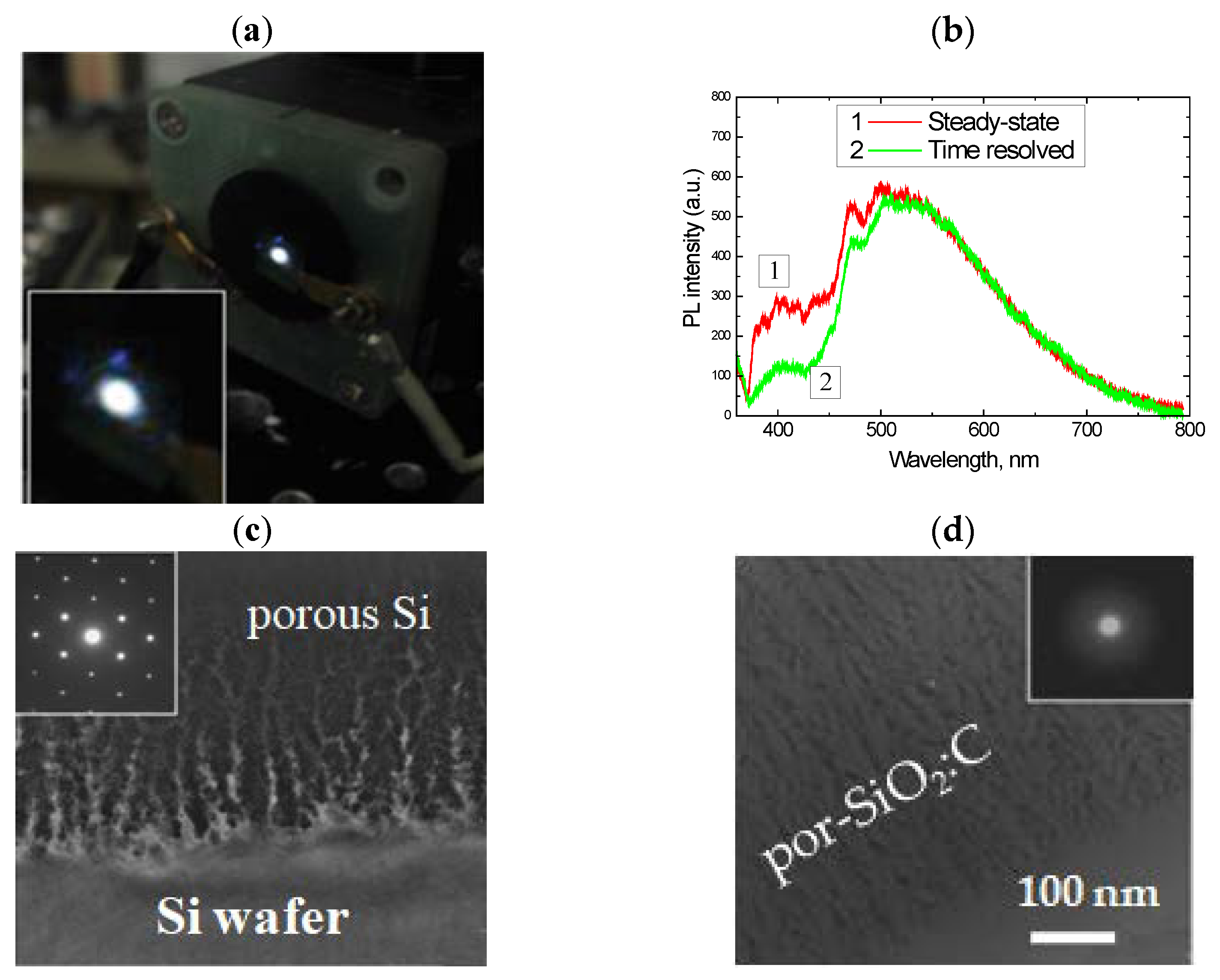
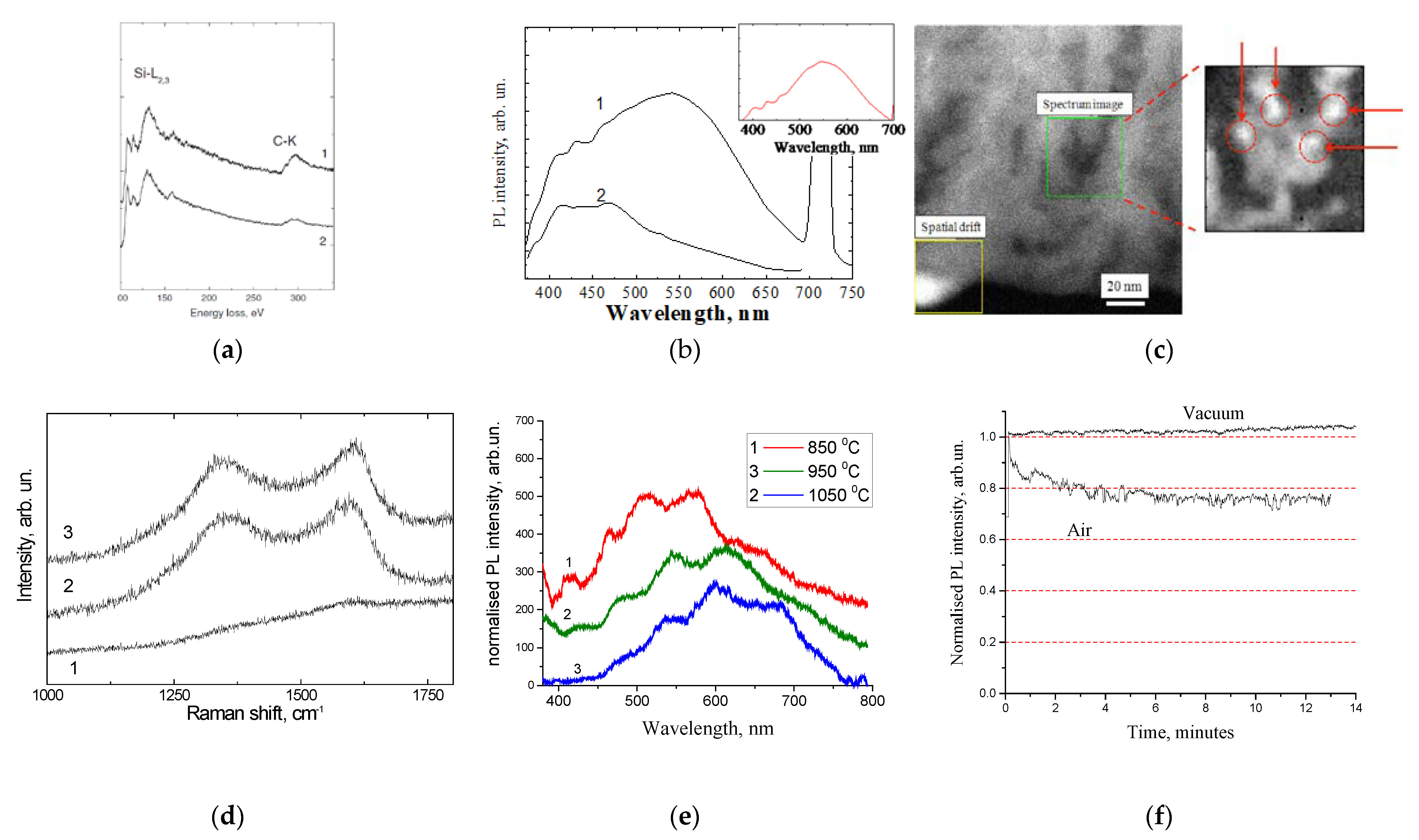
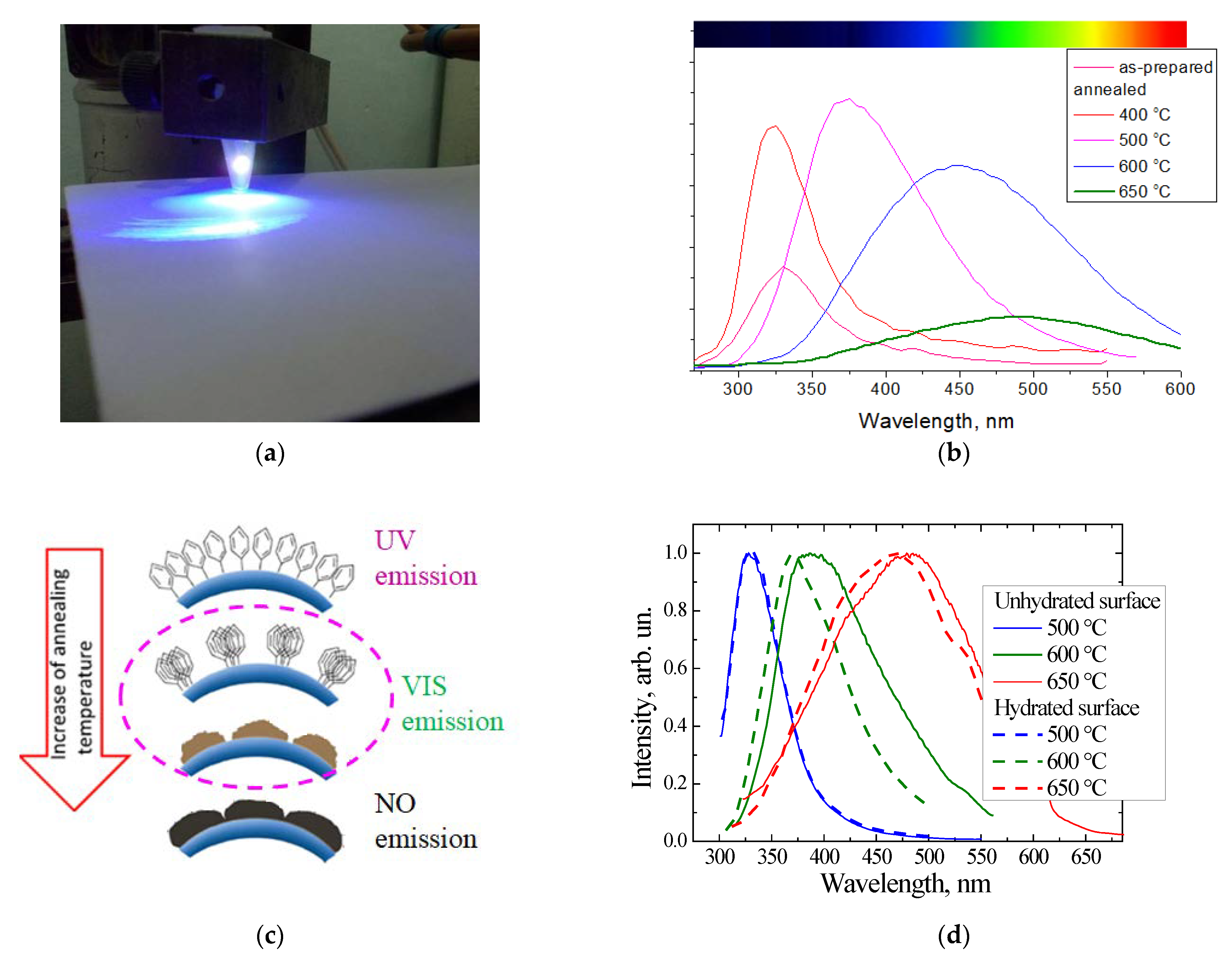
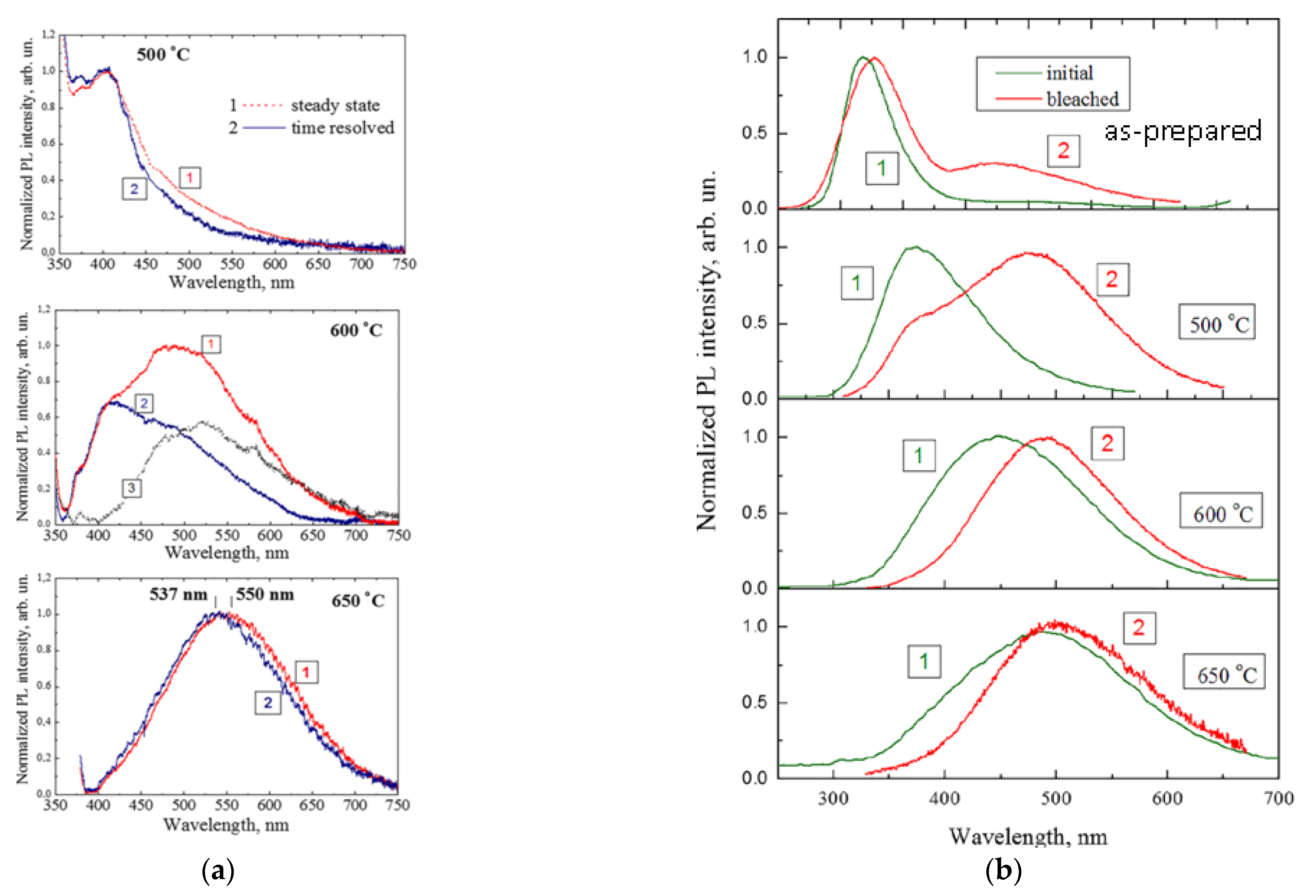
2.1. Oxidized Porous Si:C
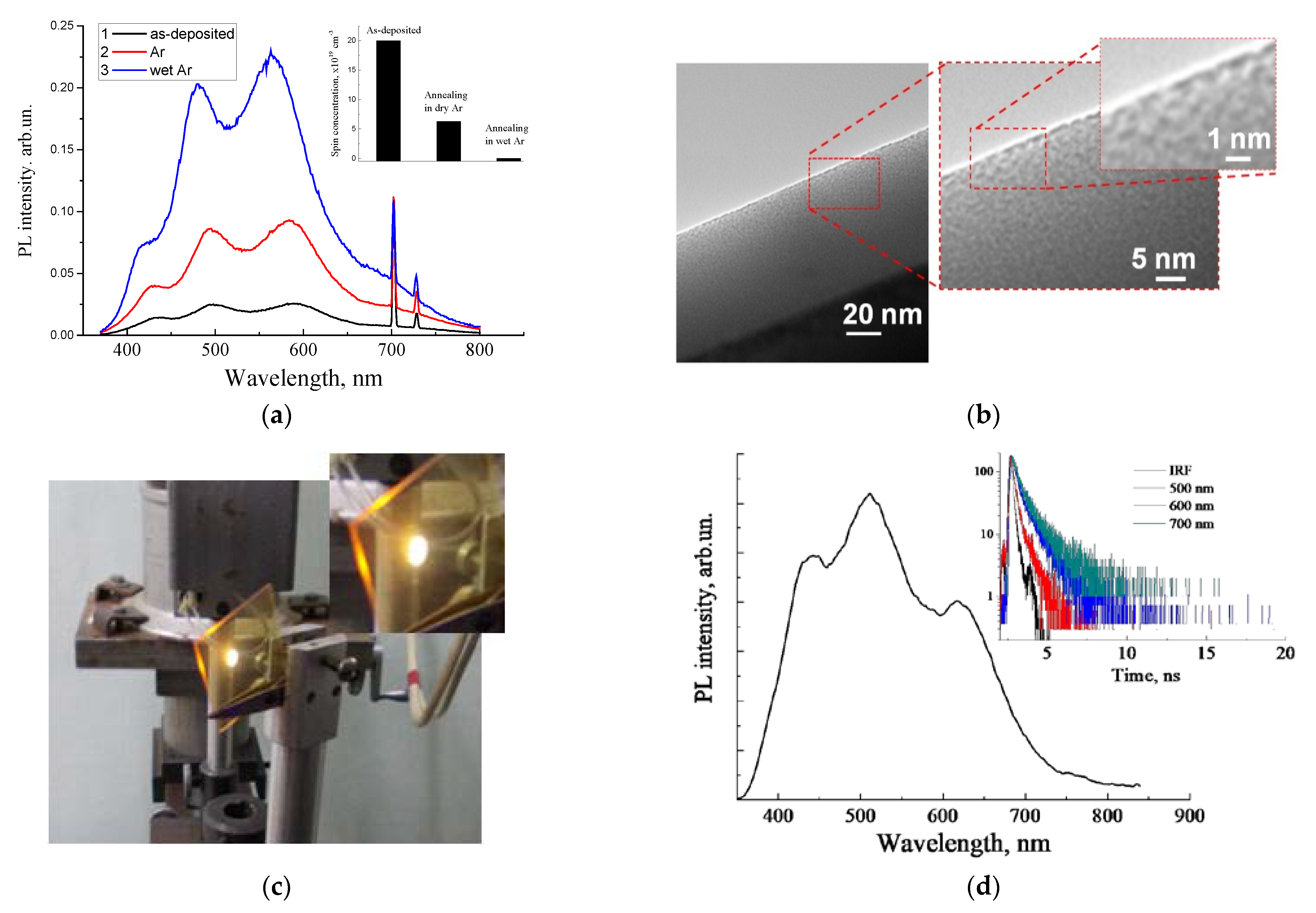
2.2. Luminescent a-SiOC:H Thin Films
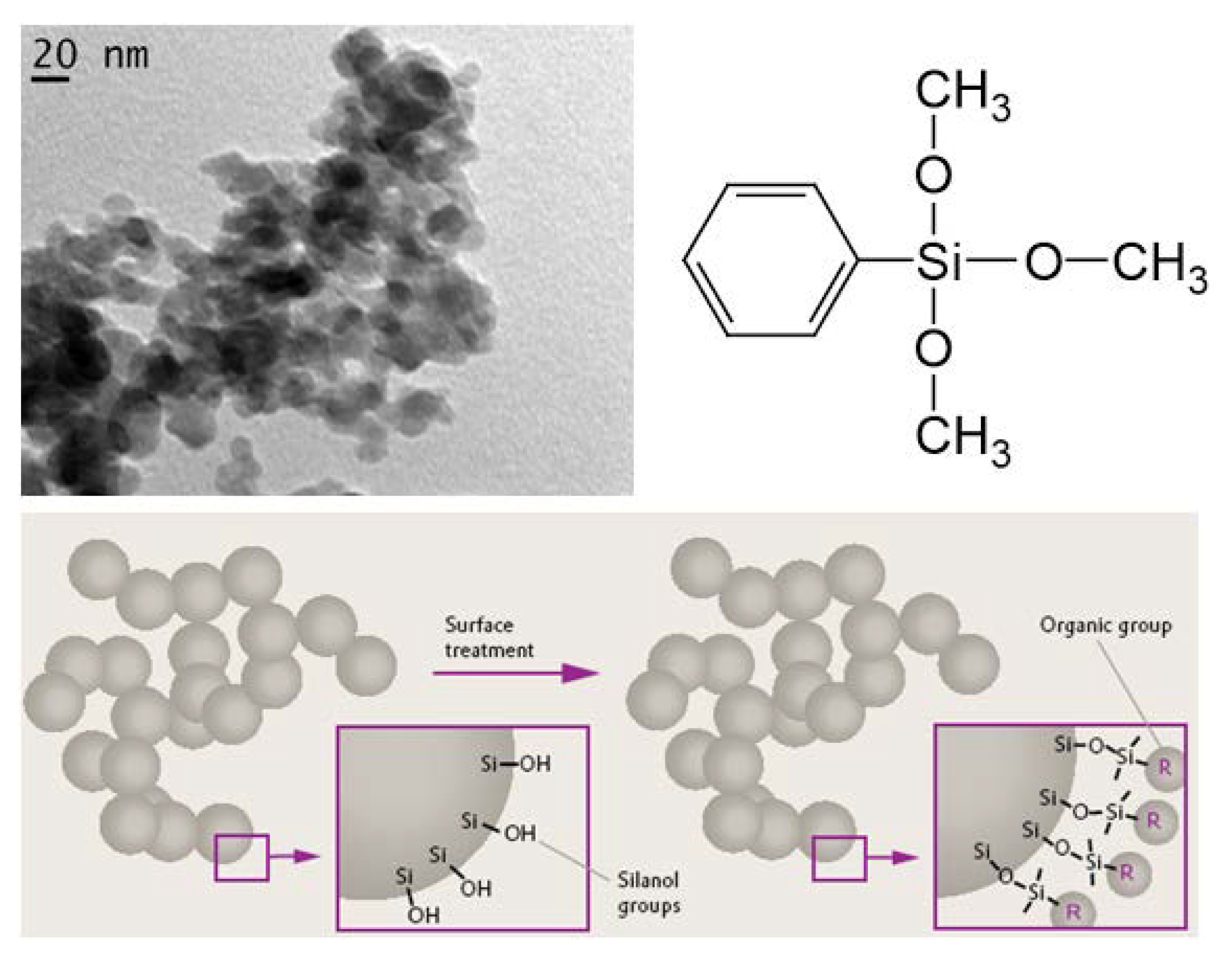
3. Luminescent Nanopowder: Carbonized Fumed Silica
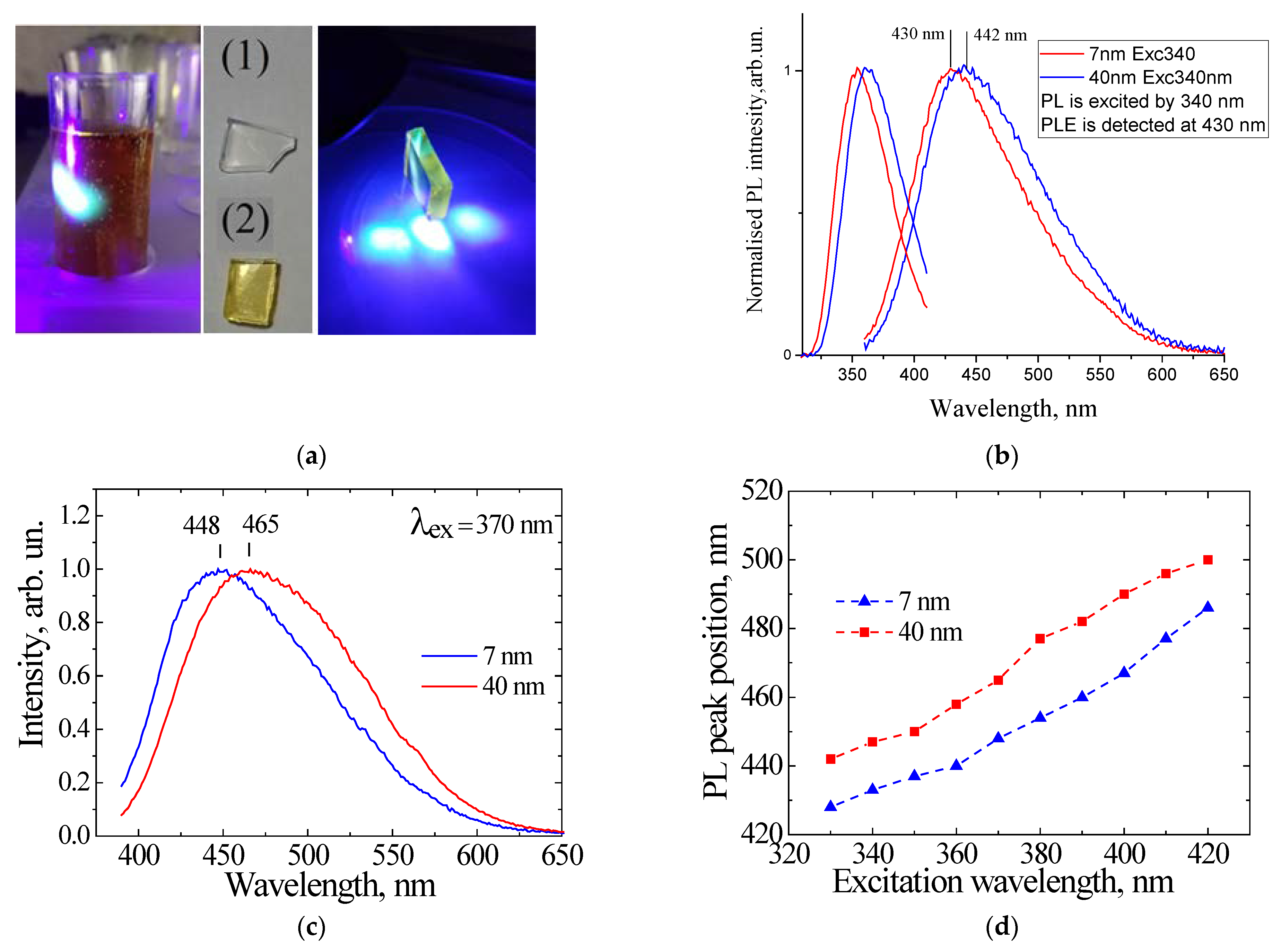
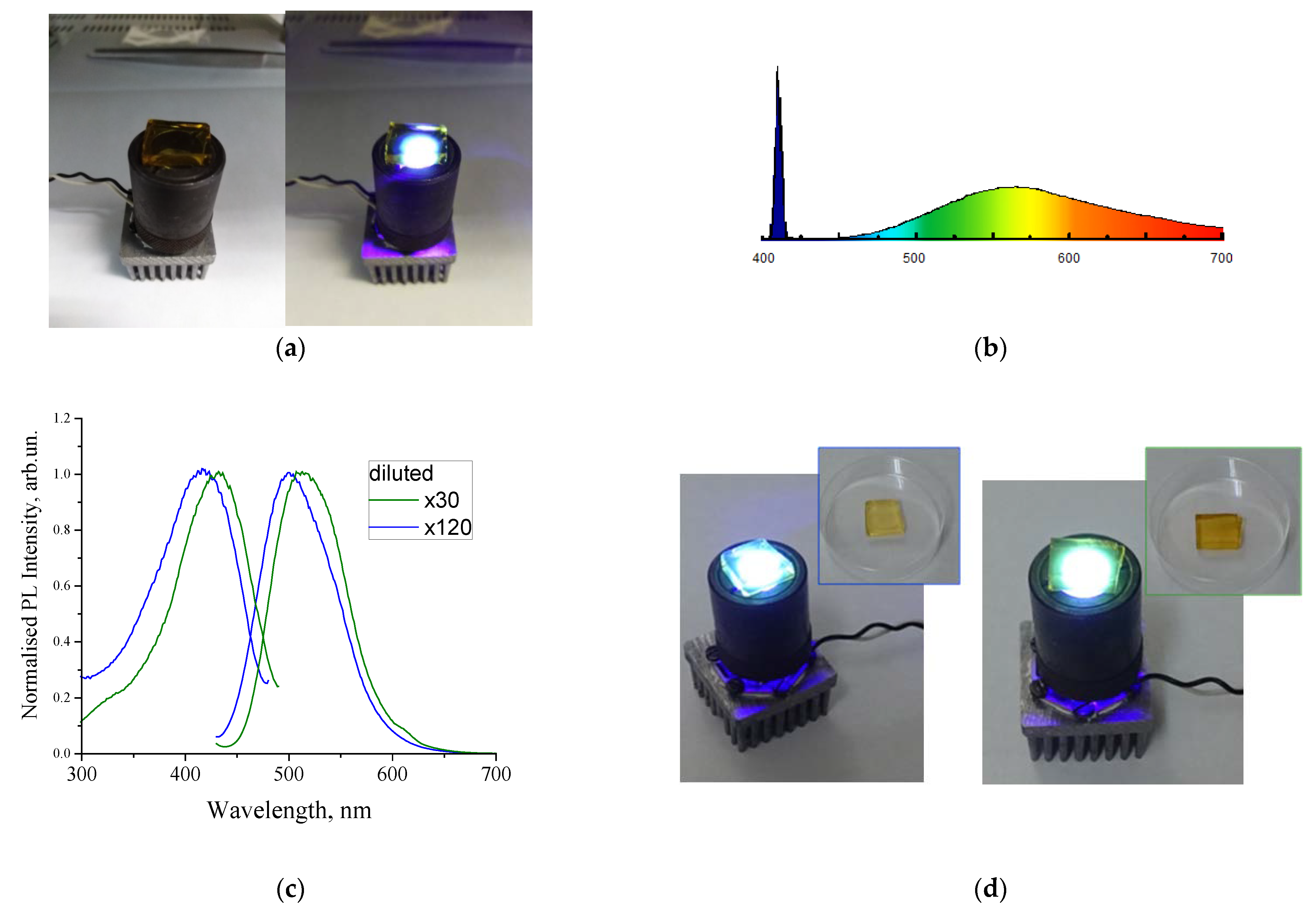
4. Sol–Gel-Derived Nanoporous Silica
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, P.; Wang, G.; Zhou, L. Luminescent sulfur quantum dots: Synthesis, properties and potential applications. ChemPhotoChem 2020, 4, 5235–5244. [Google Scholar] [CrossRef]
- Costa-Fernandez, J.M.; Pereiro, R.; Sanz-Medel, A. The use of luminescent quantum dots for optical sensing. Trends Anal. Chem. 2006, 25, 207–218. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Yu, W.W. Near infrared emitting quantum dots: Synthesis, luminescence properties and applications. J. Mater. Chem. C 2019, 7, 13662–13679. [Google Scholar] [CrossRef]
- Purcell-Milton, F.; Gun’ko, Y.K. Quantum dots for luminescent solar concentrators. J. Mater. Chem. 2012, 22, 16687–16697. [Google Scholar] [CrossRef]
- Sreenivasan, V.K.A.; Zvyagin, A.V.; Goldys, E.M. Luminescent nanoparticles and their applications in the life sciences. J. Phys. Condens. Matter. 2013, 25, 194101. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Shi, D.; Guo, Z.; Bedford, N. Basic properties of nanomaterials. In Nanomaterials and Devices; Shi, D., Guo, Z., Bedford, N., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Tsinghua University Press: Beijing, China, 2015; pp. 1–23. [Google Scholar] [CrossRef]
- Shi, D.; Guo, Z.; Bedford, N. Semiconductor quantum dots. In Nanomaterials and Devices; Shi, D., Guo, Z., Bedford, N., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Tsinghua University Press: Beijing, China, 2015; pp. 83–104. [Google Scholar] [CrossRef]
- Strauss, V.; Margraf, J.T.; Dolle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T.; et al. Carbon nanodots: Towards a comprehensive understanding of their photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrion, C.; Valcarce, L.M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794–7807. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Tuerhong, M.; Yang, X.; Yin, X.-B. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Li, J.-L.; Tang, B.; Yuan, B.; Sun, L.; Wang, X.-G. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials 2013, 34, 9519–9534. [Google Scholar] [CrossRef]
- Elvati, P.; Baumeister, E.; Violi, A. Graphene quantum dots: Effect of size, composition and curvature on their assembly. RSC Adv. 2017, 7, 17704–17710. [Google Scholar] [CrossRef] [Green Version]
- Jorns, M.; Pappas, D. A review of fluorescent carbon dots, their synthesis, physical and chemical characteristics, and applications. Nanomaterials 2021, 11, 1448. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Dekaliuk, M.O.; Viagin, O.; Malyukin, Y.V.; Demchenko, A.P. Fluorescent carbon nanomaterials: ‘‘quantum dots’’ or nanoclusters? Phys. Chem. Chem. Phys. 2014, 16, 16075–16084. [Google Scholar] [CrossRef]
- Fu, M.; Erhart, F.; Wang, Y.; Milowska, K.Z.; Reckmeier, C.; Rogach, A.L.; Stolyarczyk, J.K.; Urban, A.S.; Feldman, J. Carbon dots: A unique fluorescent cocktail of polycyclic aromatic hydrocarbons. Nano Lett. 2015, 15, 6030–6035. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. Engl. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, M.; Liu, Y.; He, F.; Gao, F.; Su, Y.; Wei, H.; Zhang, Y. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 2014, 6, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Banerjee, S.; Das, N.C. Advancement in science and technology of carbon dot-polymer hybrid composites: A review. Funct. Compos. Struct. 2019, 1, 022001. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Singh, N. Cell microenvironment pH sensing in 3D microgels using fluorescent carbon dots. ACS Biomater. Sci. Eng. 2017, 3, 3620–3627. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Li, H.; Zhao, W. Synthesis of green-emitting carbon quantum dots with double carbon sources and their application as a fluorescent probe for selective detection of Cu2+ ions. RSC Adv. 2020, 10, 2536–2544. [Google Scholar] [CrossRef] [Green Version]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, X.; Fan, Y.; Guo, X.; Zhou, L.; Lv, Y.; Lin, J. One-step microwave synthesis of N-doped hydroxyl functionalized carbon dots with ultra-high fluorescence quantum yields. Nanoscale 2016, 8, 15281–15287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; An, X.; Gong, J. Novel pH-sensitive N doped carbon dots with both long fluorescence lifetime and high quantum yield. RSC Adv. 2015, 5, 32319–32322. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Shi, L.; Liu, Y.; Meng, X.; Xu, H.; Xu, Y.; Liu, B.; Fang, X.; Li, H.-B.; Ding, T. Supramolecular interactions via hydrogen bonding contributing to citric-acid derived carbon dots with high quantum yield and sensitive photoluminescence. RSC Adv. 2017, 7, 20345–20353. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Kataoka, M.; Yamamoto, K. Photoluminescence spectra of carbon clusters embedded in SiO2. Jpn. J. Appl. Phys. 1993, 2, L274–L276. [Google Scholar] [CrossRef]
- Green, W.H.; Le, K.P.; Grey, J.; Au, T.T.; Sailor, M.J. White phosphors from a silicate carboxylate sol-gel precursor that lack metal activator ions. Science 1997, 276, 1826–1828. [Google Scholar] [CrossRef]
- Yamada, N. Photoluminescence in carbon/silica gel nanocomposites. In Supercarbon: Synthesis, Properties and Applications; Springer: New York, NY, USA, 1998; pp. 211–225. [Google Scholar]
- Yu, Y.H.; Wong, S.P.; Wilson, I.H. Visible photoluminescence in carbon-implanted thermal SiO2 films. Phys. Status Solidi A 1998, 168, 531–534. [Google Scholar] [CrossRef]
- Saha, A.; Raj, R.; Williamson, D.L. A model for the nanodomains in polymer-derived SiCO. J. Am. Ceram. Soc. 2006, 89, 2188–2195. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Xu, X.; Zhuang, J.; Zhang, H.; Zhang, X.; Hu, C.; Lei, B.; Kaminski, C.F.; Liu, Y. Carbon dot-silica nanoparticle composites for ultralong lifetime phosphorescence imaging in tissue and cells at room remperature. Chem. Mater. 2019, 31, 9887–9894. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Zhu, Z.; Luo, J.; Wu, Z.; Wang, Z. High-efficient, spherical and thermal-stable carbon dots@silica fluorescent composite as rare earth-free phosphors for white LED. Ceram. Int. 2020, 46, 14706–14712. [Google Scholar] [CrossRef]
- Stepanidenko, E.; Khavlyuk, P.; Arefina, I.; Cherevkov, S.; Xiong, Y.; Döring, A.; Varygin, G.; Kurdyukov, D.; Eurov, D.; Golubev, V.; et al. Strongly luminescent composites based on carbon dots embedded in a nanoporous silicate glass. Nanomaterials 2020, 10, 1063. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, W.; Wang, S.; Li, C.; Qian, M.; Chen, J.; Huang, R. Facile incorporation of dispersed fluorescent carbon nanodots into mesoporous silica nanosphere for pH-triggered drug delivery and imaging. Carbon 2016, 108, 146–153. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, S.; Jiang, K.; Wang, Y.; Liu, Y.; Wu, S.; Li, Z.; Shu, Q.; Lin, H. In situ synthesis of fluorescent mesoporous silica–carbon dot nanohybrids featuring folate receptor-overexpressing cancer cell targeting and drug delivery. Nano-Micro Lett. 2019, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, M.; Chen, L.; Du, Y.; Jiang, H.; Huo, T.; Yang, Y.; Guo, W.; Wang, Y.; Huang, R. Biodegradable mesoporous silica achieved via carbon nanodots-incorporated framework swelling for debris-mediated photothermal synergistic immunotherapy. Nano Lett. 2019, 19, 8409–8417. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ostermeyer, G.P.; Du, D.; Lin, Y. Carbon nanodot-hybridized silica nanospheres assisted immunoassay for sensitive detection of Escherichia coli. Sens. Actuators B Chem. 2021, 349, 130730. [Google Scholar] [CrossRef]
- Liu, C.; Bao, L.; Tang, B.; Zhao, J.-Y.; Zhang, Z.-L.; Xiong, L.-H.; Hu, J.; Wu, L.-L.; Pang, D.-W. Fluorescence-converging carbon nanodots-hybridized silica nanosphere. Small 2016, 12, 4702–4706. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Tang, J.; Zhang, Y.; Jiang, T.; Gong, X.; Peng, C.; Wei, J.; Yang, J.; Wang, Y.; Wang, X.; et al. Incorporation of well-dispersed sub-5 nm graphitic pencil nanodots into ordered mesoporous frameworks. Nat. Chem. 2016, 8, 171–178. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Liu, K.-K.; Wu, X.-Y.; Lou, Q.; Sui, L.-Z.; Dong, L.; Yuan, K.-J.; Shan, C.-X. Lifetime-engineered carbon nanodots for time division duplexing. Adv. Sci. 2021, 8, 2003433. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Gou, S.-S.; Liu, K.-K.; Wu, W.-J.; Guo, C.-Z.; Lu, S.-Y.; Zang, J.-H.; Wu, X.-Y.; Lou, Q.; Dong, L.; et al. Ultralong and efficient phosphorescence from silica confined carbon nanodots in aqueous solution. Nano Today 2020, 34, 100900. [Google Scholar] [CrossRef]
- Tian, Y.; Ran, Z.; Yang, W. Carbon dot-silica composite nanoparticle: An excitation-independent fluorescence material with tunable fluorescence. RSC Adv. 2017, 7, 43839–43844. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zheng, C.; Guo, Q.; Huang, D.; Wu, X.; Chen, L. Characterization and enhanced nonlinear optical limiting response in carbon nanodots dispersed in solid-state hybrid organically modified silica gel glasses. Opt. Mater. 2018, 76, 335–343. [Google Scholar] [CrossRef]
- Baeg, J.-O.; Yadav, R.K.; Kumar, A.; Park, N.-J.; Yadav, D. New carbon nanodots-silica hybrid photocatalyst for highly selective solar fuel production from CO2. ChemCatChem 2017, 9, 3153–3159. [Google Scholar]
- Vasin, A.V.; Ishikawa, Y.; Shibata, N.; Salonen, J.; Lehto, V.-P. Strong white photoluminescence from carbon-Incorporated silicon oxide fabricated by preferential oxidation of silicon in nano-structured Si:C layer. Jpn. J. Appl. Phys. 2007, 46, L465–L467. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Vasin, A.V.; Salonen, J.; Muto, S.; Lysenko, V.S.; Nazarov, A.N.; Shibata, N.; Lehto, V.-P. Color control of white photoluminescence from carbon incorporated silicon oxide. J. Appl. Phys. 2008, 104, 083522-1–083522-6. [Google Scholar] [CrossRef]
- Vasin, A.V.; Muto, S.; Ishikawa, Y.; Salonen, J.; Nazarov, A.N.; Lysenko, V.S.; Okholin, P. Attribution of white-light emitting centers with carbonized surface in nano-structured SiO2:C layers. Thin Solid Films 2011, 519, 4008–4011. [Google Scholar] [CrossRef]
- Savchenko, D.; Vasin, A.; Muto, S.; Kalabukhova, E.; Nazarov, A. EPR study of porous Si:C and SiO2:C layers. Phys. Status Solidi B 2018, 255, 1700559. [Google Scholar] [CrossRef]
- Vasin, A.; Rusavsky, A.; Nazarov, A.; Lysenko, V.; Rudko, G.; Piryatinski, Y.; Blonsky, I.; Salonen, J.; Makila, E.; Starik, S. Excitation effects and luminescence stability in porous SiO2:C layers. Phys. Status Solidi A 2012, 209, 1015–1021. [Google Scholar] [CrossRef]
- Sundaram, K.B.; Alizadeh, Z.; Chow, L. The effects of oxidation on the optical properties of amorphous SiC films. Mater. Sci. Eng. B 2002, 90, 47–49. [Google Scholar] [CrossRef]
- Vasin, A.V.; Ishikawa, Y.; Kolesnik, S.P.; Konchits, A.A.; Lysenko, V.S.; Nazarov, A.N.; Rudko, G.Y. Light-emitting properties of amorphous Si:C:O:H layers fabricated by oxidation of carbon-rich a-Si:C:H films. Solid State Sci. 2009, 11, 1833–1837. [Google Scholar] [CrossRef]
- Vasin, A.V.; Muto, S.; Ishikawa, Y.; Rusavsky, A.V.; Kimura, T.; Lysenko, V.S.; Nazarov, A.N. Comparative study of annealing and oxidation effects inSiC:H and a-SiC thin films deposited by radio-frequency magnetron sputtering techniques. Thin Solid Films 2011, 519, 2218–2222. [Google Scholar] [CrossRef]
- Wang, Y.; Townsend, P.D. Common mistakes in luminescence analysis. J. Phys. Conf. Ser. 2012, 398, 012003. [Google Scholar] [CrossRef] [Green Version]
- Vivaldo, I.; Ambrosio, R.C.; López, R.; Flores-Méndez, J.; Sánchez-Gaspariano, L.A.; Moreno, M.; Candia, F. Enhanced photoluminescence of hydrogenated amorphous silicon carbide thin films by means of a fast thermal annealing process. Materials 2020, 13, 2643. [Google Scholar] [CrossRef]
- Vasin, A.V.; Rusavsky, A.V.; Kysil, D.V.; Prucnal, S.; Piryatinsky, Y.P.; Starik, S.P.; Nasieka, I.; Strelchuk, V.V.; Lysenko, V.S.; Nazarov, A.N. The effect of deposition processing on structural and luminescent properties of a-SiOC:H thin films fabricated by RF-magnetron sputtering. J. Lumin. 2017, 191, 102–106. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of carbon dots in optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Vasin, A.V.; Adlung, M.; Tertykh, V.A.; Kysil, D.; Gallis, S.; Nazarov, A.N.; Lysenko, V.S. Broad band (UV-VIS) photoluminescence from carbonized fumed silica: Emission, excitation and kinetic properties. J. Lumin. 2017, 190, 141–147. [Google Scholar] [CrossRef]
- Vasin, A.V.; Kysil, D.V.; Lajaunie, L.; Rudko, G.Y.; Lysenko, V.S.; Sevostianov, S.V.; Tertykh, V.A.; Piryatinski, Y.P.; Cannas, M.; Vaccaro, L.; et al. Multiband light emission and nanoscale chemical analyses of carbonized fumed silica. J. Appl. Phys. 2018, 124, 105108. [Google Scholar] [CrossRef] [Green Version]
- Vasin, A.V.; Muto, S.; Ishikawa, Y.; Kysil, D.V.; Sevostianov, S.V.; Isaieva, O.F.; Rudko, G.Y.; Yatskiv, R.; Starik, S.; Tertykh, V.A.; et al. Evolution from UV emission of phenyl groups to visible emission of pyrolytic nanocarbons dispersed in fumed silica: Alternative insight into photoluminescence of carbon nanodots. J. Lumin. 2020, 219, 116926. [Google Scholar] [CrossRef]
- Vasin, A.V.; Kysil, D.V.; Isaeva, O.F.; Rudko, G.Y.; Virnyi, D.V.; Sevostianov, S.V.; Tertykh, V.A.; Piryatinski, Y.P.; Starik, S.; Vaccaro, L.; et al. Effect of Hydration Procedure of Fumed Silica Precursoron the Formation of Luminescent Carbon Centers inSiO2:C Nanocomposites. Phys. Status Solidi A 2019, 219, 1800560. [Google Scholar] [CrossRef]
- Hartmann, J.-M.; Auwera, J.V.; Boulet, C.; Birot, M.; Dourges, M.-A.; Toupance, T.; El Hamzaoui, H.; Ausset, P.; Carré, Y.; Kocon, L.; et al. Infrared absorption by molecular gases to probe porous materials and comparisons with other techniques. Micropor. Mesopor. Mat. 2017, 237, 31–37. [Google Scholar] [CrossRef]
- Vasin, A.V.; Kysil, D.V.; Sevostianov, S.V.; Isaieva, O.; Rudko, G.Y.; Yatskiv, R.; El Hamzaoui, H.; Capoen, B.; Bouazaoui, M.; Tertykh, V.; et al. Porous silica as a nanotemplate for the solid state and liquid phase synthesis of luminescent carbon dots. ECS Trans. 2020, 97, 91–96. [Google Scholar] [CrossRef]
- Mikhraliieva, A.; Zaitsev, V.; Aucélio, R.Q.; da Motta, H.B.; Nazarkovsky, M. Benefit of porous silica nanoreactor in preparation of fluorescence carbon dots from citric acid. Nano Express 2020, 1, 010011. [Google Scholar] [CrossRef]
- Vasin, A.; Kysil, D.; Sevostianov, S.; Isaieva, O.; Rudko, G.; Capoen, B.; Bouazaoui, M.; El Hamzaoui, H.; Tertykh, V.; Starik, S.; et al. Liquid-phase synthesis of hydrophilic luminescent carbon dots using porous silica as a nanotemplate. Phys. Status Solidi A 2021, 218, 2000817. [Google Scholar] [CrossRef]
- Patent, Z.A. The Process for Obtaining of Fluoralkylated Carbon Quantum Dots. WO2020121119. 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020121119 (accessed on 12 September 2021).
- Lisnyak, V.V.; Zaderko, A.N.; Mariychuk, R.; Lysenko, V.; Boldyrieva, O.Y.; Skryshevsky, V.A.; Mussabek, G.; Taurbayev, Y.; Zhylkybayeva, N.; Tananiko, O.Y. Preparation and characterization of F-, O-, and N-containing carbon nanoparticles for pH sensing. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Chao, D.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Yellow emissive carbon dots with quantum yield up to 68.6% from manganese ions. Carbon 2018, 135, 253–259. [Google Scholar] [CrossRef]
- Khan, W.U.; Wang, D.; Zhang, W.; Tang, Z.; Ma, X.; Ding, X.; Du, S.; Wang, Y. High quantum yield green-emitting carbon dots for Fe(III) detection, biocompatible fluorescent ink and cellular imaging. Sci. Rep. 2017, 7, 14866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Zhu, Z.; Li, X.; Zhang, T.; Zhang, W.; Chen, M.; Zhou, X. Facile synthesis of yellow emissive carbon dots with high quantum yield and their application in construction of fluorescence-labeled shape memory nanocomposite. J. Alloys Compd. 2020, 834, 154399. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward high-efficient red emissive carbon dots: Facile preparation, unique properties, and applications as multifunctional theranostic agents. Chem. Mater. 2016, 28, 8659–8668. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% efficient red emissive carbon quantum dots for high color rendering and stable warm white-light-emitting diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward efficient orange emissive carbon nanodots through conjugated sp2-domain controlling and surface charges engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasin, A.; Kysil, D.; Rusavsky, A.; Isaieva, O.; Zaderko, A.; Nazarov, A.; Lysenko, V. Synthesis and Luminescent Properties of Carbon Nanodots Dispersed in Nanostructured Silicas. Nanomaterials 2021, 11, 3267. https://doi.org/10.3390/nano11123267
Vasin A, Kysil D, Rusavsky A, Isaieva O, Zaderko A, Nazarov A, Lysenko V. Synthesis and Luminescent Properties of Carbon Nanodots Dispersed in Nanostructured Silicas. Nanomaterials. 2021; 11(12):3267. https://doi.org/10.3390/nano11123267
Chicago/Turabian StyleVasin, Andrii, Dmytro Kysil, Andriy Rusavsky, Oksana Isaieva, Alexander Zaderko, Alexei Nazarov, and Volodymyr Lysenko. 2021. "Synthesis and Luminescent Properties of Carbon Nanodots Dispersed in Nanostructured Silicas" Nanomaterials 11, no. 12: 3267. https://doi.org/10.3390/nano11123267
APA StyleVasin, A., Kysil, D., Rusavsky, A., Isaieva, O., Zaderko, A., Nazarov, A., & Lysenko, V. (2021). Synthesis and Luminescent Properties of Carbon Nanodots Dispersed in Nanostructured Silicas. Nanomaterials, 11(12), 3267. https://doi.org/10.3390/nano11123267




