Biogenic Synthesis of Iron Oxide Nanoparticles Using Enterococcus faecalis: Adsorption of Hexavalent Chromium from Aqueous Solution and In Vitro Cytotoxicity Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation, Screening, and Identification of Enterococcus faecalis_RMSN6 and Biosynthesis of Fe3O4 Nanoparticle
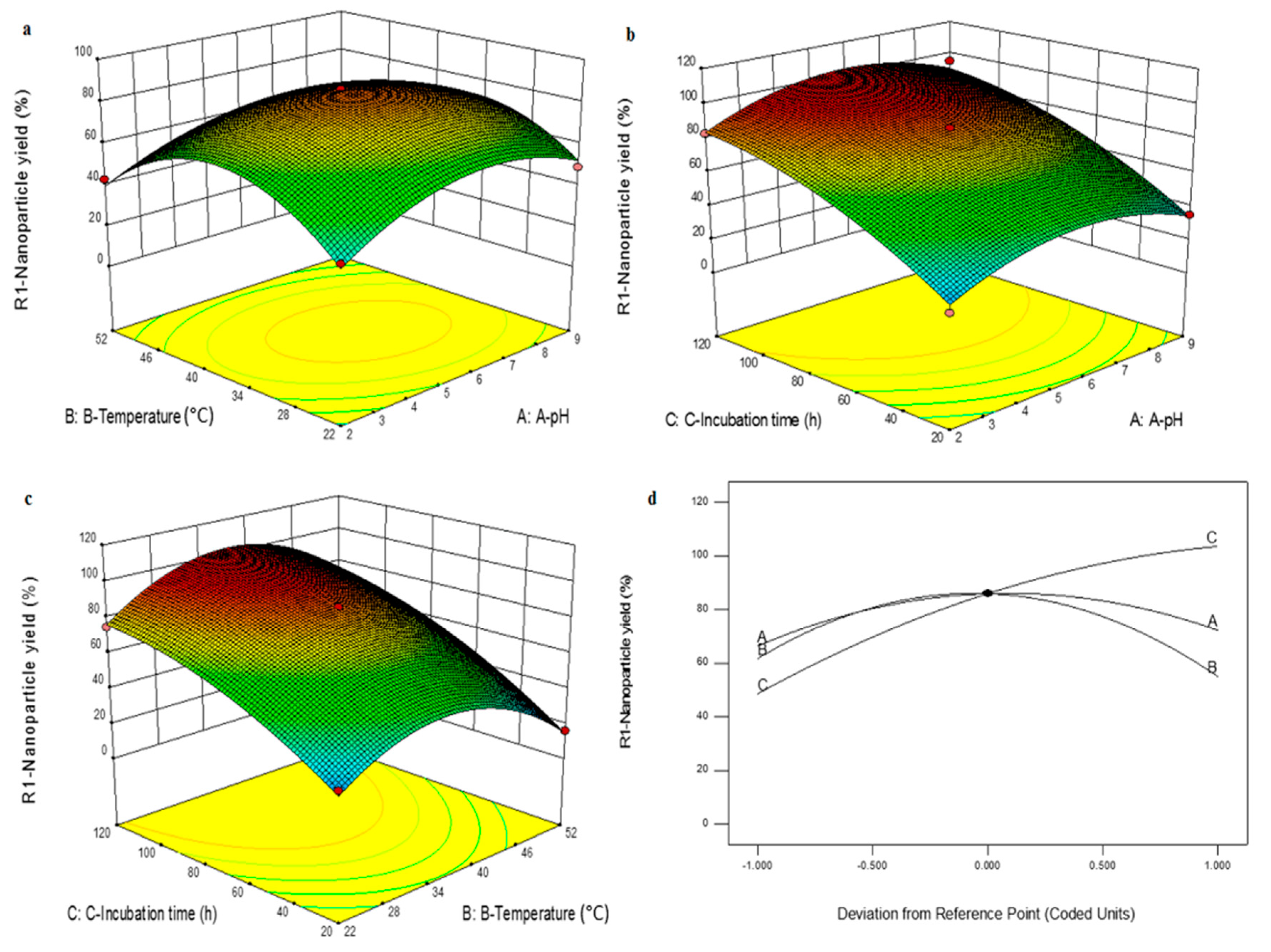
2.3. Box-Behnken Design (BBD)
2.4. Material Characterization
2.5. Adsorption Investigation
2.6. MTT Assay Using A549 Cell Lines
2.7. Statistical Analysis
3. Results and Discussion
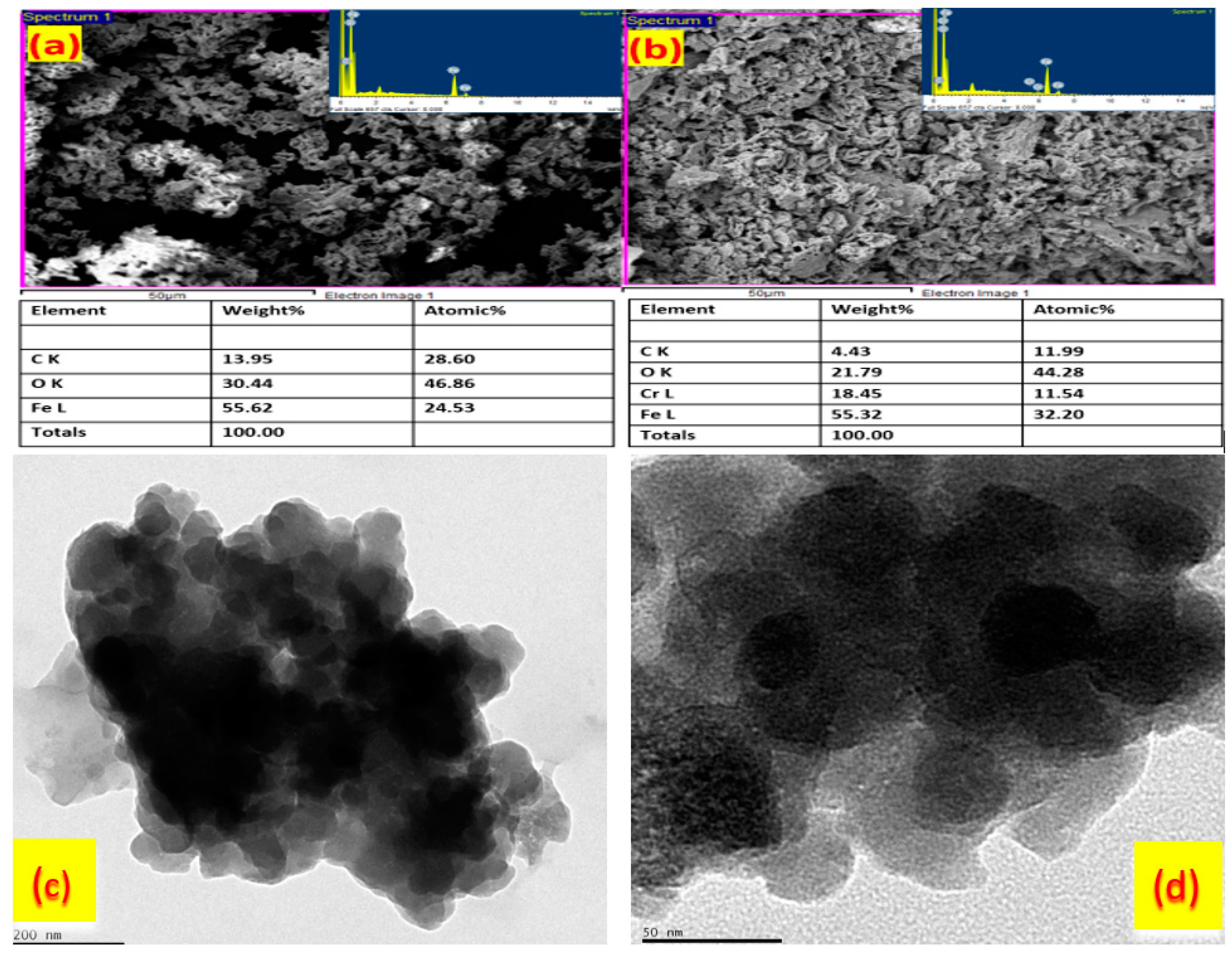
3.1. Synthesis and Physical Characterizations of Bio-Synthesized Fe3O4 Nanoparticles
3.2. Effect of Various Parameters on Cr(VI) Removal
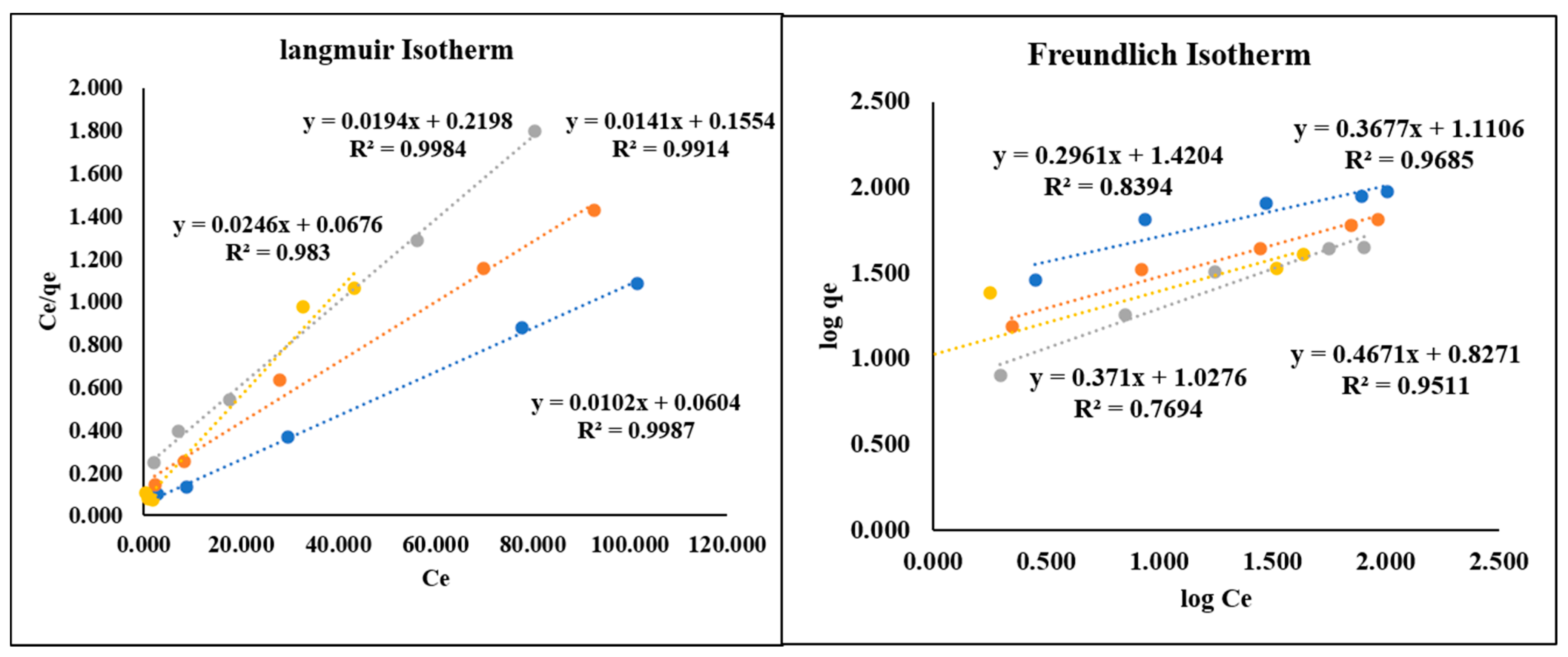
3.3. Kinetic and Adsorption Isotherm Studies
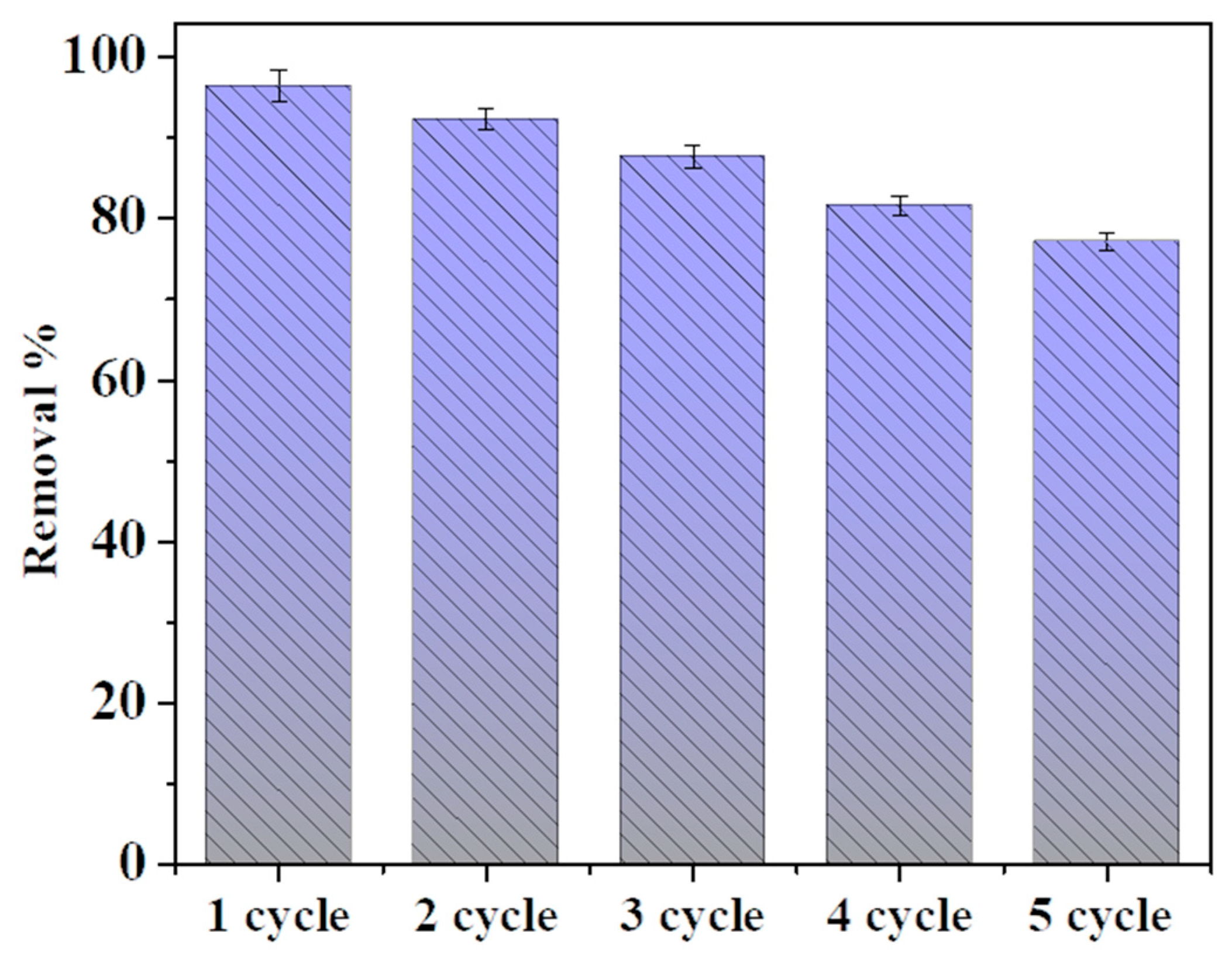
3.4. Adsorption Regeneration and Reuse
3.5. Cytotoxicity Studies on A549 Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic nanoparticles in cancer therapy and diagnosis. Adv. Healthc. Mater. 2020, 9, e1901058. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic nanoparticles in biology and medicine: Past, present, and future trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Naha, P.C.; Hsu, J.C.; Kim, J.; Shah, S.; Bouché, M.; Si-Mohamed, S.; Rosario-Berrios, D.N.; Douek, P.; Hajfathalian, M.; Yasini, P. Dextran-coated cerium oxide nanoparticles: A computed tomography contrast agent for imaging the gastrointestinal tract and inflammatory bowel disease. ACS Nano 2020, 14, 10187–10197. [Google Scholar] [CrossRef]
- Koudan, E.V.; Zharkov, M.N.; Gerasimov, M.V.; Karshieva, S.S.; Shirshova, A.D.; Chrishtop, V.V.; Kasyanov, V.A.; Levin, A.A.; Parfenov, V.A.; Karalkin, P.A. Magnetic patterning of tissue spheroids using polymer microcapsules containing iron oxide nanoparticles. ACS Biomater. Sci. Eng. 2021, 7, 5206–5214. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.S.; Rodrigues, M.T.; Domingues, R.M.; Costa, R.R.; Paz, E.; Rodríguez-Abreu, C.; Freitas, P.; Almeida, B.G.; Carvalho, M.A.; Gonçalves, C. Development of inhalable superparamagnetic iron oxide nanoparticles (SPIONs) in microparticulate system for antituberculosis drug delivery. Adv. Healthc. Mater. 2018, 7, 1800124. [Google Scholar] [CrossRef] [Green Version]
- Jabir, M.S.; Nayef, U.M.; Abdulkadhim, W.K.; Taqi, Z.J.; Sulaiman, G.M.; Sahib, U.I.; Al-Shammari, A.M.; Wu, Y.-J.; El-Shazly, M.; Su, C.C. Fe 3 O 4 nanoparticles capped with PEG induce apoptosis in breast cancer AMJ13 cells via mitochondrial damage and reduction of NF-κB translocation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1241–1259. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Xiang, H.; Xu, X.; Zou, J.; Lu, C. Biocompatible superparamagnetic europium-doped iron oxide nanoparticle clusters as multifunctional nanoprobes for multimodal in vivo imaging. ACS Appl. Mater. Interfaces 2021, 13, 33850–33861. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Zhang, M.; Tan, W.; Qiu, G.; Zheng, L. Improved removal capacity of magnetite for Cr(IV) by electrochemical reduction. J. Hazard. Mater. 2019, 374, 26–34. [Google Scholar] [CrossRef]
- Nuzhina, J.V.; Shtil, A.A.; Prilepskii, A.Y.; Vinogradov, V.V. Preclinical evaluation and clinical translation of magnetite-based nanomedicines. J. Drug Deliv. Sci. Technol. 2019, 54, 101282. [Google Scholar] [CrossRef]
- Lin, K.; Wang, L.; Tian, F.; Du, K.; Chang, Y.; Han, L.; Yao, P. A facile and controllable protocol for simultaneous synthesis of magnetite nanoparticles and luminescent carbon dots. J. Alloys Compd. 2018, 769, 360–366. [Google Scholar] [CrossRef]
- Mattei, J.-G.; Grammatikopoulos, P.; Zhao, J.; Singh, V.; Vernieres, J.; Steinhauer, S.; Porkovich, A.; Danielson, E.; Nordlund, K.; Djurabekova, F. Gas-phase synthesis of trimetallic nanoparticles. Chem. Mater. 2019, 31, 2151–2163. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods–a comparative review. Methods 2019, 31, 2151–2163. [Google Scholar] [CrossRef]
- Wang, L.; Dong, L.; Li, L.; Weng, Z.; Xu, H.; Yu, M.; Wang, Z. Fabrication of periodically micropatterned magnetite nanoparticles by laser-interference-controlled electrodeposition. J. Mater. Sci. 2018, 53, 3239–3249. [Google Scholar] [CrossRef] [Green Version]
- Darezereshki, E.; Darban, A.K.; Abdollahy, M.; Jamshidi, A. Synthesis of magnetite nanoparticles from iron ore tailings using a novel reduction-precipitation method. J. Alloys Compd. 2018, 749, 336–343. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Surendran, L.; Sudhagar, B.; DS, R.S.K.; Bupesh, G. Hydrothermal assisted Eichhornia crassipes mediated synthesis of magnetite nanoparticles (E-Fe3O4) and its antibiofilm activity. Mater. Res. Express 2019, 6, 095405. [Google Scholar]
- Kim, Y.; Roh, Y. Effects of microbial growth conditions on synthesis of magnetite nanoparticles using indigenous Fe (III)-reducing bacteria. Minerals 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, M.; Nouri, M.; Mohammadi, A.; Mosavari, N.; Constable, P. Preparation of immunomagnetic beads coupled with a rhodamine hydrazine immunosensor for the detection of Mycobacterium avium subspecies paratuberculosis in bovine feces, milk, and colostrum. J. Dairy Sci. 2021, 104, 6944–6960. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Jimenez-Lopez, C.; Neal, A.L.; Rull-Perez, F.; Rodriguez-Navarro, A.; Fernandez-Vivas, A.; Iañez-Pareja, E. Magnetite biomineralization induced by Shewanellaoneidensis. Geochim. Cosmochim. Acta 2010, 74, 967–979. [Google Scholar] [CrossRef]
- Klueglein, N.; Lösekann-Behrens, T.; Obst, M.; Behrens, S.; Appel, E.; Kappler, A. Magnetite formation by the novel Fe (III)-reducing Geothrixfermentans strain HradG1 isolated from a hydrocarbon-contaminated sediment with increased magnetic susceptibility. Geomicrobiol. J. 2013, 30, 863–873. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Balaji, R.; Renganathan, V.; Chen, S.M.; Singh, V. Selective and High-Performance Electrochemical Sensor for Cadmium Ions Based on Intimate Binary Spinel CoMn2O4 Nanostructures. ChemistrySelect 2019, 4, 13123–13130. [Google Scholar] [CrossRef]
- Ahmad, M.; Manzoor, K.; Ikram, S. Versatile nature of hetero-chitosan based derivatives as biodegradable adsorbent for heavy metal ions; a review. Int. J. Biol. Macromol. 2017, 105, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Kushima, A.; Halliday, C.; Zhou, J.; Li, J.; Hatton, T.A. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat. Commun. 2018, 9, 4701. [Google Scholar] [CrossRef]
- Wu, D.; Sui, Y.; He, S.; Wang, X.; Li, C.; Kong, H. Removal of trivalent chromium from aqueous solution by zeolite synthesized from coal fly ash. J. Hazard. Mater. 2008, 155, 415–423. [Google Scholar] [CrossRef]
- Elangovan, R.; Philip, L.; Chandraraj, K. Biosorption of hexavalent and trivalent chromium by palm flower (Borassus aethiopum). Chem. Eng. J. 2008, 141, 99–111. [Google Scholar] [CrossRef]
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- He, X.; Li, P. Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr 6+): Occurrence, sources and health risks. Expo. Health 2020, 12, 385–401. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Lei, C.; Chen, Y.-C. Evaluating the health costs of oral hexavalent chromium exposure from water pollution: A case study in Taiwan. J. Clean. Prod. 2018, 172, 819–826. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Long, F.-L.; Niu, C.-G.; Tang, N.; Guo, H.; Li, Z.-W.; Yang, Y.-Y.; Lin, L.-S. Highly efficient removal of hexavalent chromium from aqueous solution by calcined Mg/Al-layered double hydroxides/polyaniline composites. Chem. Eng. J. 2021, 404, 127084. [Google Scholar] [CrossRef]
- Daradmare, S.; Xia, M.; Kim, J.; Park, B.J. Metal–organic frameworks/alginate composite beads as effective adsorbents for the removal of hexavalent chromium from aqueous solution. Chemosphere 2021, 270, 129487. [Google Scholar] [CrossRef]
- Ahmad, W.; Qaiser, S.; Ullah, R.; Mohamed Jan, B.; Karakassides, M.A.; Salmas, C.E.; Kenanakis, G.; Ikram, R. Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater. Materials 2021, 14, 34. [Google Scholar] [CrossRef]
- Duan, W.; Chen, G.; Chen, C.; Sanghvi, R.; Iddya, A.; Walker, S.; Liu, H.; Ronen, A.; Jassby, D. Electrochemical removal of hexavalent chromium using electrically conducting carbon nanotube/polymer composite ultrafiltration membranes. J. Membr. Sci. 2017, 531, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liu, Z.; Zeng, G.; Liu, Y.; Shao, B.; Li, Z.; Liu, Y.; Zhang, W.; He, Q. Polyaniline-based adsorbents for removal of hexavalent chromium from aqueous solution: A mini review. Environ. Sci. Pollut. Res. 2018, 25, 6158–6174. [Google Scholar] [CrossRef]
- Neagu, V.; Mikhalovsky, S. Removal of hexavalent chromium by new quaternized crosslinked poly (4-vinylpyridines). J. Hazard. Mater. 2010, 183, 533–540. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Kakan, S.S.; Rajesh, N. A novel amine impregnated graphene oxide adsorbent for the removal of hexavalent chromium. Chem. Eng. J. 2013, 230, 328–337. [Google Scholar] [CrossRef]
- Vilardi, G.; Ochando-Pulido, J.M.; Verdone, N.; Stoller, M.; Di Palma, L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J. Clean. Prod. 2018, 190, 200–210. [Google Scholar] [CrossRef]
- Cheng, S.; Pan, X.; Zhang, C.; Lin, X.; Zhuang, Q.; Jiao, Y.; Dong, W.; Qi, X. UV-assisted ultrafast construction of robust Fe3O4/polydopamine/Ag Fenton-like catalysts for highly efficient micropollutant decomposition. Sci. Total. Environ. 2021, 151182. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.M.; Baloch, H.A.; Abdullah, E.C.; Mazari, S.A.; Griffin, G.J.; Srinivasan, M.P.; Tanksale, A. Iron oxide nanomaterials for the removal of heavy metals and dyes from wastewater. Nanoscale Mater. Water Purif. 2019, 2019, 447–472. [Google Scholar]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Li, F.; Dang, Z.; Zhang, L. Rapid and efficient removal of Cr(IV) by a core–shell magnetic mesoporous polydopamine nanocomposite: Roles of the mesoporous structure and redox-active functional groups. J. Mater. Chem. A 2021, 9, 13306–13319. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, C.; Li, J.; Pan, X.; Zhai, X.; Jiao, Y.; Li, Y.; Dong, W.; Qi, X. Highly efficient removal of antibiotic from biomedical wastewater using Fenton-like catalyst magnetic pullulan hydrogels. Carbohydr. Polym. 2021, 262, 117951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-inspired surface engineering for water-remediation materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef] [Green Version]
- Donot, F.; Fontana, A.; Baccou, J.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process. Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Madhuri, K.; Prabhakar, K.V. Microbial exopolysaccharides: Biosynthesis and potential applications. Orient. J. Chem. 2014, 30, 1401. [Google Scholar] [CrossRef] [Green Version]
- Velusamy, P.; Awad, Y.; Abd El-Azeem, S.; Ok, Y. Screening of heavy metal resistant bacteria isolated from hydrocarbon contaminated soil in Korea. J. Agric. Life Environ. Sci. 2011, 23, 40–43. [Google Scholar]
- Samuel, M.S.; Chidambaram, R. Hexavalent chromium biosorption studies using Penicillium griseofulvum MSR1 a novel isolate from tannery effluent site: Box–Behnken optimization, equilibrium, kinetics and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2015, 49, 156–164. [Google Scholar]
- Samuel, M.S.; Selvarajan, E.; Subramaniam, K.; Mathimani, T.; Seethappan, S.; Pugazhendhi, A. Synthesized β-cyclodextrin modified graphene oxide (β-CD-GO) composite for adsorption of cadmium and their toxicity profile in cervical cancer (HeLa) cell lines. Process. Biochem. 2020, 93, 28–35. [Google Scholar] [CrossRef]
- Chidambaram, R. Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr(IV) using fungal biomass. PLoS ONE 2015, 10, e0116884. [Google Scholar]
- Abdeen, M.; Sabry, S.; Ghozlan, H.; El-Gendy, A.A.; Carpenter, E.E. Microbial-physical synthesis of Fe and Fe3O4 magnetic nanoparticles using Aspergillus niger YESM1 and supercritical condition of ethanol. J. Nanomater. 2016, 2016, 9174891. [Google Scholar] [CrossRef] [Green Version]
- Razak, M.N.A.; Ibrahim, M.F.; Yee, P.L.; Hassan, M.A.; Abd-Aziz, S. Utilization of oil palm decanter cake for cellulase and polyoses production. Biotechnol. Bioprocess Eng. 2012, 17, 547–555. [Google Scholar] [CrossRef]
- Asuha, S.; Zhao, S.; Wu, H.; Song, L.; Tegus, O. One step synthesis of maghemite nanoparticles by direct thermal decomposition of Fe–urea complex and their properties. J. Alloys Compd. 2009, 472, L23–L25. [Google Scholar] [CrossRef]
- Aydın, Y.A.; Aksoy, N.D. Adsorption of chromium on chitosan: Optimization, kinetics and thermodynamics. Chem. Eng. J. 2009, 151, 188–194. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Sun, M.; Qiu, H.; Li, X.; Duan, H.; Luo, C. Adsorbent for chromium removal based on graphene oxide functionalized with magnetic cyclodextrin–chitosan. Colloids Surf. B Biointerfaces 2013, 107, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Bhattacharya, J.; Raj, S.; Santhanam, N.; Singh, H.; Singh, N.P. Efficient removal of Chromium (VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. Int. J. Biol. Macromol. 2019, 121, 285–292. [Google Scholar] [CrossRef]
- Samuel, M.S.; Datta, S.; Khandge, R.S.; Selvarajan, E. A state of the art review on characterization of heavy metal binding metallothioneins proteins and their widespread applications. Sci. Total Environ. 2021, 775, 145829. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Chidambaram, R.; Patel, H.; Brindhadevi, K. Clean approach for chromium removal in aqueous environments and role of nanomaterials in bioremediation: Present research and future perspective. Chemosphere 2021, 284, 131368. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Shah, S.S.; Bhattacharya, J.; Subramaniam, K.; Pradeep Singh, N.D. Adsorption of Pb(II) from aqueous solution using a magnetic chitosan/graphene oxide composite and its toxicity studies. Int. J. Biol. Macromol. 2018, 303, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Subramaniyan, V.; Bhattacharya, J.; Chidambaram, R.; Qureshi, T.; Pradeep Singh, N.D. Ultrasonic-assisted synthesis of graphene oxide—fungal hyphae: An efficient and reclaimable adsorbent for chromium(VI) removal from aqueous solution. Ultrason. Sonochem. 2018, 48, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-j.; Wang, J.-s.; Liu, Y.-g.; Li, X.; Zeng, G.-m.; Bao, Z.-l.; Zeng, X.-x.; Chen, A.-w.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, R. Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr (VI) using fungal biomass. PloS ONE 2015, 10, e0116884. [Google Scholar]
- Tianwei, T.; Xiaojing, H.; Weixia, D. Adsorption behaviour of metal ions on imprinted chitosan resin. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2001, 76, 191–195. [Google Scholar] [CrossRef]
- Thinh, N.N.; Hanh, P.T.B.; Hoang, T.V.; Hoang, V.D.; Van Khoi, N.; Dai Lam, T. Magnetic chitosan nanoparticles for removal of Cr (VI) from aqueous solution. Mater. Sci. Eng. C 2013, 33, 1214–1218. [Google Scholar] [CrossRef]
- Samuel, M.S.; Subramaniyan, V.; Bhattacharya, J.; Parthiban, C.; Chand, S.; Singh, N.P. A GO-CS@ MOF [Zn (BDC)(DMF)] material for the adsorption of chromium (VI) ions from aqueous solution. Compos. Part B Eng. 2018, 152, 116–125. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Tay, W.J.D.; Hidajat, K.; Uddin, M.S. Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr. Polym. 2013, 91, 322–332. [Google Scholar] [CrossRef]
- Wen, T.; Fan, Q.; Tan, X.; Chen, Y.; Chen, C.; Xu, A.; Wang, X. A core–shell structure of polyaniline coated protonic titanate nanobelt composites for both Cr (VI) and humic acid removal. Polym. Chem. 2016, 7, 785–794. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhao, G.; Song, G.; Chen, D.; Chen, H.; Xie, J.; Hayat, T.; Alsaedi, A.; Wang, X. Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium (VI) from aqueous solutions. Chem. Eng. J. 2018, 334, 569–578. [Google Scholar] [CrossRef]
- Samuel, M.S.; Shah, S.S.; Subramaniyan, V.; Qureshi, T.; Bhattacharya, J.; Singh, N.P. Preparation of graphene oxide/chitosan/ferrite nanocomposite for Chromium (VI) removal from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Wang, J.; Yu, S.; Chen, Z.; Hayat, T.; Wang, X. Magnetic porous carbonaceous material produced from tea waste for efficient removal of As (V), Cr (VI), humic acid, and dyes. ACS Sustain. Chem. Eng. 2017, 5, 4371–4380. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, M.S.; Datta, S.; Chandrasekar, N.; Balaji, R.; Selvarajan, E.; Vuppala, S. Biogenic Synthesis of Iron Oxide Nanoparticles Using Enterococcus faecalis: Adsorption of Hexavalent Chromium from Aqueous Solution and In Vitro Cytotoxicity Analysis. Nanomaterials 2021, 11, 3290. https://doi.org/10.3390/nano11123290
Samuel MS, Datta S, Chandrasekar N, Balaji R, Selvarajan E, Vuppala S. Biogenic Synthesis of Iron Oxide Nanoparticles Using Enterococcus faecalis: Adsorption of Hexavalent Chromium from Aqueous Solution and In Vitro Cytotoxicity Analysis. Nanomaterials. 2021; 11(12):3290. https://doi.org/10.3390/nano11123290
Chicago/Turabian StyleSamuel, Melvin S., Saptashwa Datta, Narendhar Chandrasekar, Ramachandran Balaji, Ethiraj Selvarajan, and Srikanth Vuppala. 2021. "Biogenic Synthesis of Iron Oxide Nanoparticles Using Enterococcus faecalis: Adsorption of Hexavalent Chromium from Aqueous Solution and In Vitro Cytotoxicity Analysis" Nanomaterials 11, no. 12: 3290. https://doi.org/10.3390/nano11123290
APA StyleSamuel, M. S., Datta, S., Chandrasekar, N., Balaji, R., Selvarajan, E., & Vuppala, S. (2021). Biogenic Synthesis of Iron Oxide Nanoparticles Using Enterococcus faecalis: Adsorption of Hexavalent Chromium from Aqueous Solution and In Vitro Cytotoxicity Analysis. Nanomaterials, 11(12), 3290. https://doi.org/10.3390/nano11123290








