Recent Breakthroughs and Advancements in NOx and SOx Reduction Using Nanomaterials-Based Technologies: A State-of-the-Art Review
Abstract
1. Introduction
2. NOx and SOx Emission from Combustion
2.1. Adverse Effect of NOx
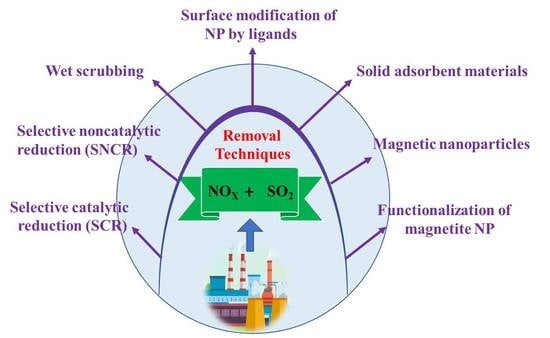
2.2. NOx Treatment Techniques
2.2.1. Selective Catalytic Reduction (SCR)
2.2.2. Selective Noncatalytic Reduction (SNCR)
2.2.3. Limitations of SCR and SNCR
2.2.4. Common Solid Adsorbent Materials
3. Low Temperature-Based Abatement Technique
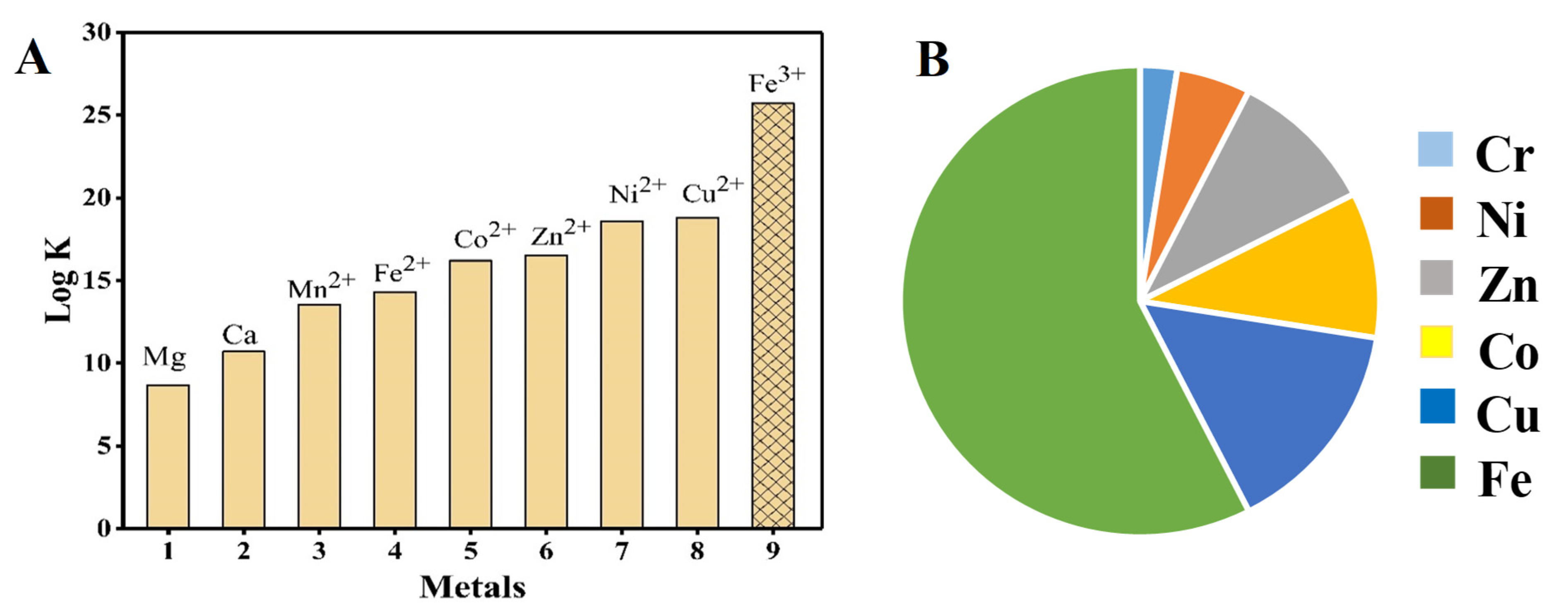
3.1. Metal Ligand Absorption
3.2. Metal Ligand Stability
3.3. Principle of Gas Absorption
4. Wet Scrubbing
4.1. Chemically Absorption by Metal-Ligand System
4.2. NOx Removal by Different Techniques
5. Challenges of Wet Scrubbing Method
5.1. Regeneration of Fe-EDTA
5.2. Secondary Pollutant
6. Drawback of Wet Scrubbing
7. Iron Oxide Nanoparticles
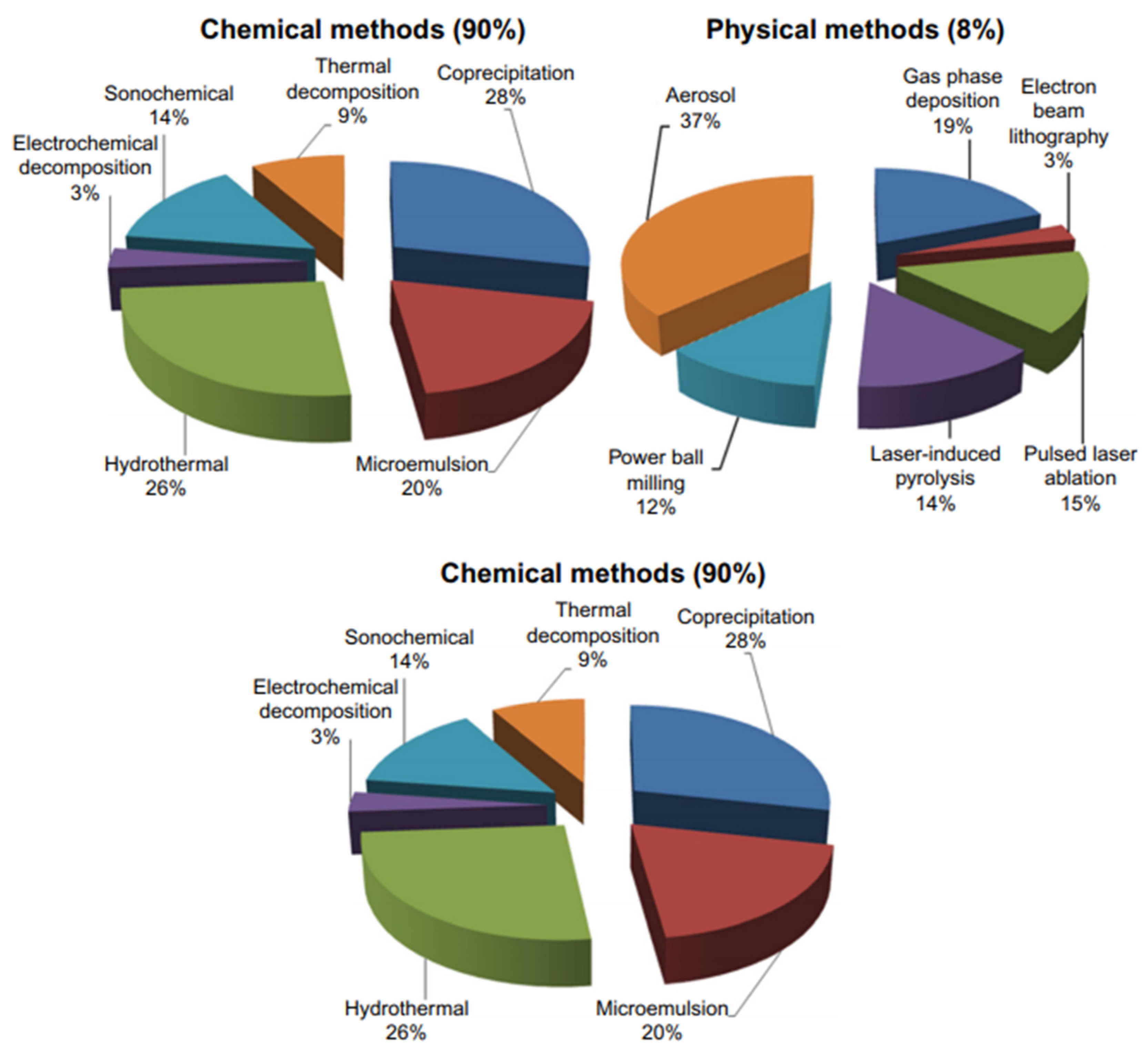
7.1. Synthesis of Magnetic Iron-Oxide Nanoparticles
7.2. Solvothermal Synthesis
7.3. LaMer and Dinegar Model
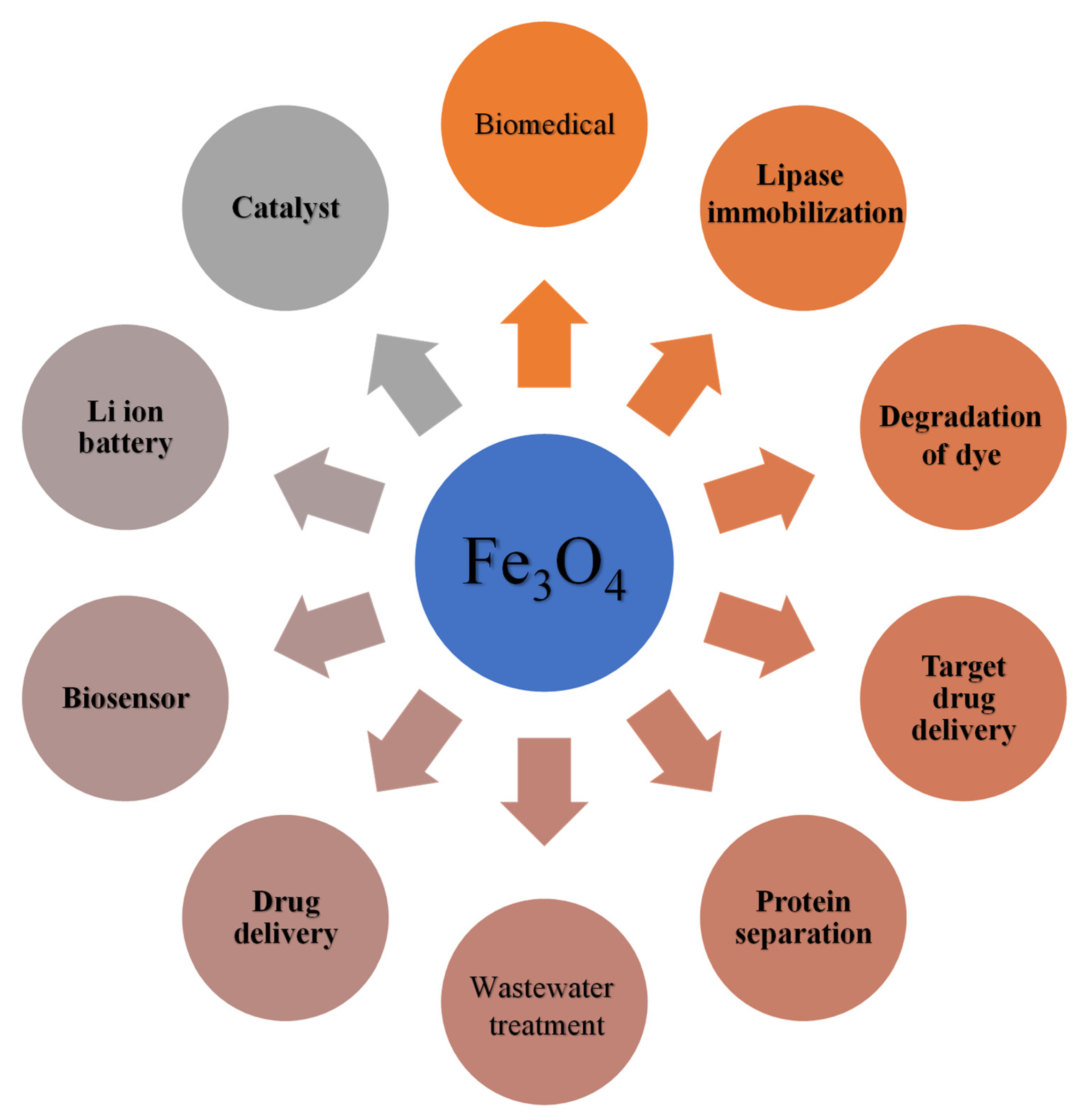
8. General Applications of Magnetite (Fe3O4)
9. Functionalization of Magnetite NP
9.1. Functionalized Magnetic NP for NOx and SOx Removal
9.2. Surface Modification of NP by Ligands
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Suchecki, T.; Kumazawa, H. Application of hydrazine to regeneration of post-absorption solutions in combined SO2/NOx removal from flue gases by a complex method. Sep. Technol. 1994, 4, 763–770. [Google Scholar]
- Sharif, H.M.A.; Li, T.; Mahmood, N.; Ahmad, M.; Xu, J.; Mahmood, A.; Djellabi, R.; Yang, B. Thermally Activated Epoxy-functionalized Carbon as an Electrocatalyst for Efficient NOx Reduction. Carbon 2021, 182, 516–524. [Google Scholar] [CrossRef]
- Manconi, I.; van der Maas, P.; Lens, P.N. Effect of sulfur compounds on biological reduction of nitric oxide in aqueous Fe(II)EDTA2− solutions. Nitric Oxide 2006, 15, 40–49. [Google Scholar] [CrossRef]
- Raza, S.; Zhang, J.; Ali, I.; Li, X.; Liu, C. Recent trends in the development of biomass-based polymers from renewable resources and their environmental applications. J. Taiwan Inst. Chem. Eng. 2020, 115, 293–303. [Google Scholar] [CrossRef]
- Ruth, L.A. Energy from municipal solid waste: A comparison with coal combustion technology. Prog. Energy Combust. Sci. 1998, 24, 545–564. [Google Scholar] [CrossRef]
- Park, J.-H.; Ahn, J.-W.; Kim, K.-H.; Son, Y.-S. Historic and futuristic review of electron beam technology for the treatment of SO2 and NOx in flue gas. Chem. Eng. J. 2019, 355, 351–366. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Cheng, H.-Y.; Haider, M.R.; Khan, K.; Yang, L.; Wang, A.-J. NO Removal with Efficient Recovery of N2O by Using Recyclable Fe3O4@EDTA@Fe(II) Complex: A Novel Approach toward Resource Recovery from Flue Gas. Environ. Sci. Technol. 2018, 53, 1004–1013. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, R.; Yuan, B.; Jiang, J. Simultaneous removal of SO2, NO and Hg 0 through an integrative process utilizing a cost-effective complex oxidant. J. Hazard. Mater. 2016, 301, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Rownaghi, A.A.; Monjezi, S.; Lively, R.P.; Jones, C.W. SOx/NOx Removal from Flue Gas Streams by Solid Adsorbents: A Review of Current Challenges and Future Directions. Energy Fuels 2015, 29, 5467–5486. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Farooq, M.; Hussain, I.; Ali, M.; Mujtaba, M.; Sultan, M.; Yang, B. Recent innovations for scaling up microbial fuel cell systems: Significance of physicochemical factors for electrodes and membranes materials. J. Taiwan Inst. Chem. Eng. 2021, 129, 207–226. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.; Fatah, N.; Hamid, M.; Ibrahim, M.; Aziz, M.; Setiabudi, H. A highly competitive system for CO methanation over an active metal-free fibrous silica mordenite via in-situ ESR and FTIR studies. Energy Convers. Manag. 2020, 211, 112754. [Google Scholar] [CrossRef]
- Ramachandran, B.; Herman, R.G.; Choi, S.; Stenger, H.G.; Lyman, C.E.; Sale, J.W. Testing zeolite SCR catalysts under protocol conditions for NOx abatement from stationary emission sources. Catal. Today 2000, 55, 281–290. [Google Scholar] [CrossRef]
- Sims, R.E.; Rogner, H.-H.; Gregory, K. Carbon emission and mitigation cost comparisons between fossil fuel, nuclear and renewable energy resources for electricity generation. Energy Policy 2003, 31, 1315–1326. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.; Hamid, M.; Hassan, N. Recent advances in catalytic systems in the prism of physicochemical properties to remediate toxic CO pollutants: A state-of-the-art review. Chemosphere 2021, 277, 130285. [Google Scholar] [CrossRef]
- Richter, A.; Burrows, J.P.; Nüss, H.; Granier, C.; Niemeier, U. Increase in tropospheric nitrogen dioxide over China observed from space. Nat. Cell Biol. 2005, 437, 129–132. [Google Scholar] [CrossRef]
- Guo, Q.; He, Y.; Sun, T.; Wang, Y.; Jia, J. Simultaneous removal of NOx and SO2 from flue gas using combined Na2SO3 assisted electrochemical reduction and direct electrochemical reduction. J. Hazard. Mater. 2014, 276, 371–376. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of Urban Fine Particulate Matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Yasukawa, R.; Tabata, K.; Ueda, K.; Niwa, M. A highly durable catalytic NOx reduction in the presence of SOx using periodic two steps, an operation in oxidizing conditions and a relatively short operation in reducing conditions. Appl. Catal. B Environ. 1999, 21, 121–131. [Google Scholar] [CrossRef]
- Muzio, L.; Quartucy, G. Implementing NOx control: Research to application. Prog. Energy Combust. Sci. 1997, 23, 233–266. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Mahmood, A.; Djellabi, R.; Cheng, H.-Y.; Dong, H.; Ajibade, F.O.; Ali, I.; Yang, B.; Wang, A.-J. Utilization of electrochemical treatment and surface reconstruction to achieve long lasting catalyst for NOx removal. J. Hazard. Mater. 2021, 401, 123440. [Google Scholar] [CrossRef]
- Shi, K.; Liang, B.; Guo, Q.; Zhao, Y.; Sharif, H.M.A.; Li, Z.; Chen, E.; Wang, A. Accelerated bioremediation of a complexly contaminated river sediment through ZVI-electrode combined stimulation. J. Hazard. Mater. 2021, 413, 125392. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Dora, J.; Gostomczyk, M.A.; Jakubiak, M.; Kordylewski, W.; Mista, W.; Tkaczuk, M. Parametric studies of the effectiveness of NO oxidation process by ozone. Chem. Process Eng. 2009, 30, 621–634. [Google Scholar]
- Pasel, J.; Käßner, P.; Montanari, B.; Gazzano, M.; Vaccari, A.; Makowski, W.; Lojewski, T.; Dziembaj, R.; Papp, H. Transition metal oxides supported on active carbons as low temperature catalysts for the selective catalytic reduction (SCR) of NO with NH. Appl. Catal. B Environ. 1998, 18, 199–213. [Google Scholar] [CrossRef]
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef]
- Nakajima, F.; Hamada, I. The state-of-the-art technology of NOx control. Catal. Today 1996, 29, 109–115. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.A.; Hassan, N.S.; Farooq, M.; Mujtaba, M.A.; Hamid, M.Y.S.; Sharif, H.M.A.; Nabgan, W.; Aziz, M.A.H.; Owgi, A.H.K. Contemporary thrust and emerging prospects of catalytic systems for substitute natural gas production by CO methanation. Fuel 2021, 122604. [Google Scholar] [CrossRef]
- Murayama, T.; Chen, J.; Hirata, J.; Matsumoto, K.; Ueda, W. Hydrothermal synthesis of octahedra-based layered niobium oxide and its catalytic activity as a solid acid. Catal. Sci. Technol. 2014, 4, 4250–4257. [Google Scholar] [CrossRef]
- Okazaki, S.; Kuroha, H.; Okuyama, T. Effect of Nb2o5addition on the Catalytic Activity of Feoxfor Reduction of Noxwith Nh3and O. Chem. Lett. 1985, 14, 45–48. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Si, Z.; Weng, D.; Ma, J.; Xu, T. Impacts of niobia loading on active sites and surface acidity in NbOx/CeO2–ZrO2 NH3–SCR catalysts. Appl. Catal. B Environ. 2015, 179, 380–394. [Google Scholar] [CrossRef]
- Mosrati, J.; Atia, H.; Eckelt, R.; Lund, H.; Agostini, G.; Bentrup, U.; Rockstroh, N.; Keller, S.; Armbruster, U.; Mhamdi, M. Nb-Modified Ce/Ti Oxide Catalyst for the Selective Catalytic Reduction of NO with NH3 at Low Temperature. Catalysts 2018, 8, 175. [Google Scholar] [CrossRef]
- Li, W.; Guo, R.-T.; Wang, S.-X.; Pan, W.-G.; Chen, Q.-L.; Li, M.-Y.; Sun, P.; Liu, S.-M. The enhanced Zn resistance of Mn/TiO2 catalyst for NH3-SCR reaction by the modification with Nb. Fuel Process. Technol. 2016, 154, 235–242. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; He, H.; Shi, X.; Mo, J.; Wu, Z. Manganese–niobium mixed oxide catalyst for the selective catalytic reduction of NOx with NH3 at low temperatures. Chem. Eng. J. 2014, 250, 390–398. [Google Scholar] [CrossRef]
- Javed, M.T.; Irfan, N.; Gibbs, B. Control of combustion-generated nitrogen oxides by selective non-catalytic reduction. J. Environ. Manag. 2007, 83, 251–289. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Mahmood, N.; Wang, S.; Hussain, I.; Hou, Y.-N.; Yang, L.-H.; Zhao, X.; Yang, B. Recent advances in hybrid wet scrubbing techniques for NOx and SO2 removal: State of the art and future research. Chemosphere 2021, 273, 129695. [Google Scholar] [CrossRef]
- Miller, B.G. Coal Energy Systems; Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Krum, K.R.K.; Jensen, M.; Li, S.; Norman, T.; Marshall, P.; Wu, H.; Glarborg, P. Selective Noncatalytic Reduction of NOx Using Ammonium Sulfate. Energy Fuels 2021, 35, 12392–12402. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.-Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NO x with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Chien, T.-W.; Chu, H. Removal of SO2 and NO from flue gas by wet scrubbing using an aqueous NaClO2 solution. J. Hazard. Mater. 2000, 80, 43–57. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, W.; Wu, Z. Simultaneous absorption of NO and SO2 by FeIIEDTA combined with Na2SO3 solution. Chem. Eng. J. 2007, 132, 227–232. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wang, Y.; Lyu, Y.-K. Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem. Eng. J. 2019, 361, 1161–1172. [Google Scholar] [CrossRef]
- Jin, D.-S.; Deshwal, B.-R.; Park, Y.-S.; Lee, H.-K. Simultaneous removal of SO2 and NO by wet scrubbing using aqueous chlorine dioxide solution. J. Hazard. Mater. 2006, 135, 412–417. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn–Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Eubank, J.F.; Wuttke, S.; Xiao, B.; Wheatley, P.S.; Bazin, P.; LaValley, J.-C.; Daturi, M.; Vimont, A.; De Weireld, G.; et al. Nitric Oxide Adsorption and Delivery in Flexible MIL-88(Fe) Metal–Organic Frameworks. Chem. Mater. 2013, 25, 1592–1599. [Google Scholar] [CrossRef]
- Ma, C.; Yi, H.; Tang, X.; Zhao, S.; Yang, K.; Song, L.; Zhang, Y.; Wang, Y. Improving simultaneous removal efficiency of SO2 and NOx from flue gas by surface modification of MgO with organic component. J. Clean. Prod. 2019, 230, 508–517. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Zhao, C. CO2 Absorption Using Dry Potassium-Based Sorbents with Different Supports. Energy Fuels 2009, 23, 4683–4687. [Google Scholar] [CrossRef]
- Chang, J.C.S.; Kaplan, N. SO2 removal by limestone dual alkali. Environ. Prog. 1984, 3, 267–274. [Google Scholar] [CrossRef]
- Barman, S.; Philip, L. Integrated System for the Treatment of Oxides of Nitrogen from Flue Gases. Environ. Sci. Technol. 2006, 40, 1035–1041. [Google Scholar] [CrossRef]
- Koh, H.K.; Geller, A.C.; VanderWeele, T.J. Deaths from COVID-19. JAMA 2021, 325, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Yang, X.; Chen, Y.; Hu, X.; Wu, X. CeMn/TiO2 catalysts prepared by different methods for enhanced low-temperature NH3-SCR catalytic performance. Chem. Eng. Sci. 2021, 238, 116588. [Google Scholar] [CrossRef]
- Djellabi, R.; Yang, B.; Xiao, K.; Gong, Y.; Cao, D.; Sharif, H.M.A.; Zhao, X.; Zhu, C.; Zhang, J. Unravelling the mechanistic role of Ti-O-C bonding bridge at titania/lignocellulosic biomass interface for Cr(VI) photoreduction under visible light. J. Colloid Interface Sci. 2019, 553, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhou, L.; Li, W.; Hu, D.; Wen, J.; Huang, B. Improving the Performance of Gd Addition on Catalytic Activity and SO2 Resistance over MnOx/ZSM-5 Catalysts for Low-Temperature NH3-SCR. Catalysts 2021, 11, 324. [Google Scholar] [CrossRef]
- Cooper, C.E. Nitric oxide and iron proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 290–309. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H.; Machida, H. Absorption of dilute NO into aqueous solutions of Na2SO3 with added Fe(II)NTA and reduction kinetics of Fe(III)NTA by Na2SO. Ind. Eng. Chem. Res. 1987, 26, 2016–2019. [Google Scholar] [CrossRef]
- Littlejohn, D.; Chang, S.G. Reaction of ferrous chelate nitrosyl complexes with sulfite and bisulfite ions. Ind. Eng. Chem. Res. 1990, 29, 10–14. [Google Scholar] [CrossRef][Green Version]
- van der Maas, P.; Peng, S.; Klapwijk, B.; Lens, P. Enzymatic versus Nonenzymatic Conversions during the Reduction of EDTA-Chelated Fe(III) in BioDeNOx Reactors. Environ. Sci. Technol. 2005, 39, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-F.; Hou, Y.-N.; Guo, J.; Sharif, H.M.A.; Huang, C.; Zhao, J.; Li, H.; Song, Y.; Lu, C.; Han, Y.; et al. Rational design of biogenic PdxAuy nanoparticles with enhanced catalytic performance for electrocatalysis and azo dyes degradation. Environ. Res. 2022, 204, 112086. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, J.; Li, M.; Zhang, S.; Li, S.; Li, W. Bioelectrochemical Reduction of Fe(II)EDTA–NO in a Biofilm Electrode Reactor: Performance, Mechanism, and Kinetics. Environ. Sci. Technol. 2016, 50, 3846–3851. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.H.; Harkness, J.B.L. Enhanced flue-gas denitrification using ferrous.cntdot.EDTA and a polyphenolic compound in an aqueous scrubber system. Energy Fuels 1991, 5, 244–248. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H.; Takada, Y. Chemical reactions accompanying absorption of nitric oxide into aqueous mixed solutions of iron(II)-EDTA and sodium sulfite. Ind. Eng. Chem. Fundam. 1984, 23, 60–64. [Google Scholar] [CrossRef]
- Buisman, C.J.N.; Dijkman, H.; Verbraak, P.L.; Den Hartog, A.J. Process for Purifying Flue Gas Containing Nitrogen Oxides. U.S. Patent 5,891,408, 6 April 1999. [Google Scholar]
- Sada, E.; Kumazawa, H.; Kudo, I.; Kondo, T. Individual and Simultaneous Absorption of Dilute NO and SO2 in Aqueous Slurries of MgSO3 with FeII-EDTA. Ind. Eng. Chem. Process. Des. Dev. 1980, 19, 377–382. [Google Scholar] [CrossRef]
- Maas, P.V.D.; Van de Sandt, T.; Klapwijk, B.; Lens, P. Biological reduction of nitric oxide in aqueous Fe(II) EDTA solutions. Biotechnol. Prog. 2003, 19, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, L.-L.; Mi, X.-H.; Jiang, J.-L.; Li, W. NOxRemoval from Simulated Flue Gas by Chemical Absorption−Biological Reduction Integrated Approach in a Biofilter. Environ. Sci. Technol. 2008, 42, 3814–3820. [Google Scholar] [CrossRef]
- Mahmood, A. Photovoltaic and Charge Transport Behavior of Diketopyrrolopyrrole Based Compounds with A–D–A–D–A Skeleton. J. Clust. Sci. 2019, 30, 1123–1130. [Google Scholar] [CrossRef]
- Suchecki, T.T.; Mathews, B.; Kumazawa, H. Kinetic Study of Ambient-Temperature Reduction of FeIIIedta by Na2S2O. Ind. Eng. Chem. Res. 2005, 44, 4249–4253. [Google Scholar] [CrossRef]
- Mok, Y.S.; Lee, H.-J. Removal of sulfur dioxide and nitrogen oxides by using ozone injection and absorption–reduction technique. Fuel Process. Technol. 2006, 87, 591–597. [Google Scholar] [CrossRef]
- Mogili, P.; Kleiber, P.; Young, M.; Grassian, V. N2O5 hydrolysis on the components of mineral dust and sea salt aerosol: Comparison study in an environmental aerosol reaction chamber. Atmos. Environ. 2006, 40, 7401–7408. [Google Scholar] [CrossRef]
- Daniel, A.L.; Dilip, R. Ahuja. Relative contributions of greenhouse gas emissions to global warming. Nature 1990, 344, 529–531. [Google Scholar]
- Usman, M.; Byrne, J.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef]
- Kong, L.; Lu, X.; Bian, X.; Zhang, W.; Wang, C. Constructing Carbon-Coated Fe3O4 Microspheres as Antiacid and Magnetic Support for Palladium Nanoparticles for Catalytic Applications. ACS Appl. Mater. Interfaces 2011, 3, 35–42. [Google Scholar] [CrossRef]
- Ali, I.; Li, J.; Peng, C.; Qasim, M.; Khan, Z.M.; Naz, I.; Sultan, M.; Rauf, M.; Iqbal, W.; Sharif, H.M.A. 3-Dimensional membrane capsules: Synthesis modulations for the remediation of environmental pollutants—A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 10, 1–62. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, C.; Meng, D.; Diao, G. In situ synthesis of silver nanostructures on magnetic Fe3O4@C core–shell nanocomposites and their application in catalytic reduction reactions. J. Mater. Chem. A 2013, 1, 2118–2125. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.-J. Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications. Chem. Rev. 2019, 119, 1666–1762. [Google Scholar] [CrossRef] [PubMed]
- Palchoudhury, S.; An, W.; Xu, Y.; Qin, Y.; Zhang, Z.; Chopra, N.; Holler, R.A.; Turner, C.H.; Bao, Y. Synthesis and Growth Mechanism of Iron Oxide Nanowhiskers. Nano Lett. 2011, 11, 1141–1146. [Google Scholar] [CrossRef]
- Vreeland, E.C.; Watt, J.; Schober, G.B.; Hance, B.G.; Austin, M.J.; Price, A.D.; Fellows, B.D.; Monson, T.C.; Hudak, N.S.; Maldonado-Camargo, L.; et al. Enhanced Nanoparticle Size Control by Extending LaMer’s Mechanism. Chem. Mater. 2015, 27, 6059–6066. [Google Scholar] [CrossRef]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Nizamuddin, S.; Baloch, H.A.; Mubarak, N.M.; Dumbre, D.K.; Inamuddin, A.A.M.; Bhutto, A.W.; Srinivasan, M.; Griffin, G.J. Synthesis of magnetic carbon nanocomposites by hydrothermal carbonization and pyrolysis. Environ. Chem. Lett. 2018, 16, 821–844. [Google Scholar] [CrossRef]
- Devi, S.M.; Nivetha, A.; Prabha, I. Superparamagnetic Properties and Significant Applications of Iron Oxide Nanoparticles for Astonishing Efficacy—A Review. J. Supercond. Nov. Magn. 2019, 32, 127–144. [Google Scholar] [CrossRef]
- Ali, J.; Wang, L.; Waseem, H.; Sharif, H.M.A.; Djellabi, R.; Zhang, C.; Pan, G. Bioelectrochemical recovery of silver from wastewater with sustainable power generation and its reuse for biofouling mitigation. J. Clean. Prod. 2019, 235, 1425–1437. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.-L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Mahmood, A.; Cheng, H.-Y.; Djellabi, R.; Ali, J.; Jiang, W.-L.; Wang, S.-S.; Haider, M.R.; Mahmood, N.; Wang, A.-J. Fe3O4 Nanoparticles Coated with EDTA and Ag Nanoparticles for the Catalytic Reduction of Organic Dyes from Wastewater. ACS Appl. Nano Mater. 2019, 2, 5310–5319. [Google Scholar] [CrossRef]
- Yang, L.-H.; Cheng, H.-Y.; Ding, Y.-C.; Su, S.-G.; Wang, B.; Zeng, R.; Sharif, H.M.A.; Wang, A.-J. Enhanced treatment of coal gasification wastewater in a membraneless sleeve-type bioelectrochemical system. Bioelectrochemistry 2019, 129, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, T.; Wu, C.; Qiu, L.; Hu, R.; Li, J.; Cansiz, S.; Zhang, L.; Cui, C.; Zhu, G.; et al. Facile Surface Functionalization of Hydrophobic Magnetic Nanoparticles. J. Am. Chem. Soc. 2014, 136, 12552–12555. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Wang, J.-L. Machine learning for high performance organic solar cells: Current scenario and future prospects. Energy Environ. Sci. 2021, 14, 90–105. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Yu, X.-Y.; Jin, Z.; Jia, Y.; Xu, W.-H.; Luo, T.; Zhu, B.-J.; Liu, J.-H.; Huang, X.-J. Ultra high adsorption capacity of fried egg jellyfish-like γ-AlOOH(Boehmite)@SiO2/Fe3O4 porous magnetic microspheres for aqueous Pb(II) removal. J. Mater. Chem. 2011, 21, 16550–16557. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, J.-L. A time and resource efficient machine learning assisted design of non-fullerene small molecule acceptors for P3HT-based organic solar cells and green solvent selection. J. Mater. Chem. A 2021, 9, 15684–15695. [Google Scholar] [CrossRef]
- Wang, H.-M.; Ma, Y.-P.; Chen, X.-Y.; Xu, S.-Y.; Chen, J.-D.; Zhang, Q.-L.; Zhao, B.; Ning, P. Promoting effect of SO2−4 functionalization on the performance of Fe2O3 catalyst in the selective catalytic reduction of NOx with NH3. J. Fuel Chem. Technol. 2020, 48, 584–593. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.-p.; Qin, S.-n.; Guo, J.-r.; Zhang, Y.-q.; Wang, J.-c.; Zhang, J.-c.; Fan, B.-g.; Jin, Y. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals. Bioresource Technol. 2021, 332, 125086. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Hasegawa, J.-Y.; Li, S.; Shen, Y.; Li, H.; Shi, L.; Zhang, D. SO2-Tolerant Selective Catalytic Reduction of NOx over Meso-TiO2@Fe2O3@Al2O3 Metal-Based Monolith Catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Huang, J.; Feng, Z.; Xiong, Q.; Ye, Z.; Chen, Z.; Li, X.; Yu, Z. Self-supported nickel-doped molybdenum carbide nanoflower clusters on carbon fiber paper for an efficient hydrogen evolution reaction. Nanoscale 2021, 13, 8264–8274. [Google Scholar] [CrossRef]
- Jing, G.; Zhou, J.; Zhou, Z.; Lin, T. Reduction of Fe(III)EDTA− in a NOx scrubbing solution by magnetic Fe3O4–chitosan microspheres immobilized mixed culture of iron-reducing bacteria. Bioresour. Technol. 2012, 108, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Z.; Jing, G. Synthesis of Fe3O4 poly (styrene–glycidyl methacrylate) magnetic porous microspheres and application in the immobilization of Klebsiella sp. FD-3 to reduce Fe(III) EDTA in a NOx scrubbing solution. Bioresour. Technol. 2013, 130, 750–756. [Google Scholar] [CrossRef]
- Yao, G.-H.; Gui, K.-T.; Wang, F. Low-Temperature De-NOx by Selective Catalytic Reduction Based on Iron-Based Catalysts. Chem. Eng. Technol. 2010, 33, 1093–1098. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, L.; Dong, X.; Yu, H.; Wang, T.; Wang, J.; Wang, R.; Yu, W.; Liu, G. Fe3O4/rGO nanocomposite: Synthesis and enhanced NO x gas-sensing properties at room temperature. RSC Adv. 2016, 6, 37085–37092. [Google Scholar] [CrossRef]
- Irfan, M.; Sevim, M.; Koçak, Y.; Balci, M.; Metin, Ö.; Ozensoy, E. Enhanced Photocatalytic NOx Oxidation and Storage Under Visible-Light Irradiation by Anchoring Fe3O4 Nanoparticles on Mesoporous Graphitic Carbon Nitride (mpg-C3N4). Appl. Catal. B Environ. 2019, 249, 126–137. [Google Scholar] [CrossRef]
- Li, Y.; Yi, H.; Tang, X.; Liu, X.; Wang, Y.; Cui, B.; Zhao, S. Study on the performance of simultaneous desulfurization and denitrification of Fe3O4-TiO2 composites. Chem. Eng. J. 2016, 304, 89–97. [Google Scholar] [CrossRef]
- Santiago, R.; Mossin, S.; Bedia, J.; Fehrmann, R.; Palomar, J. Methanol-Promoted Oxidation of Nitrogen Oxide (NOx) by Encapsulated Ionic Liquids. Environ. Sci. Technol. 2019, 53, 11969–11978. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.S.; Shen, W. Rod-Shaped Fe2O3as an Efficient Catalyst for the Selective Reduction of Nitrogen Oxide by Ammonia. Angew. Chem. Int. Ed. 2012, 51, 2989–2993. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Liao, C.; Zhao, J.; Feng, S.; Li, P.; Liu, X.; Yang, J.; Shih, K. Magnetic Rattle-Type Fe3O4@CuS Nanoparticles as Recyclable Sorbents for Mercury Capture from Coal Combustion Flue Gas. ACS Appl. Nano Mater. 2018, 1, 4726–4736. [Google Scholar] [CrossRef]
- van der Maas, P.; Brink, P.V.D.; Klapwijk, B.; Lens, P. Acceleration of the Fe(III)EDTA− reduction rate in BioDeNOx reactors by dosing electron mediating compounds. Chemosphere 2009, 75, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.-S. A Novel Aluminum–Graphite Dual-Ion Battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef]
- Warner, C.L.; Addleman, R.S.; Cinson, A.D.; Droubay, T.C.; Engelhard, M.H.; Nash, M.A.; Yantasee, W.; Warner, M.G. High-Performance, Superparamagnetic, Nanoparticle-Based Heavy Metal Sorbents for Removal of Contaminants from Natural Waters. ChemSusChem 2010, 3, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.-Z.; Kang, Y.-R.; Sun, Z.-S.; Wang, X.-D.; Meng, Y.; Hong, M.-H.; Xie, W.-F. Cauliflower-shaped Bi2O3–ZnO heterojunction with superior sensing performance towards ethanol. J. Alloy. Compd. 2021, 854, 157152. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpää, M. EDTA-cross-linked β-cyclodextrin: An environmentally friendly bifunctional adsorbent for simultaneous adsorption of metals and cationic dyes. Environ. Sci. Technol. 2015, 49, 10570–10580. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karimzadeh, I.; Ganjali, M.R. Ethylenediaminetetraacetic acid capped superparamagnetic iron oxide (Fe3O4) nanoparticles: A novel preparation method and characterization. J. Magn. Magn. Mater. 2017, 439, 312–319. [Google Scholar] [CrossRef]
- Kim, G.; Choi, W. Charge-transfer surface complex of EDTA-TiO2 and its effect on photocatalysis under visible light. Appl. Catal. B Environ. 2010, 100, 77–83. [Google Scholar] [CrossRef]
- Wang, G.; Tomasella, F.P. Ion-pairing HPLC methods to determine EDTA and DTPA in small molecule and biological pharmaceutical formulations. J. Pharm. Anal. 2016, 6, 150–156. [Google Scholar] [CrossRef]







| Name | Formula | Characteristic | Source | When and Where | Health Problems | Atmospheric Problems |
|---|---|---|---|---|---|---|
| Sulpher dioxide | SO2 | Colour less | Coal plants, vehicles, industries | London 1952, Beijing, China 1985 | Damages Lungs tissue, asthma | Smog, ozone formation, acid rain |
| Nitric oxide | NO | Colorless | Coal plants, vehicles, industries Combustion, nitric acid plants | Los Angeles 1940, Beijing, China 1985 | Chronic respiratory, visibility, lungs damage, breast cancer, asthma attacks | Smog, ozone formation, acid rain, secondary pollutants |
| Nitrogen dioxides | NO2 | Reddish-brown |
| Nitrogen Oxides | Color | Solubility (g·dm−3) [23] | State | Density (g·dm−3) |
|---|---|---|---|---|
| NO | Colorless | 0.032 | Gas | 1.3402 |
| N2O | Colorless | 0.111 | Gas | 1.8 |
| NO2 | Red-brown | 213.0 | Gas | 3.4 |
| N2O4 | Transparent | 213.0 | Liquid | 1492.7 (273 K) |
| N2O5 | White | 500.0 | Solid | 20,508 K |
| Chemical Reduction | Biological Reduction | ||||
|---|---|---|---|---|---|
| Reductants | Year | Ref. | Reductants | Year | Ref. |
| Na2SO3 | 1984 | [60] | Ethanol | 1999 | [61] |
| SO32− | 1980 | [62] | Acetate | 2003 | [63] |
| HSO32− | 1990 | [55] | Glucose | 2007 | [64] |
| Polyphenolic compounds | 1991 | [59] | Gallic acid pyrogallol and tannic acid | 1991 | [65] |
| Hydrazine | 1994 | [1] | |||
| Na2S2O4 | 2005 | [66] | |||
| Na2S | 2006 | [67] | |||
| Name | Oxidation State | Examples |
|---|---|---|
| Ferric oxides | Fe(III) | Ferrihydrite, goethite, lepidocrocite |
| Ferrous oxides | Fe(II) | Fe(II)O, Fe(II)(OH)2 |
| Mixed-valent iron oxides | Fe(III) and Fe(II) | Magnetite, green rust (GR) |
| System/Material | Purpose | Reductant/Name of Technique | Year | Ref. |
|---|---|---|---|---|
| Fe3O4-Chitosan | NOx removal | C6H12O6, microorganisms (Biological reduction) | 2012 | [94] |
| Fe3O4-poly(styrene–glycidylmethacrylate) | NOx removal | By microorganisms (Biological reduction) | 2013 | [95] |
| Fe3O4 | De-NOx | SCR | 2010 | [96] |
| Fe3O4/rGO | NOx-sensing | Injection of NO in reacting chamber | 2016 | [97] |
| Fe3O4/mpg-C3N4 | NOx oxidation and storage | Photocatalytic oxidation under visible light | 2019 | [98] |
| Fe3O4-TiO2 | NOx removal | Adsorption fixed-bed reactor and High temperature | 2016 | [99] |
| Fe3O4 and Fe2O3 | De-NOx | SCR by NH3 | 2009 | [100] |
| Rod-Shaped Fe2O3 | NOx reduction | SCR by NH3 | 2012 | [101] |
| Fe3O4@CuS | Hg capture (Flue gas treatment) | Adsorption fixed-bed reactor | 2018 | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.; Hussain, I.; Mehmud, I.; Umair, M.; Hu, S.; Sharif, H.M.A. Recent Breakthroughs and Advancements in NOx and SOx Reduction Using Nanomaterials-Based Technologies: A State-of-the-Art Review. Nanomaterials 2021, 11, 3301. https://doi.org/10.3390/nano11123301
Ali M, Hussain I, Mehmud I, Umair M, Hu S, Sharif HMA. Recent Breakthroughs and Advancements in NOx and SOx Reduction Using Nanomaterials-Based Technologies: A State-of-the-Art Review. Nanomaterials. 2021; 11(12):3301. https://doi.org/10.3390/nano11123301
Chicago/Turabian StyleAli, Moazzam, Ijaz Hussain, Irfan Mehmud, Muhammad Umair, Sukai Hu, and Hafiz Muhammad Adeel Sharif. 2021. "Recent Breakthroughs and Advancements in NOx and SOx Reduction Using Nanomaterials-Based Technologies: A State-of-the-Art Review" Nanomaterials 11, no. 12: 3301. https://doi.org/10.3390/nano11123301
APA StyleAli, M., Hussain, I., Mehmud, I., Umair, M., Hu, S., & Sharif, H. M. A. (2021). Recent Breakthroughs and Advancements in NOx and SOx Reduction Using Nanomaterials-Based Technologies: A State-of-the-Art Review. Nanomaterials, 11(12), 3301. https://doi.org/10.3390/nano11123301