Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids
Abstract
:1. Introduction
- zero-dimensional (0D) particles (e.g., quantum dots or core-shell particles);
- one-dimensional (1D) moieties (nanowires; nanorods; nanotubes, including carbon and oxide materials; nanoribbons, etc.);
- two-dimensional (2D) materials (flat structures, self-organized monolayers, nanodiscs, graphene);
- three-dimensional (3D) structures (dendritic structures, etc.).
2. Peculiarities of the Processes of Electrochemical Synthesis of Nanostructures in Ionic Liquids and the Methods Used
- preparation of nanomaterials with a given structure and properties;
- the use of nanomaterials for a specific purpose, taking into account their structure and properties;
- control of the structure and properties of nanomaterials both during their production and in the course of their application.
- simultaneous formation and immobilization of nanoparticles;
- immobilization of metal ions with subsequent reduction to metal nanoparticles;
- preparation of metal nanoparticles with their subsequent immobilization on a substrate.
- The dependence of the DEL capacity on the electrode potential is bell-shaped, and there are no signs of compliance with the Gui–Chapman theory [64];
- Significant overscreening effects (excessive shielding) are observed at small electrode polarizations;
- At large polarizations, the effect of “lattice saturation” (maximum increase in ion packing) is observed;
- The thickness of the DEL in IL is larger than one layer of particles.
3. Production of Carbon Nanomaterials
4. Silicon and Germanium Nanoparticles
5. Metal Nanoparticles
- To investigate in situ the effects of the modulating factors, including cations, anions, metal salts, water content and additives on the nucleation and growth in ILs; such methods as in situ SEM and in situ TEM are efficient.
- To deeply and systematically study the variation in the interfacial structure of the ILs-electrified substrate with modulating factors, including the nature of the cations and anions, metal salts, water content and additives by a combination of experiments and simulation methods.
- To precisely characterize the influence of anions, metal salts, water content and additives on the process, with spectroscopic methods such as UV/Vis spectroscopy, Raman spectroscopy and extended X-ray absorption fine structure (EXAFS) being recommended for the study.
- To find the relationship between the modulating factors and the quality of the deposited materials, enabling the easy tuning of electrodeposition in ILs.
6. Nanomaterials Based on Oxides
- the necessity of pretreatment (annealing, polishing, etc.);
- a two-stage method for producing nanotubes;
- duration (more than 30 min to obtain primary ordered pores);
- single use of electrolytes that do not always meet the criteria of “green chemistry.”
7. Electropolymerization in Ionic Liquids
7.1. Nano-Sized Polymer Films
7.2. Electropolymerization of Ionic Liquids
8. Nanoscale Composites
9. Conclusions
- designer solvents for various processes;
- media for electrochemical reactions, catalysis [185], electrocatalysis and electrodeposition;
- electrolytes for power sources and generators, such as supercapacitors, and batteries for solar cells or fuel cells (ILs with high proton conductivity have been found);
- liquids for electric wetting;
- liquid organic components for self-assembled nanoplasmon devices;
- electronic valve media for monomolecular devices and electrolytes for electronics and superconductors;
- media and electrolytes for chemical and electrochemical sensors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, C.M. (Ed.) Handbook of Functionalized Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-816787-8. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Applications of Nanotechnology in Daily Life. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 113–143. ISBN 978-0-12-813586-0. [Google Scholar]
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; Von Gleich, A.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef]
- Bulychev, N.A.; Kazaryan, M.A.; Nikiforov, V.N.; Shevchenko, S.N.; Yakunin, V.G.; Timoshenko, V.; Bychenko, A.B.; Sredin, V.G. Peculiarities of metal oxide nanoparticles obtained in acoustoplasma discharge. Tech. Phys. Lett. 2016, 42, 495–497. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Kadir, A.A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef] [Green Version]
- Endres, F. Electrodeposited silicon from ionic liquids. In Silicon Nanomaterials Sourcebook: Arrays, Functional Materials, and Industrial Nanosilicon; Series in Material Science and Engineering; Sattler, K., Ed.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 12; pp. 311–328. ISBN 978-1-4987-6378-3. [Google Scholar]
- Feng, G.; Chen, M.; Bi, S.; Goodwin, Z.A.H.; Postnikov, E.B.; Brilliantov, N.; Urbakh, M.; Kornyshev, A.A. Free and Bound States of Ions in Ionic Liquids, Conductivity, and Underscreening Paradox. Phys. Rev. X 2019, 9, 021024. [Google Scholar] [CrossRef] [Green Version]
- Strate, A.; Neumann, J.; Niemann, T.; Stange, P.; Khudozhitkov, A.E.; Stepanov, A.G.; Paschek, D.; Kolokolov, D.I.; Ludwig, R. Counting cations involved in cationic clusters of hydroxy-functionalized ionic liquids by means of infrared and solid-state NMR spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 6861–6867. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Petrii, O.A. Electrosynthesis of nanostructures and nanomaterials. Russ. Chem. Rev. 2015, 84, 159–193. [Google Scholar] [CrossRef]
- Fahmi, A.; Pietsch, T.; Mendoza, C.; Cheval, N. Functional hybrid materials. Mater. Today 2009, 12, 44–50. [Google Scholar] [CrossRef]
- Petrii, O.A.; Tsirlina, G.A. Size effects in electrochemistry. Russ. Chem. Rev. 2001, 70, 285–298. [Google Scholar] [CrossRef]
- Campbell, F.W.; Compton, R.G. The use of nanoparticles in electroanalysis: An updated review. Anal. Bioanal. Chem. 2009, 396, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Drensler, S.; Walkner, S.; Mardare, C.C.; Hassel, A.W. On the pH-sensing properties of differently prepared tungsten oxide films: On the PH-Sensing Properties of Differently Prepared Tungsten Oxide Films. Phys. Status Solidi A 2014, 211, 1340–1345. [Google Scholar] [CrossRef]
- Petrii, O.A. Zero charge potentials of platinum metals and electron work functions (Review). Russ. J. Electrochem. 2013, 49, 401–422. [Google Scholar] [CrossRef]
- Ehrenburg, M.R.; Molodkina, E.B.; Broekmann, P.; Rudnev, A.V. Underpotential Deposition of Silver on Au(111) from an Air- and Water-Stable Ionic Liquid Visualized by In-Situ STM. ChemElectroChem 2018, 6, 1149–1156. [Google Scholar] [CrossRef]
- Kleijn, S.E.F.; Lai, S.C.S.; Koper, M.T.M.; Unwin, P.R. Electrochemistry of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 3558–3586. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Song, P.; Ruan, M.; Xu, W. Recent progress in two-dimensional nanomaterials: Synthesis, engineering, and applications. FlatChem 2019, 18, 100133. [Google Scholar] [CrossRef]
- Di Bari, G.A. Electrodeposition of Nickel. In Modern Electroplating; Schlesinger, M., Paunovic, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 79–114. ISBN 978-0-470-60263-8. [Google Scholar]
- Tsyntsaru, N.; Cesiulis, H.; Donten, M.; Sort, J.; Pellicer, E.; Podlaha-Murphy, E.J. Modern trends in tungsten alloys electrodeposition with iron group metals. Surf. Eng. Appl. Electrochem. 2012, 48, 491–520. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Wang, H.-W. Electrochemical Synthesis of Nickel Oxide Nanoparticulate Films on Nickel Foils for High-Performance Electrode Materials of Supercapacitors. Int. J. Electrochem. Sci. 2012, 7, 4405–4417. [Google Scholar]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Buzzeo, M.C.; Evans, R.G.; Compton, R.G. Non-Haloaluminate Room-Temperature Ionic Liquids in Electrochemistry—A Review. ChemPhysChem 2004, 5, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, N.; El Abedin, A.S.Z.; Endres, F. In Situ STM Investigation of Gold Reconstruction and of Silicon Electrodeposition on Au(111) in the Room Temperature Ionic Liquid 1-Butyl-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. B 2006, 110, 6250–6256. [Google Scholar] [CrossRef] [PubMed]
- Endres, F.; Bukowski, M.; Hempelmann, R.; Natter, H. Electrodeposition of Nanocrystalline Metals and Alloys from Ionic Liquids. Angew. Chem. Int. Ed. 2003, 42, 3428–3430. [Google Scholar] [CrossRef] [PubMed]
- Golgovici, F.; Anicai, L.; Florea, A.; Visan, T. Electrochemical Synthesis of Conducting Polymers Involving Deep Eutectic Solvents. Curr. Nanosci. 2020, 16, 478–494. [Google Scholar] [CrossRef]
- Prasad, K.R.; Miura, N. Electrochemically deposited nanowhiskers of nickel oxide as a high-power pseudocapacitive electrode. Appl. Phys. Lett. 2004, 85, 4199–4201. [Google Scholar] [CrossRef]
- Kilimnik, A.B.; Ostrozhkova, E.J.; Bakunin, E.S. Method of Producing Ultramicrodispersed Nickel Oxide Powder Using AC. RU 2503748 C2, 10 January 2014. [Google Scholar]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Riley, D.J. Electrochemistry in nanoparticle science. Curr. Opin. Colloid Interface Sci. 2002, 7, 186–192. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Miller, A.E.; Chang, H.C.; Banerjee, G.; Yuzhakov, V.V.; Yue, D.-F.; Ricker, R.E.; Jones, S.N.; Eastman, J.A.; Baugher, E.M.; et al. Electrochemically assembled quasi-periodic quantum dot arrays. Nanotechnology 1996, 7, 360–371. [Google Scholar] [CrossRef] [Green Version]
- Nazneen, F.; Galvin, P.; Arrigan, D.W.M.; Thompson, M.; Benvenuto, P.; Herzog, G. Electropolishing of medical-grade stainless steel in preparation for surface nano-texturing. J. Solid State Electrochem. 2011, 16, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Yuzhakov, V.V.; Chang, H.-C.; Miller, A.E. Pattern formation during electropolishing. Phys. Rev. B 1997, 56, 12608–12624. [Google Scholar] [CrossRef] [Green Version]
- Yuzhakov, V.V.; Takhistov, P.V.; Miller, A.E.; Chang, H.-C. Pattern selection during electropolishing due to double-layer effects. Chaos: Interdiscip. J. Nonlinear Sci. 1999, 9, 62–77. [Google Scholar] [CrossRef] [PubMed]
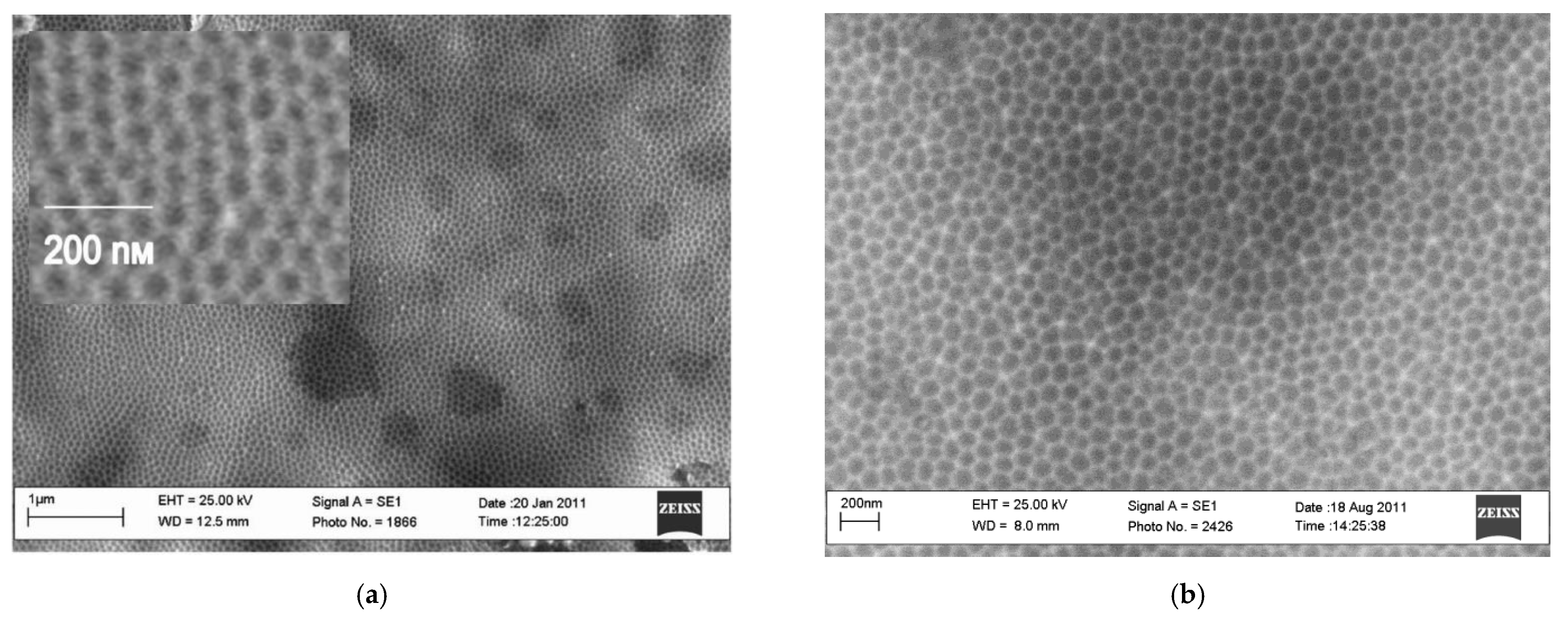
- Lebedeva, O.; Kudryavtsev, I.; Kultin, D.; Dzhungurova, G.; Kalmykov, K.; Kustov, L. Self-Organized Hexagonal Nanostructures on Nickel and Steel Formed by Anodization in 1-Butyl-3-methylimidazolium bis(triflate)imide Ionic Liquid. J. Phys. Chem. C 2014, 118, 21293–21298. [Google Scholar] [CrossRef]
- Torriero, A.A.J. (Ed.) Electrochemistry in Ionic Liquids; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-15131-1. [Google Scholar]
- Torriero, A.A.J.; Shiddiky, M.J.A. (Eds.) Electrochemical Properties and Applications of Ionic Liquids; Nova Science Publishers: New York, NY, USA, 2011; ISBN 978-1-61761-381-4. [Google Scholar]
- Macfarlane, D.R.; Seddon, K.R. Ionic Liquids—Progress on the Fundamental Issues. Aust. J. Chem. 2007, 60, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, M.V.; Kornyshev, A.A. Ionic Liquids at Electrified Interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Lane, G.H. Electrochemical reduction mechanisms and stabilities of some cation types used in ionic liquids and other organic salts. Electrochim. Acta 2012, 83, 513–528. [Google Scholar] [CrossRef]
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M.; Mei, T.X. Investigating the electrochemical windows of ionic liquids. J. Ind. Eng. Chem. 2013, 19, 106–112. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Janiak, C. Metal Nanoparticle Synthesis in Ionic Liquids. In Ionic Liquids (ILs) in Organometallic Catalysis; Topics in Organometallic Chemistry; Dupont, J., Kollár, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 51, pp. 17–54. ISBN 978-3-662-47856-1. [Google Scholar]
- Richter, K.; Campbell, P.S.; Baecker, T.; Schimitzek, A.; Yaprak, D.; Mudring, A.-V. Ionic liquids for the synthesis of metal nanoparticles: Ionic Liquids for the Synthesis of Metal Nanoparticles. Phys. Status Solidi B 2013, 250, 1152–1164. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Nanoparticles in ionic liquids: Interactions and organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef]
- Litschauer, M.; Neouze, M.-A. Nanoparticles connected through an ionic liquid-like network. J. Mater. Chem. 2007, 18, 640–646. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.-Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marullo, S.; D’Anna, F.; Rizzo, C.; Billeci, F. Ionic liquids: “normal” solvents or nanostructured fluids? Org. Biomol. Chem. 2021, 19, 2076–2095. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, B.; Perlt, E. Ionic Liquids II; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-89794-3. [Google Scholar]
- Manojkumar, K.; Sivaramakrishna, A.; Vijayakrishna, K. A short review on stable metal nanoparticles using ionic liquids, supported ionic liquids, and poly(ionic liquids). J. Nanopart. Res. 2016, 18, 103. [Google Scholar] [CrossRef]
- Janiak, C. Ionic Liquids for the Synthesis and Stabilization of Metal Nanoparticles. Z. Naturforsch. B J. Chem. Sci. 2013, 68, 1059–1089. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Self-Organization of Room-Temperature Ionic Liquids Exhibiting Liquid-Crystalline Bicontinuous Cubic Phases: Formation of Nano-Ion Channel Networks. J. Am. Chem. Soc. 2007, 129, 10662–10663. [Google Scholar] [CrossRef]
- Wegner, S.; Janiak, C. Metal Nanoparticles in Ionic Liquids. Top. Curr. Chem. 2017, 375, 65. [Google Scholar] [CrossRef]
- Jitvisate, M.; Seddon, J.R.T. Local Structure and Flow Properties of Ionic Liquids on Charged and Inert Substrates. J. Phys. Chem. C 2016, 120, 4860–4865. [Google Scholar] [CrossRef]
- Kislenko, S.A. Double Electric Layer in Strongly Non-Ideal Ionic Liquid [BMIm] [PF6]. Ph.D. Dissertation, Institute for High Temperatures of the Russian Academy of Sciences, Moscow, Russia, 2010. Available online: https://static.freereferats.ru/_avtoreferats/01004893632.pdf (accessed on 18 November 2011).
- Arano, K.; Begic, S.; Chen, F.; Rakov, D.; Mazouzi, D.; Gautier, N.; Kerr, R.; Lestriez, B.; Le Bideau, J.; Howlett, P.C.; et al. Tuning the Formation and Structure of the Silicon Electrode/Ionic Liquid Electrolyte Interphase in Superconcentrated Ionic Liquids. ACS Appl. Mater. Interfaces 2021, 13, 28281–28294. [Google Scholar] [CrossRef]
- Kislenko, S.A.; Moroz, Y.O.; Karu, K.; Ivaništšev, V.B.; Fedorov, M.V. Calculating the Maximum Density of the Surface Packing of Ions in Ionic Liquids. Russ. J. Phys. Chem. A 2018, 92, 999–1005. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Towards understanding the structure and capacitance of electrical double layer in ionic liquids. Electrochim. Acta 2008, 53, 6835–6840. [Google Scholar] [CrossRef]
- Black, J.M.; Okatan, M.; Feng, G.; Cummings, P.T.; Kalinin, S.V.; Balke, N. Topological defects in electric double layers of ionic liquids at carbon interfaces. Nano Energy 2015, 15, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Gomes, C.; Costa, R.; Pereira, C.M.; Silva, A.F. The electrical double layer at the ionic liquid/Au and Pt electrode interface. RSC Adv. 2014, 4, 28914–28921. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Luan, Y.; Mu, T. Ionic liquids for synthesis of inorganic nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Chong, A.L.; Forsyth, M.; Kar, M.; Vijayaraghavan, R.; Somers, A.; Pringle, J.M. New dimensions in salt–solvent mixtures: A 4th evolution of ionic liquids. Faraday Discuss. 2017, 206, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Di Carmine, G.; Abbott, A.P.; D’Agostino, C. Deep eutectic solvents: Alternative reaction media for organic oxidation reactions. React. Chem. Eng. 2021, 6, 582–598. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, W.; Klein, J.; Gurkan, B. Do Deep Eutectic Solvents Behave Like Ionic Liquid Electrolytes? A Perspective from the Electrode-Electrolyte Interface. J. Electrochem. Soc. 2021, 168, 026503. [Google Scholar] [CrossRef]
- Kim, J.H.; Shawky, A.; Yasuda, S.; Murakoshi, K. Selective Synthesis of Graphitic Carbon and Polyacetylene by Electrochemical Reduction of Halogenated Carbons in Ionic Liquid at Room Temperature. Electrochim. Acta 2015, 176, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Novoselova, I.A.; Kuleshov, S.V.; Volkov, S.V.; Bykov, V.N. Electrochemical synthesis, morphological and structural characteristics of carbon nanomaterials produced in molten salts. Electrochim. Acta 2016, 211, 343–355. [Google Scholar] [CrossRef]
- Wang, X.; Sharif, F.; Liu, X.; Licht, G.; Lefler, M.; Licht, S. Magnetic carbon nanotubes: Carbide nucleated electrochemical growth of ferromagnetic CNTs from CO2. J. CO2 Util. 2020, 40, 101218. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Carbon-Based Nanomaterials Functionalized with Ionic Liquids for Microextraction in Sample Preparation. Separations 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Ischenko, A.A.; Fetisov, G.V.; Aslalnov, L.A. Nanosilicon: Properties, Synthesis, Applications, Methods of Analysis and Control; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-07375-5. [Google Scholar]
- Hong, Y.J.; Saroj, R.K.; Park, W.I.; Yi, G.-C. One-dimensional semiconductor nanostructures grown on two-dimensional nanomaterials for flexible device applications. APL Mater. 2021, 9, 060907. [Google Scholar] [CrossRef]
- Platek, A.; Piwek, J.; Fic, K.; Schubert, T.; Gentile, P.; Bidan, G.; Frackowiak, E. Electrochemical performance of silicon nanostructures in low-temperature ionic liquids for microelectronic applications. J. Mater. Chem. A 2017, 5, 22708–22716. [Google Scholar] [CrossRef]
- Meng, X.; Al-Salman, R.; Zhao, J.; Borissenko, N.; Li, Y.; Endres, F. Electrodeposition of 3D Ordered Macroporous Germanium from Ionic Liquids: A Feasible Method to Make Photonic Crystals with a High Dielectric Constant. Angew. Chem. Int. Ed. 2009, 48, 2703–2707. [Google Scholar] [CrossRef]
- Al-Salman, R.; Mallet, J.; Molinari, M.; Fricoteaux, P.; Martineau, F.; Troyon, M.; El Abedin, S.Z.; Endres, F. Template assisted electrodeposition of germanium and silicon nanowires in an ionic liquid. Phys. Chem. Chem. Phys. 2008, 10, 6233–6237. [Google Scholar] [CrossRef]
- Mallet, J.; Molinari, M.; Martineau, F.; Delavoie, F.; Fricoteaux, P.; Troyon, M. Growth of Silicon Nanowires of Controlled Diameters by Electrodeposition in Ionic Liquid at Room Temperature. Nano Lett. 2008, 8, 3468–3474. [Google Scholar] [CrossRef]
- Al-Salman, R.; Endres, F. Template-assisted electrodeposition of SixGe1−x nanowires with varying length and composition from two different ionic liquids. J. Mater. Chem. 2009, 19, 7228–7231. [Google Scholar] [CrossRef]
- Homma, T.; Komadina, J.; Nakano, Y.; Ouchi, T.; Akiyoshi, T.; Iishibashi, Y.; Nishimura, Y.; Nishida, T.; Fukunaka, Y. Templated Electrodeposition of Si Nanowires from Ionic Liquid. ECS Trans. 2012, 41, 9–15. [Google Scholar] [CrossRef]
- Juzeliu<monospace>ū</monospace>nas, E.; Fray, D.J. Silicon Electrochemistry in Molten Salts. Chem. Rev. 2019, 120, 1690–1709. [Google Scholar] [CrossRef]
- Endres, F.; MacFarlane, D.; Abbott, A. (Eds.) Electrodeposition from Ionic Liquids, 1st ed.; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-3-527-31565-9. [Google Scholar]
- Katayama, Y.; Yokomizo, M.; Miura, T.; Kishi, T. Preparation of a Novel Fluorosilicate Salt for Electrodeposition of Silicon at Low Temperature. Electrochemistry 2001, 69, 834–836. [Google Scholar] [CrossRef] [Green Version]
- El Abedin, S.Z.; Borissenko, N.; Endres, F. Electrodeposition of nanoscale silicon in a room temperature ionic liquid. Electrochem. Commun. 2004, 6, 510–514. [Google Scholar] [CrossRef]
- Freyland, W.; Zell, C.A.; El Abedin, S.Z.; Endres, F. Nanoscale electrodeposition of metals and semiconductors from ionic liquids. Electrochim. Acta 2003, 48, 3053–3061. [Google Scholar] [CrossRef]
- Al-Salman, R.; El Abedin, S.Z.; Endres, F. Electrodeposition of Ge, Si and SixGe1−x from an air- and water-stable ionic liquid. Phys. Chem. Chem. Phys. 2008, 10, 4650–4657. [Google Scholar] [CrossRef]
- Schmuck, M.; Balducci, A.; Rupp, B.; Kern, W.; Passerini, S.; Winter, M. Alloying of electrodeposited silicon with lithium—A principal study of applicability as anode material for lithium ion batteries. J. Solid State Electrochem. 2008, 14, 2203–2207. [Google Scholar] [CrossRef]
- Tsuyuki, Y.; Takai, H.; Fukunaka, Y.; Homma, T. Formation of Si thin films by electrodeposition in ionic liquids for solar cell applications. Jpn. J. Appl. Phys. 2018, 57, 08RB11. [Google Scholar] [CrossRef]
- Shah, N.K.; Pati, R.K.; Ray, A.; Mukhopadhyay, I. Electrodeposition of Si from an Ionic Liquid Bath at Room Temperature in the Presence of Water. Langmuir 2017, 33, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.P. Electrodeposition of Silicon from Nonaqueous Solvents. J. Electrochem. Soc. 2005, 152, C795–C802. [Google Scholar] [CrossRef]
- Nishimura, Y.; Fukunaka, Y. Electrochemical reduction of silicon chloride in a non-aqueous solvent. Electrochim. Acta 2007, 53, 111–116. [Google Scholar] [CrossRef]
- Munisamy, T.; Bard, A.J. Electrodeposition of Si from organic solvents and studies related to initial stages of Si growth. Electrochim. Acta 2010, 55, 3797–3803. [Google Scholar] [CrossRef]
- Tchalala, M.R.; El-Demellawi, J.K.; Abou-Hamad, E.; Retamal, J.R.D.; Varadhan, P.; He, J.-H.; Chaieb, S. Hybrid electrolytes based on ionic liquids and amorphous porous silicon nanoparticles: Organization and electrochemical properties. Appl. Mater. Today 2017, 9, 10–20. [Google Scholar] [CrossRef]
- Serrà, A.; Vallés, E. Advanced electrochemical synthesis of multicomponent metallic nanorods and nanowires: Fundamentals and applications. Appl. Mater. Today 2018, 12, 207–234. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Vallés, E. Facile electrochemical synthesis, using microemulsions with ionic liquid, of highly mesoporous CoPt nanorods with enhanced electrocatalytic performance for clean energy. Int. J. Hydrogen Energy 2015, 40, 8062–8070. [Google Scholar] [CrossRef] [Green Version]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Transition metal nanoparticles in ionic liquids: Synthesis and stabilization. J. Mol. Liq. 2018, 276, 826–849. [Google Scholar] [CrossRef]
- Liu, Q.X.; El Abedin, S.Z.; Endres, F. Electrodeposition of Nanocrystalline Aluminum: Breakdown of Imidazolium Cations Modifies the Crystal Size. J. Electrochem. Soc. 2008, 155, D357–D362. [Google Scholar] [CrossRef]
- El Abedin, S.Z.; Moustafa, E.M.; Hempelmann, R.; Natter, H.; Endres, F. Additive free electrodeposition of nanocrystalline aluminium in a water and air stable ionic liquid. Electrochem. Commun. 2005, 7, 1111–1116. [Google Scholar] [CrossRef]
- El Abedin, S.Z.; Moustafa, E.M.; Hempelmann, R.; Natter, H.; Endres, F. Electrodeposition of Nano- and Microcrystalline Aluminium in Three Different Air and Water Stable Ionic Liquids. ChemPhysChem 2006, 7, 1535–1543. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, L.; Luo, L.; Wang, S.; Wang, X. Deposition mechanism of aluminum on uranium in AlCl3-1-ethyl-3-methylimidazolium chloride ionic liquid by galvanic displacement. J. Appl. Electrochem. 2018, 48, 827–834. [Google Scholar] [CrossRef]
- He, P.; Liu, H.; Li, Z.; Liu, Y.; Xu, X.; Li, J. Electrochemical Deposition of Silver in Room-Temperature Ionic Liquids and Its Surface-Enhanced Raman Scattering Effect. Langmuir 2004, 20, 10260–10267. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Sun, I.-W. Electrochemistry of Cd(II) in the basic 1-ethyl-3-methylimidazolium chloride/tetrafluoroborate room temperature molten salt. Electrochim. Acta 2000, 45, 3163–3170. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Sun, I.-W. Electrochemical study of copper in a basic 1-ethyl-3-methylimidazolium tetrafluoroborate room temperature molten salt. Electrochim. Acta 1999, 45, 441–450. [Google Scholar] [CrossRef]
- Yang, M.-H.; Sun, I.-W. Electrodeposition of antimony in a water-stable 1-ethyl-3-methylimidazolium chloride tetrafluoroborate room temperature ionic liquid. J. Appl. Electrochem. 2003, 33, 1077–1084. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Katayama, Y.; Miura, T. Electrochemical Preparation of Nickel and Iron Nanoparticles in a Hydrophobic Ionic Liquid. Electrochem. Solid-state Lett. 2011, 14, D110–D115. [Google Scholar] [CrossRef]
- Kustov, L.M.; Lebedeva, O.K.; Kultin, D.; Root, N.V.; Kazansky, V.B. Electrochemical modification of steel by platinum nanoparticles. Dokl. Chem. 2016, 470, 297–301. [Google Scholar] [CrossRef]
- Tarasov, A.; Root, N.; Lebedeva, O.; Kultin, D.; Kiwi-Minsker, L.; Kustov, L. Platinum Nanoparticles on Sintered Metal Fibers Are Efficient Structured Catalysts in Partial Methane Oxidation into Synthesis Gas. ACS Omega 2020, 5, 5078–5084. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Sun, I.-W. Electrodeposition of PtZn in a Lewis acidic ZnCl2–1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2004, 49, 3251–3258. [Google Scholar] [CrossRef]
- Huang, J.-F.; Sun, I.-W. Fabrication and Surface Functionalization of Nanoporous Gold by Electrochemical Alloying/Dealloying of Au-Zn in an Ionic Liquid, and the Self-Assembly ofL-Cysteine Monolayers. Adv. Funct. Mater. 2005, 15, 989–994. [Google Scholar] [CrossRef]
- Huang, J.-F.; Sun, I.-W. Formation of Nanoporous Platinum by Selective Anodic Dissolution of PtZn Surface Alloy in a Lewis Acidic Zinc Chloride-1-Ethyl-3-methylimidazolium Chloride Ionic Liquid. Chem. Mater. 2004, 16, 1829–1831. [Google Scholar] [CrossRef]
- Dhas, N.A.; Raj, C.P.; Gedanken, A. Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles. Chem. Mater. 1998, 10, 1446–1452. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.-R.; Lee, K.J.; Stott, N.E.; Kim, D. Large-scale synthesis of copper nanoparticles by chemically controlled reduction for applications of inkjet-printed electronics. Nanotechnology 2008, 19, 415604. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.K.; Gaikwad, S.; Adhyapak, P.V.; Singh, N.; Marimuthu, R. Synthesis and characterization of copper nanoparticles. Mater. Lett. 2007, 61, 4711–4714. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, I.; Freyland, W. Electrodeposition of Ti Nanowires on Highly Oriented Pyrolytic Graphite from an Ionic Liquid at Room Temperature. Langmuir 2003, 19, 1951–1953. [Google Scholar] [CrossRef]
- Mukhopadhyay, I.; Aravinda, C.L.; Borissov, D.; Freyland, W. Electrodeposition of Ti from TiCl4 in the ionic liquid l-methyl-3-butyl-imidazolium bis (trifluoro methyl sulfone) imide at room temperature: Study on phase formation by in situ electrochemical scanning tunneling microscopy. Electrochim. Acta 2005, 50, 1275–1281. [Google Scholar] [CrossRef]
- El Abedin, S.Z.; Farag, H.K.; Moustafa, E.M.; Welz-Biermann, U.; Endres, F. Electroreduction of tantalum fluoride in a room temperature ionic liquid at variable temperatures. Phys. Chem. Chem. Phys. 2005, 7, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.C. Electrochemical fabrication of dendritic silver–copper bimetallic nanomaterials in protic ionic liquid for electrocarboxylation. J. Taiwan Inst. Chem. Eng. 2018, 87, 158–164. [Google Scholar] [CrossRef]
- Al-Salman, R.; Sommer, H.; Brezesinski, T.; Janek, J. Template-Free Electrochemical Synthesis of High Aspect Ratio Sn Nanowires in Ionic Liquids: A General Route to Large-Area Metal and Semimetal Nanowire Arrays? Chem. Mater. 2015, 27, 3830–3837. [Google Scholar] [CrossRef]
- Rudnev, A.V. Electrodeposition of lanthanides from ionic liquids and deep eutectic solvents. Russ. Chem. Rev. 2020, 89, 1463–1482. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Zhang, S.; Lu, X.; Zhang, X. Electrodeposition in Ionic Liquids. ChemPhysChem 2015, 17, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Costovici, S.; Petica, A.; Dumitru, C.-S.; Cojocaru, A.; Anicai, L. Electrochemical Synthesis on ZnO Nanopowder Involving Choline Chloride Based Ionic Liquids. Chem. Eng. Trans. 2014, 41, 343–348. [Google Scholar] [CrossRef]
- Alammar, T.; Shekhah, O.; Wohlgemuth, J.; Mudring, A.-V. Ultrasound-assisted synthesis of mesoporous β-Ni(OH)2 and NiO nano-sheets using ionic liquids. J. Mater. Chem. 2012, 22, 18252–18260. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Yamada, Y. Shape- and Size-Controlled Nanomaterials for Artificial Photosynthesis. ChemSusChem 2013, 6, 1834–1847. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.J.; Varma, A.R.S.; Dionysiou, D.D. Thermally Stable Nanocrystalline TiO2 Photocatalysts Synthesized via Sol−Gel Methods Modified with Ionic Liquid and Surfactant Molecules. Chem. Mater. 2006, 18, 5377–5384. [Google Scholar] [CrossRef]
- Ding, K.; Miao, Z.; Liu, Z.; Zhang, Z.; Han, B.; An, G.; Miao, A.S.; Xie, Y. Facile Synthesis of High Quality TiO2 Nanocrystals in Ionic Liquid via a Microwave-Assisted Process. J. Am. Chem. Soc. 2007, 129, 6362–6363. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, X.; Yan, Z.; Zhu, L. Ionic Liquid-Assisted Synthesis of Large-Scale TiO2 Nanoparticles with Controllable Phase by Hydrolysis of TiCl4. ACS Nano 2008, 3, 115–122. [Google Scholar] [CrossRef]
- Wender, H.; Feil, A.F.; Diaz, L.B.; Ribeiro, C.S.; Machado, G.J.; Migowski, P.; Weibel, D.E.; Dupont, J.; Teixeira, S.R. Self-Organized TiO2 Nanotube Arrays: Synthesis by Anodization in an Ionic Liquid and Assessment of Photocatalytic Properties. ACS Appl. Mater. Interfaces 2011, 3, 1359–1365. [Google Scholar] [CrossRef]
- Anicai, L.; Petica, A.; Patroi, D.; Marinescu, V.; Prioteasa, P.; Costovici, S. Electrochemical synthesis of nanosized TiO2 nanopowder involving choline chloride based ionic liquids. Mater. Sci. Eng. B 2015, 199, 87–95. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Selvam, T.; Schmuki, P. Electrochemical synthesis of self-organized TiO2 nanotubular structures using an ionic liquid (BMIM-BF4). Electrochim. Acta 2008, 54, 643–648. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef]
- Abbas, W.A.; Abdullah, I.H.; Ali, B.A.; Ahmed, N.; Mohamed, A.M.; Rezk, M.Y.; Ismail, N.; Mohamed, M.A.; Allam, N.K. Recent advances in the use of TiO2 nanotube powder in biological, environmental, and energy applications. Nanoscale Adv. 2019, 1, 2801–2816. [Google Scholar] [CrossRef] [Green Version]
- Ruiquan, Y.; Longfei, J.; Xufei, Z.; Ye, S.; Dongliang, Y.; Aijun, H. Theoretical derivation of ionic current and electronic current and comparison between fitting curves and measured curves. RSC Adv. 2012, 2, 12474–12481. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Gong, T.; Su, J.; Zhang, W.; Song, Y.; Zhu, X. Comparative study on the anodizing process of Ti and Zr and oxide morphology. Ceram. Int. 2021, 47, 23332–23337. [Google Scholar] [CrossRef]
- Zhou, Q.; Tian, M.; Ying, Z.; Dan, Y.; Tang, F.; Zhang, J.; Zhu, J.; Zhu, X. Dense films formed during Ti anodization in NH4F electrolyte: Evidence against the field-assisted dissolution reactions of fluoride ions. Electrochem. Commun. 2020, 111. [Google Scholar] [CrossRef]
- Antonietti, M.; Kuang, D.-B.; Smarsly, B.; Zhou, Y. Ionic Liquids for the Convenient Synthesis of Functional Nanoparticles and Other Inorganic Nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4988–4992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.Y.; Antonietti, M. Synthesis of Very Small TiO2 Nanocrystals in a Room-Temperature Ionic Liquid and Their Self-Assembly toward Mesoporous Spherical Aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef]
- Qu, J.; Luo, H.; Dai, S. Method for Synthesis of Titanium Dioxide Nanotubes Using Ionic Liquids. U.S. Patent 8,585,886, 19 November 2013. [Google Scholar]
- Lebedeva, O.K.; Kultin, D.J.; Root, N.V.; Kustov, L.M.; Dzhungurova, G.E.; Kalmykov, K.B.; Dunaev, S.F. Electrochemical Method for Producing Nano-Sized Titanium (IV) Oxide Structures. RU Patent 2,602,126, 10 November 2016. [Google Scholar]
- Lebedeva, O.K.; Kultin, D.Y.; Root, N.V.; Kustov, L.M.; Dzhungurova, G.E.; Kalmykov, K.B.; Dunaev, S.F. Electrochemical Method of Producing Nano-Sized Structures of Nickel(II) Oxide. RU Patent 2,592,892, 27 July 2016. [Google Scholar]
- Fröschl, T.; Hörmann, U.; Kubiak, P.; Kučerová, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Hüsing, N.; Kaiser, U.; Landfester, K.; et al. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef]
- Lebedeva, O.K.; Root, N.V.; Kultin, D.Y.; Kalmykov, K.B.; Kustov, L.M. Formation of Nanostructures on the Nickel Metal Surface in Ionic Liquid under Anodizing. Russ. J. Phys. Chem. A 2018, 92, 965–967. [Google Scholar] [CrossRef]
- Lebedeva, O.K.; Kultin, D.Y.; Root, N.V.; Kustov, L.M.; Dunaev, S.F.; Kazansky, V.B. Synthesis of Nanotitania on the Surface of Titanium Metal in Ionic Liquids: Role of Water Additions. Dokl. Chem. 2018, 479, 41–44. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kudryavtsev, I.; Root, N.; Kustov, L. The role of initial hexagonal self-ordering in anodic nanotube growth in ionic liquid. Electrochem. Commun. 2017, 75, 78–81. [Google Scholar] [CrossRef]
- Kalmykov, K.B.; Dmitrieva, N.E.; Lebedeva, O.K.; Root, N.V.; Kultin, D.; Kustov, L.M. Formation of a regular cellular structure on the surface of Zr67Ni30Si3 alloy at electrochemical polishing in ionic liquids. Russ. Chem. Bull. 2016, 65, 2801–2804. [Google Scholar] [CrossRef]
- Lebedeva, O.; Jungurova, G.; Kultin, D.; Kustov, L.; Zakharov, A.; Kalmikov, K.; Chernikova, E.; Krasovskiy, V. Ionic liquids based on the imidazolium cation in platinum and titanium electropolishing. Green Chem. 2011, 13, 1004–1008. [Google Scholar] [CrossRef]
- Sopha, H.; Macak, J.M. Recent advancements in the synthesis, properties, and applications of anodic self-organized TiO2 nanotube layers. In Nanostructured Anodic Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–209. ISBN 978-0-12-816706-9. [Google Scholar]
- Root, N.V. Synthesis of Surface Nanostructures on Metals at Electrochemical Treatment in Ionic Liquids and Their Catalytic Properties in Partial Oxidation Reactions. Ph.D. Dissertation, Lomonosov Moscow State University, Moscow, Russia, 2017. [Google Scholar]
- Schmidt, O.G.; Eberl, K.K. Thin solid films roll up into nanotubes. Nature 2001, 410, 168. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Ketov, S.V.; Trifonov, A.S.; Churymov, A.Y. Surface structure and properties of metallic glasses. J. Alloys Compd. 2018, 742, 512–517. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kalmykov, K.; Snytko, V.; Kuznetsova, I.; Orekhov, A.; Zakharov, A.; Kustov, L. Nanorolls Decorated with Nanotubes as a Novel Type of Nanostructures: Fast Anodic Oxidation of Amorphous Fe–Cr–B Alloy in Hydrophobic Ionic Liquid. ACS Appl. Mater. Interfaces 2021, 13, 2025–2032. [Google Scholar] [CrossRef]
- Lu, W.; Fadeev, A.G.; Qi, B.; Smela, E.; Mattes, B.R.; Ding, J.; Spinks, G.M.; Mazurkiewicz, J.; Zhou, D.; Wallace, G.G.; et al. Use of Ionic Liquids for π-Conjugated Polymer Electrochemical Devices. Science 2002, 297, 983–987. [Google Scholar] [CrossRef] [Green Version]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Pringle, J.M.; Sun, J.; Annat, G.; Neil, W.; Izgorodina, E.I. Ionic Liquids in Electrochemical Devices and Processes: Managing Interfacial Electrochemistry. Accounts Chem. Res. 2007, 40, 1165–1173. [Google Scholar] [CrossRef]
- Schoetz, T.; de Leon, C.P.; Bund, A.; Ueda, M. Electro-polymerisation of 3,4-ethylenedioxythiophene on reticulated vitreous carbon in imidazolium-based chloroaluminate ionic liquid as energy storage material. Electrochem. Commun. 2018, 89, 52–56. [Google Scholar] [CrossRef]
- Lebedeva, O.K.; Kul’tin, D.Y.; Kustov, L.M.; Dunaev, S.F. Ionic Liquids in Electrochemical Processes. Ross. Khim. Zh. 2004, 48, 59–73. [Google Scholar]
- Lebedeva, O.; Kultin, D.; Root, N.; Guseynov, F.; Dunaev, S.; De Melo, F.; Kustov, L. First successful synthesis of polypyridines in ionic liquid: Role of 1-butyl-3-methylimidazolium tetrafluoroborate as electrolyte. Synth. Met. 2016, 221, 268–274. [Google Scholar] [CrossRef]
- Lebedeva, O.K.; Kultin, D.Y.; Kustov, L.M. Electrochemical Behavior of Benzene, Diphenyl, and p-Terphenyl in Room-Temperature Ionic Liquid N-Butylpyridinium Chloride–AlCl3. Russ. J. Phys. Chem. A 2021, 95, 217–220. [Google Scholar] [CrossRef]
- Lebedeva, O.K.; Kultin, D.Y.; Kustov, L.M. Electrochemical Synthesis of Polyphenylenes in Room-Temperature Ionic Liquid Butylpyridinium Chloride–AlCl3. Russ. J. Phys. Chem. A 2019, 93, 2323–2325. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Huang, X. Electrochemical polymerization of aniline in a protic ionic liquid with high proton activity. Synth. Met. 2018, 245, 18–23. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, J.; Niu, D.; Wallace, G.G.; Lu, J. Electrochemical polymerization of pyrrole in BMIMPF6 ionic liquid and its electrochemical response to dopamine in the presence of ascorbic acid. Synth. Met. 2009, 159, 1542–1545. [Google Scholar] [CrossRef]
- Kong, S.; Fontaine, O.; Roche, J.; Bouffier, L.; Kuhn, A.; Zigah, D. Electropolymerization of Polypyrrole by Bipolar Electrochemistry in an Ionic Liquid. Langmuir 2014, 30, 2973–2976. [Google Scholar] [CrossRef]
- Jensen, J.; Hösel, M.; Dyer, A.L.; Krebs, F.C. Development and Manufacture of Polymer-Based Electrochromic Devices. Adv. Funct. Mater. 2015, 25, 2073–2090. [Google Scholar] [CrossRef]
- Carbas, B.B.; Gulen, M.; Tolu, M.C.; Sonmezoglu, S. Hydrogen sulphate-based ionic liquid-assisted electro-polymerization of PEDOT catalyst material for high-efficiency photoelectrochemical solar cells. Sci. Rep. 2017, 7, 11672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Qin, G.; Yuan, X.; Xu, M.; Zheng, P.; Lu, J. Fixation of CO2 by Electrocatalytic Reduction and Electropolymerization in Ionic Liquid–H2O Solution. ChemSusChem 2008, 1, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef] [Green Version]
- Shaplov, A.S.; Morozova, S.M.; Lozinskaya, E.I.; Vlasov, P.S.; Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M.; Vygodskii, Y.S. Turning into poly(ionic liquid)s as a tool for polyimide modification: Synthesis, characterization and CO2 separation properties. Polym. Chem. 2015, 7, 580–591. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Lin, C.; An, Q.; Tao, C.; Gao, Y.; Li, G. Electrochemical polymerization of imidazolum-ionic liquids bearing a pyrrole moiety. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 4151–4161. [Google Scholar] [CrossRef]
- Lee, S.; Becht, G.A.; Lee, B.; Burns, C.T.; Firestone, M.A. Electropolymerization of a Bifunctional Ionic Liquid Monomer Yields an Electroactive Liquid-Crystalline Polymer. Adv. Funct. Mater. 2010, 20, 2063–2070. [Google Scholar] [CrossRef]
- Devasurendra, A.M.; Zhang, C.; Young, J.A.; Tillekeratne, L.M.V.; Anderson, J.L.; Kirchhoff, J.R. Electropolymerized Pyrrole-Based Conductive Polymeric Ionic Liquids and Their Application for Solid-Phase Microextraction. ACS Appl. Mater. Interfaces 2017, 9, 24955–24963. [Google Scholar] [CrossRef] [Green Version]
- Young, J.A.; Zhang, C.; Devasurendra, A.M.; Tillekeratne, L.V.; Anderson, J.L.; Kirchhoff, J.R. Conductive polymeric ionic liquids for electroanalysis and solid-phase microextraction. Anal. Chim. Acta 2016, 910, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar] [CrossRef]
- Molaakbari, E.; Mostafavi, A.; Tohidiyan, Z.; Beitollahi, H. Synthesis and application of conductive polymeric ionic liquid/Ni nanocomposite to construct a nanostructure based electrochemical sensor for determination of risperidone and methylphenidate. J. Electroanal. Chem. 2017, 801, 198–205. [Google Scholar] [CrossRef]
- Molaakbari, E.; Mostafavi, A.; Beitollahi, H.; Tohidiyan, Z. Synthesis of conductive polymeric ionic liquid/Ni nanocomposite and its application to construct a nanostructure based electrochemical sensor for determination of warfarin in the presence of tramadol. Talanta 2017, 171, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [Green Version]
- Rakov, D.A.; Chen, F.; Ferdousi, S.A.; Li, H.; Pathirana, T.; Simonov, A.N.; Howlett, P.C.; Atkin, R.; Forsyth, M. Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat. Mater. 2020, 19, 1096–1101. [Google Scholar] [CrossRef]
- Mudring, A.-V.; Alammar, T.; Bäcker, T.; Richter, K. Nanoparticle Synthesis in Ionic Liquids. In Ionic Liquids: From Knowledge to Application; ACS Symposium Series; Plechkova, N.V., Rogers, R.D., Seddon, K.R., Eds.; American Chemical Society: Washington, DC, USA, 2009; Chapter 12; Volume 1030, pp. 177–188. ISBN 978-0-8412-6997-2. [Google Scholar]
- Seitkalieva, M.M.; Samoylenko, D.E.; Lotsman, K.A.; Rodygin, K.S.; Ananikov, V.P. Metal nanoparticles in ionic liquids: Synthesis and catalytic applications. Coord. Chem. Rev. 2021, 445, 213982. [Google Scholar] [CrossRef]
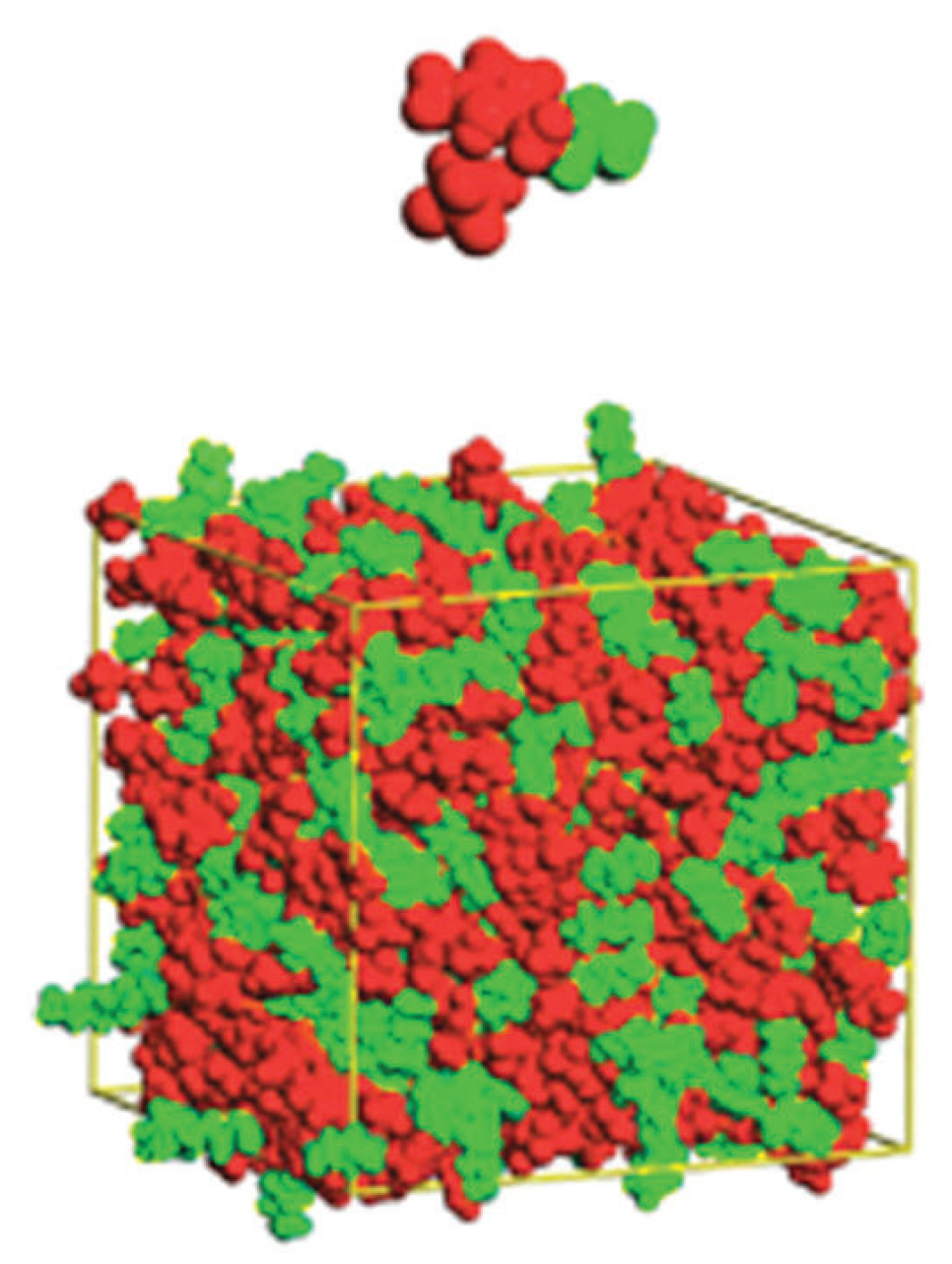















| Cation | Designation |
|---|---|
| N-Ethyl-N,N-dimethyl-2-methoxymethylammonium | [N112,1O2] |
| Triethylsulfonium | [S222] |
| N-Methoxyethyl-N-methylmorpholinium | MOEMMor |
| 1-(2-Methoxyethyl)-1-methylpyridinium | MOPMPip |
| Trihexyl(tetradecyl)phosphonium | P14,666 |
| Ethylmethylimidazolium | EMIm |
| Butylmethylimidazolium | BMIm |
| Butylmethylpyridinium | BMPy |
| Hexylmethylpyrrolidinium | HMPyrr |
| Trimethylhexylammonium | [N1116] |
| Bis(2-hydroxyethyl)ammonium | BHEA |
| 2-Hydroxyethylammonium | HEA |
| Pentylmethylimidazolium | PMIm |
| Ethylmethylpyrrolidinium | EMPyrr |
| Tetrabutylammonium | [TBA] |
| Anion | Designation |
|---|---|
| Tetrafluoroborate | BF4 |
| Hexafluorophosphate | PF6 |
| Trifluoromethanesulfonylimide | TFO |
| Bis(trifluoromethanesulfonyl)imide | [Tf2N] |
| Tris(trifluoromethanesulfonyl)methide | methide |
| Ethylsulfate | EtSO4 |
| Acetate | Ac |
| Trifluoroacetate | TFAc |
| Dicyanamide | DCA |
| Nitrate | NO3 |
| Chloride | Cl |
| Glycynate | Gly |
| Thiocyanate | SCN |
| Tris(pentafluoroethyl)trifluorophosphate | FAP |
| Fluoride | F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedeva, O.; Kultin, D.; Kustov, L. Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids. Nanomaterials 2021, 11, 3270. https://doi.org/10.3390/nano11123270
Lebedeva O, Kultin D, Kustov L. Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids. Nanomaterials. 2021; 11(12):3270. https://doi.org/10.3390/nano11123270
Chicago/Turabian StyleLebedeva, Olga, Dmitry Kultin, and Leonid Kustov. 2021. "Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids" Nanomaterials 11, no. 12: 3270. https://doi.org/10.3390/nano11123270
APA StyleLebedeva, O., Kultin, D., & Kustov, L. (2021). Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids. Nanomaterials, 11(12), 3270. https://doi.org/10.3390/nano11123270







