Sputtering Coating of Lithium Fluoride Film on Lithium Cobalt Oxide Electrodes for Reducing the Polarization of Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Sample Fabrication
2.2. Materials Characterization
2.3. Electrochemical Tests
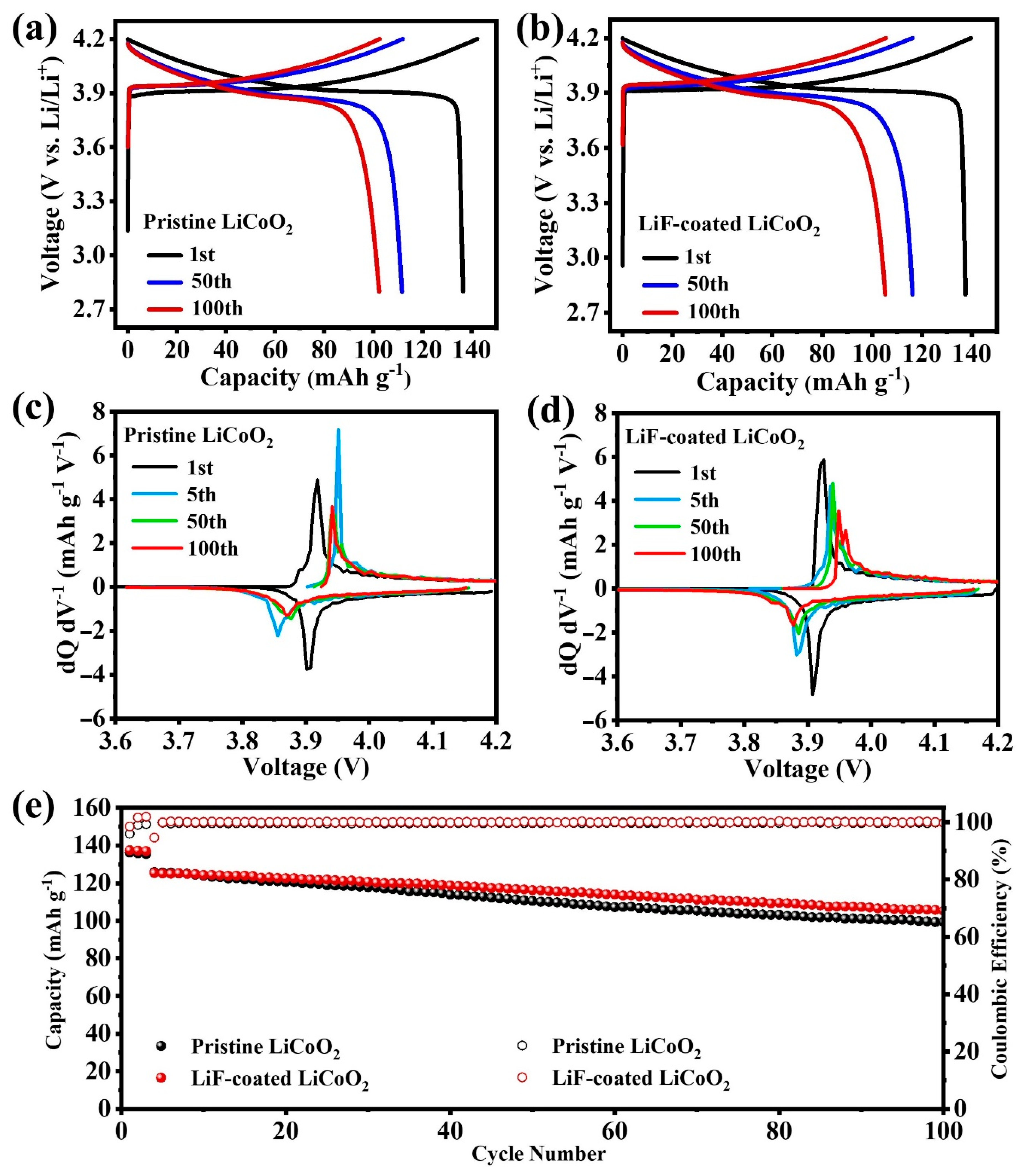
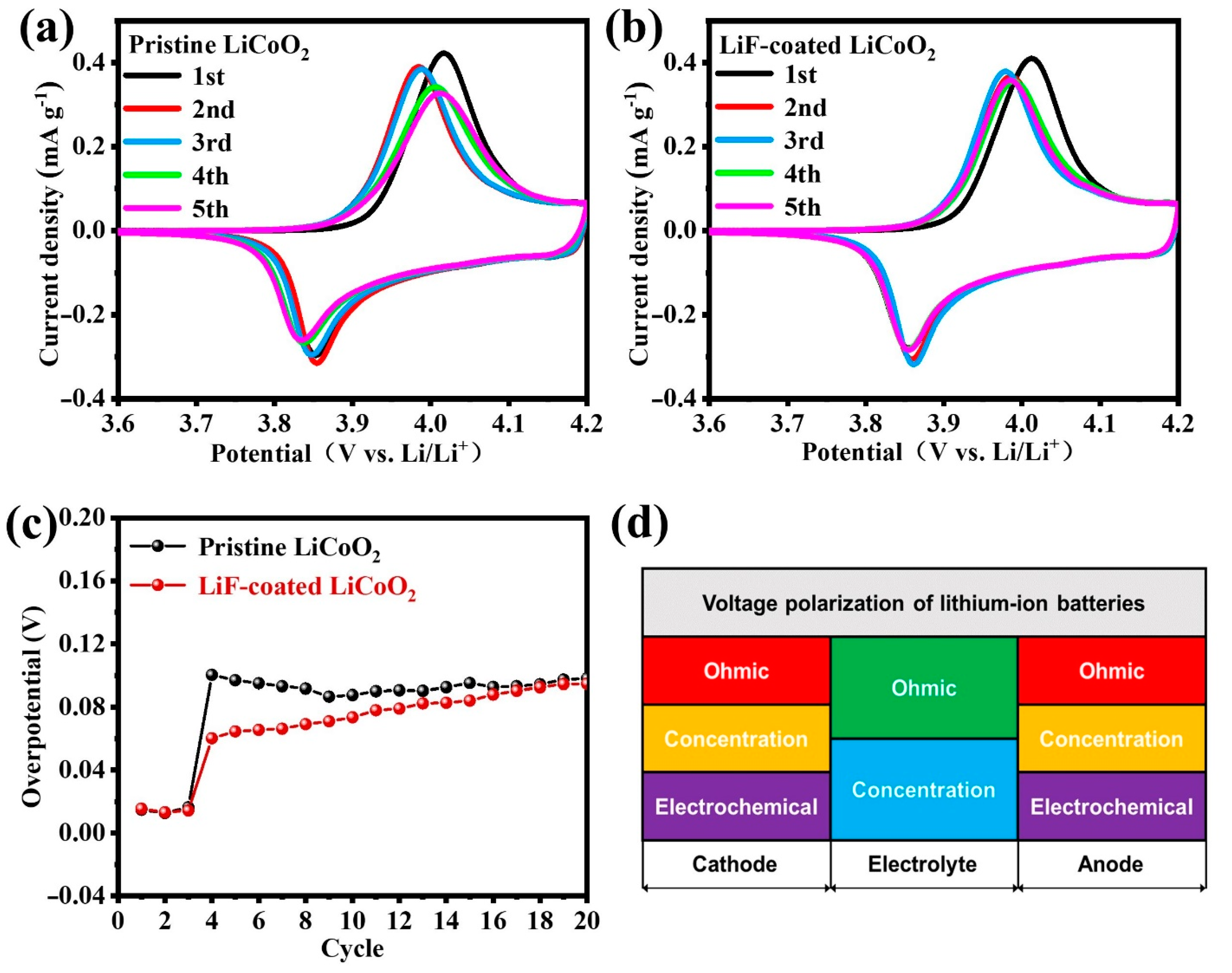
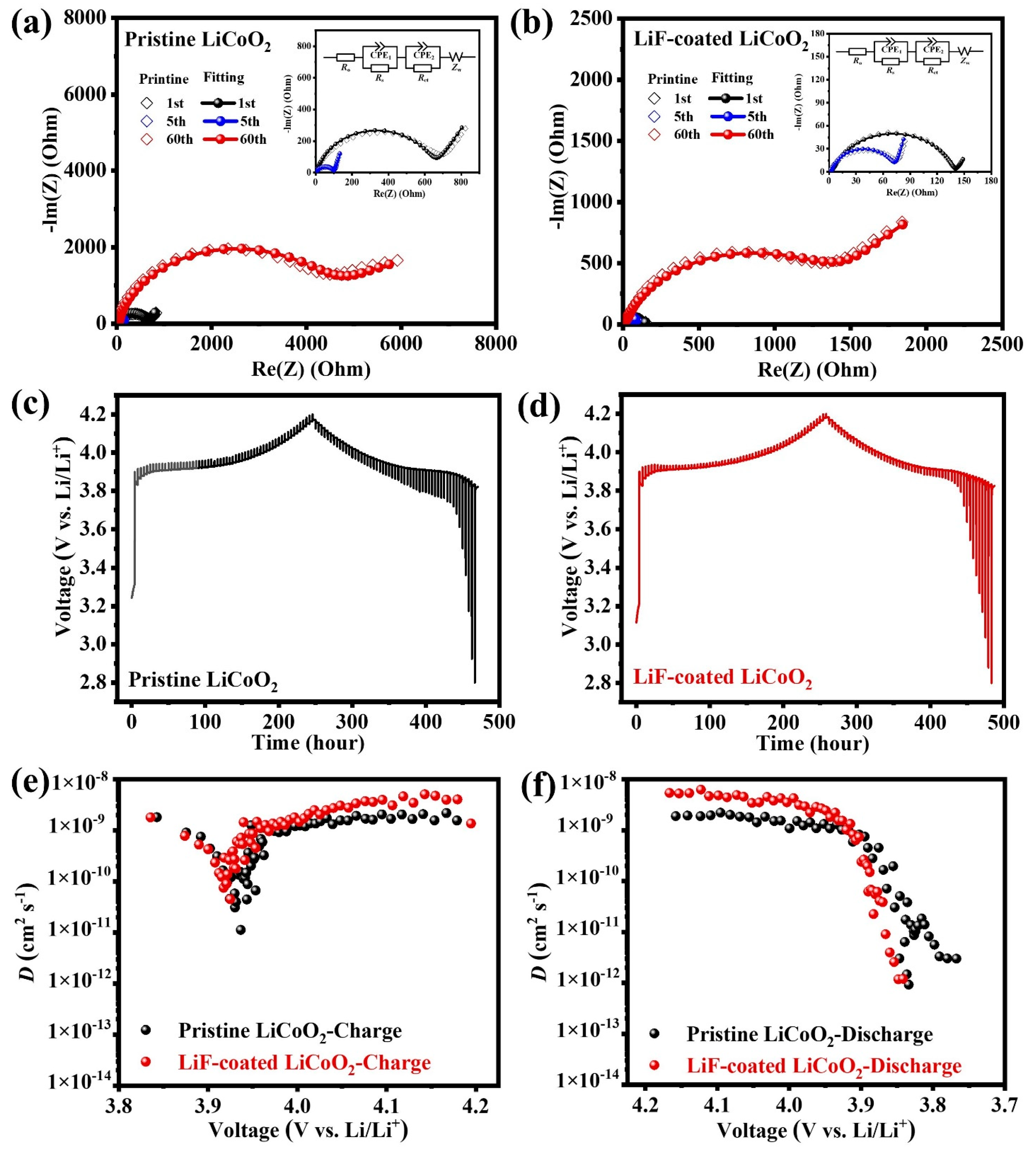
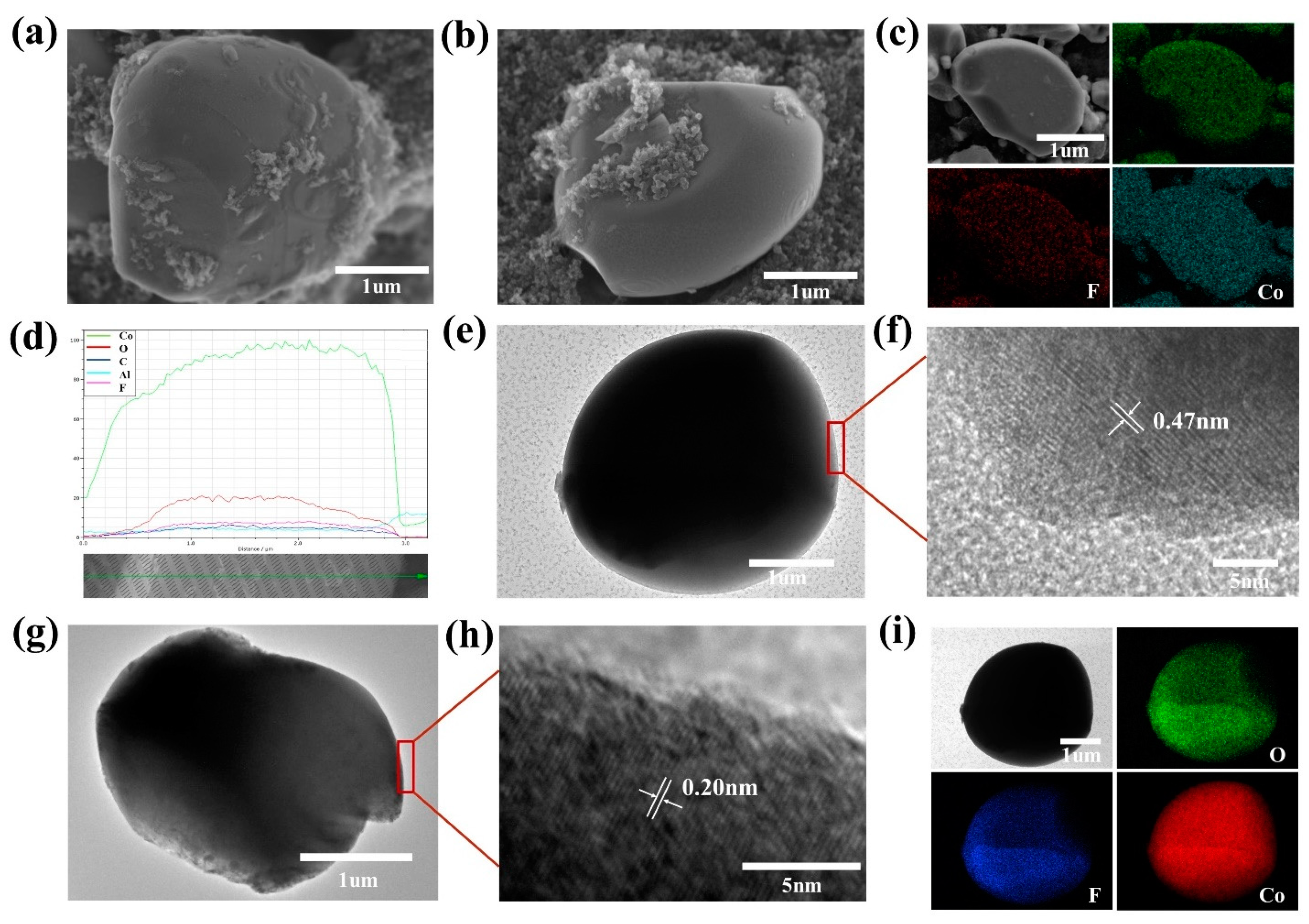
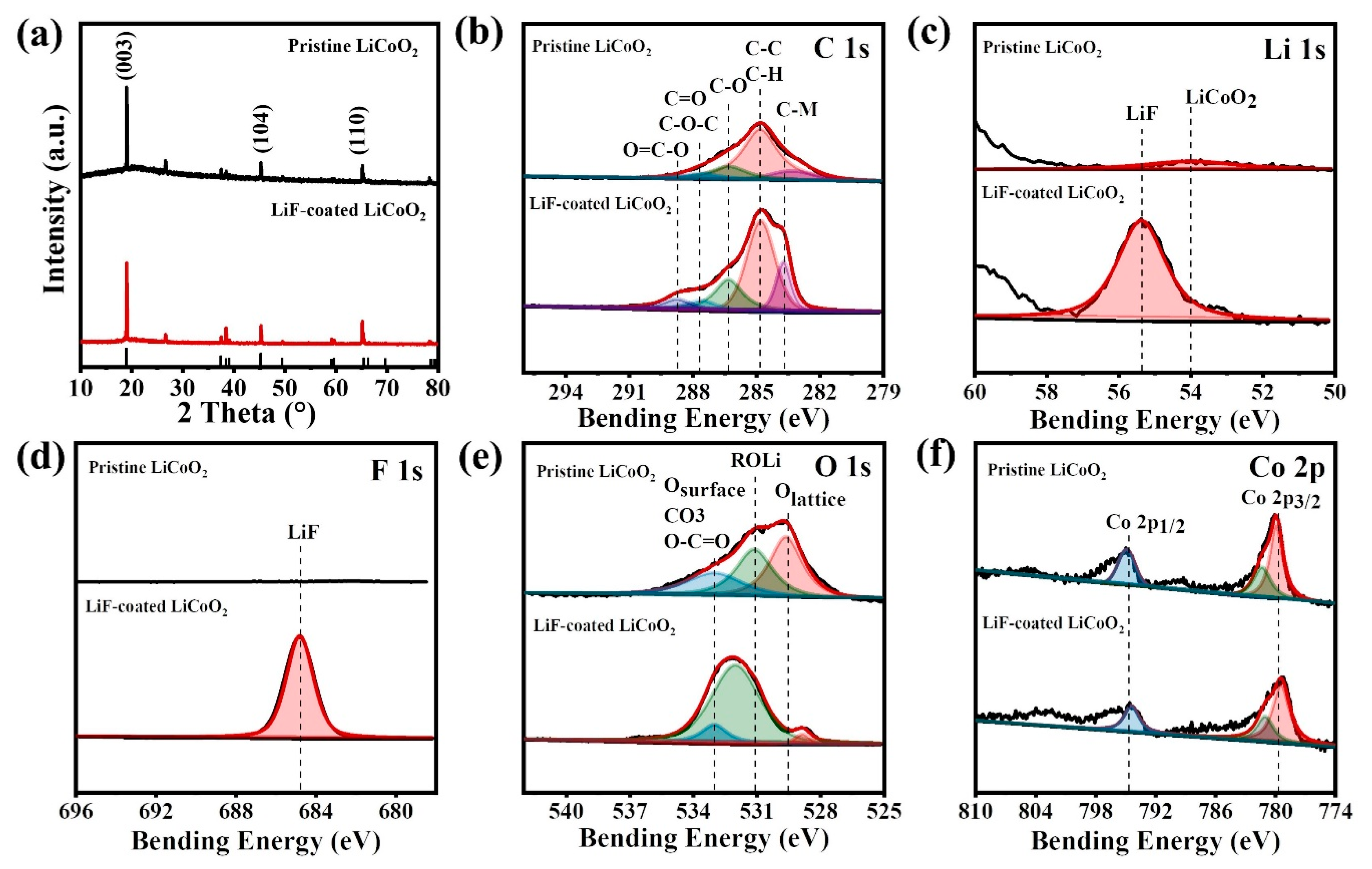
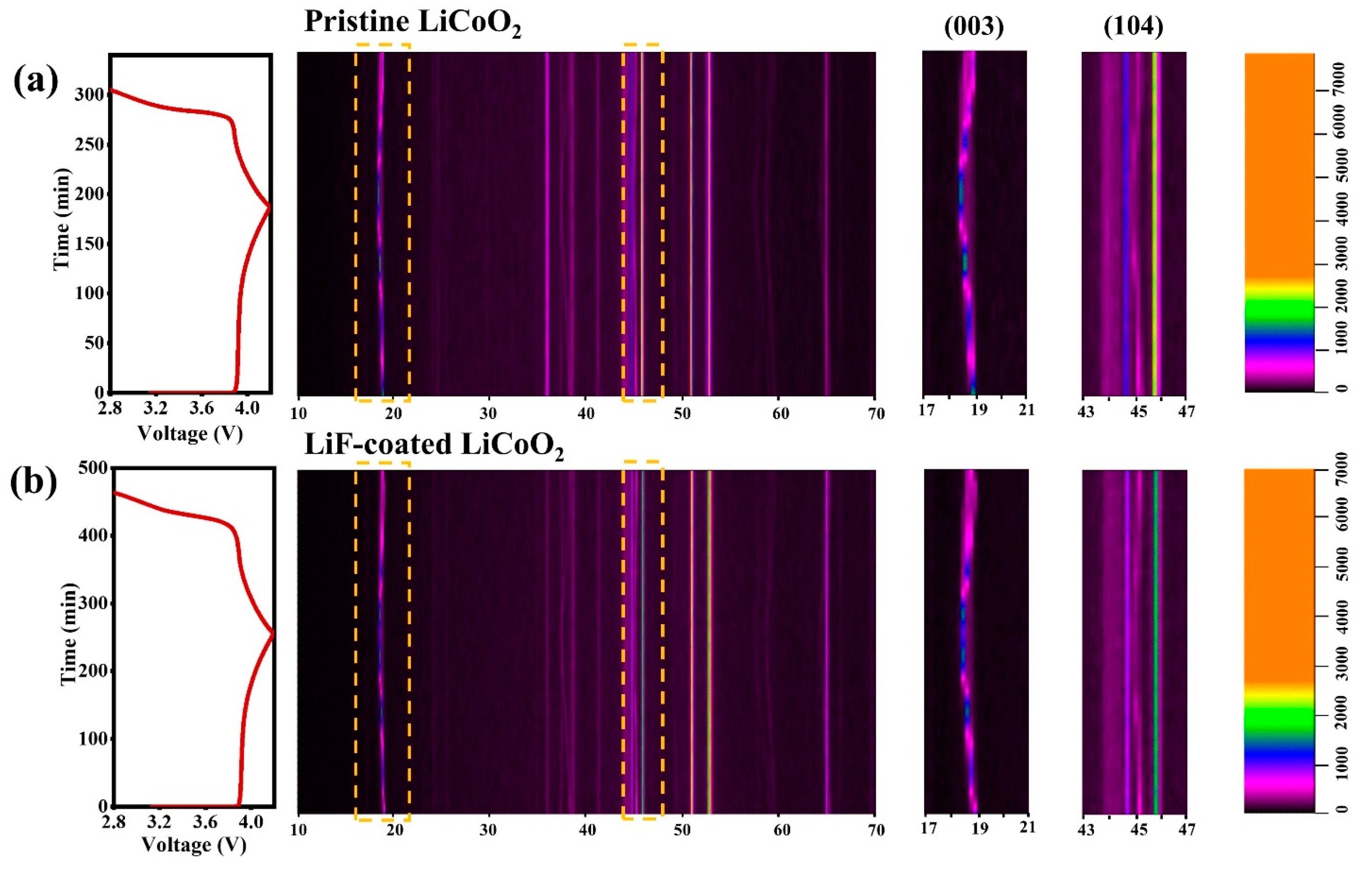
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, H.; Li, Y.; Gao, R.; Xiao, X.; Yu, Q.; Wang, C.; Waluyo, I.; Ding, J.; Hunt, A.; et al. A surface Se-substituted LiCo[O2-δSeδ] cathode with ultrastable high-voltage cycling in pouch full-cells. Adv. Mater. 2020, 32, e2005182. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.B.; Park, Y.J. Enhanced electrochemical properties of fluoridecoated LiCoO2 thin films. Nanoscale Res. Lett. 2012, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Chen, H.-C.; Jan, D.-J.; Lin, B.-C.; Hsueh, T.-H. Nanostructure distortion improvement of Al doped spinel LiMn2O4 films deposited by RF magnetron sputtering for flexible high-voltage lithium ion batteries. Mater. Res. Bull. 2021, 140, 111313. [Google Scholar] [CrossRef]
- Zhou, M. Structural and electrochemical properties of Li1.2Ni0.16Mn0.54Co0.08O2-Al2O3 composite prepared by atomic layer deposition as the cathode material for LIBs. Int. J. Electrochem. Sci. 2020, 10759–10771. [Google Scholar] [CrossRef]
- Jan, D.J.; Lee, C.C.; Yu, Y.J.; Chiang, H.W. Evaluation of lithium cobalt oxide films deposited by radio frequency magnetron sputtering as thin-film battery cathodes. Jpn. J. Appl. Phys. 2019, 58, 085501. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Dai, X.Y.; Zhou, A.J.; Xu, J.; Lu, Y.T.; Wang, L.P.; Fan, C.; Li, J.Z. Extending the high-voltage capacity of LiCoO2 cathode by direct coating of the composite electrode with Li2CO3 via magnetron sputtering. J. Phys. Chem. C 2016, 120, 422–430. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Higher, stronger, better… A review of 5-volt cathode materials for advanced lithium-ion batteries. Adv. Energy Mater. 2012, 2, 922–939. [Google Scholar] [CrossRef]
- Qian, J.; Liu, L.; Yang, J.; Li, S.; Wang, X.; Zhuang, H.L.; Lu, Y. Electrochemical surface passivation of LiCoO2 particles at ultrahigh voltage and its applications in lithium-based batteries. Nat. Commun. 2018, 9, 4918. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Blackford, M.; Ionescu, M. Characterization of alkaline-earth oxide additions to the MnO2 cathode in an aqueous secondary battery. J. Alloy. Compd. 2011, 509, 5974–5980. [Google Scholar] [CrossRef] [Green Version]
- Iriyama, Y.; Kurita, H.; Yamada, I.; Abe, T.; Ogumi, Z. Effects of surface modification by MgO on interfacial reactions of lithium cobalt oxide thin film electrode. J. Power Source 2004, 137, 111–116. [Google Scholar] [CrossRef]
- Dai, X.Y.; Wang, L.P.; Xu, J.; Wang, Y.; Zhou, A.J.; Li, J.Z. Improved electrochemical performance of LiCoO2 electrodes with ZnO coating by radio frequency magnetron sputtering. ACS Appl. Mater. Interfaces 2014, 6, 15853–15859. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.Y.; Yoon, W.-S.; Lee, H.S.; McBreen, J.; Yang, X.-Q.; Oh, S.H.; Ryu, W.H.; Lee, J.L.; Cho, W.I.; Cho, B.W. In situ XRD studies of the structural changes of ZrO2-coated LiCoO2 during cycling and their effects on capacity retention in lithium batteries. J. Power Source 2006, 163, 185–190. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, M.C.; Kim, E.S.; Shin, Y.K.; Lee, J.E.; Choi, S.; Park, K.W. TiO2-coated LiCoO2 electrodes fabricated by a sputtering deposition method for lithium-ion batteries with enhanced electrochemical performance. RSC Adv. 2019, 9, 7903–7907. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lin, D.; Liu, Y.; Li, Y.; Cui, Y. Ultrahigh-current density anodes with interconnected Li metal reservoir through overlithiation of mesoporous AlF3 framework. Sci. Adv. 2017, 3, e1701301. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Sendek, A.D.; Cubuk, E.D.; Zhang, X.; Lu, Z.; Gong, Y.; Wu, T.; Shi, F.; Liu, W.; Reed, E.J.; et al. Atomic layer deposition of stable LiAlF4 lithium ion conductive interfacial layer for stable cathode cycling. ACS Nano 2017, 11, 7019–7027. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, H.; Kim, J.-H.; Wygant, B.R.; Meyerson, M.L.; Rodriguez, R.; Liu, Y.; Kawashima, K.; Gu, D.; Peng, D.-L.; et al. Lithium fluoride coated silicon nanocolumns as anodes for lithium ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 18465–18472. [Google Scholar] [CrossRef]
- Myung, S.-T.; Izumi, K.; Komaba, S.; Sun, Y.-K.; Yashiro, H.; Kumagai, N. Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. Chem. Mater. 2005, 17, 3695–3704. [Google Scholar] [CrossRef]
- Shi, X.; Pang, Y.; Wang, B.; Sun, H.; Wang, X.; Li, Y.; Yang, J.; Li, H.W.; Zheng, S. In situ forming LiF nanodecorated electrolyte/electrode interfaces for stable all-solid-state batteries. Mater. Today Nano 2020, 10, 100079. [Google Scholar] [CrossRef]
- Tiurin, O.; Solomatin, N.; Auinat, M.; Ein-Eli, Y. Atomic layer deposition (ALD) of lithium fluoride (LiF) protective film on Li-ion battery LiMn1.5Ni0.5O4 cathode powder material. J. Power Source 2020, 448, 227373. [Google Scholar] [CrossRef]
- Kato, M.; Hayashi, T.; Hasegawa, G.; Lu, X.; Miyazaki, T.; Matsuda, Y.; Kuwata, N.; Kurihara, K.; Kawamura, J. Electrochemical properties of LiCoO2 thin film surface modified by lithium tantalate and lithium niobate coatings. Solid State Ion. 2017, 308, 54–60. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J.R. Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V. Electrochim. Acta 2004, 49, 1079–1090. [Google Scholar] [CrossRef]
- Bard, A.J. Electrochemical Methods, Fundamentals and Applications; John Wiley and Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Aurbach, D.; Markovsky, B.; Rodkin, A.; Levi, E.; Cohen, Y.S.; Kim, H.-J.; Schmidt, M. On the capacity fading of LiCoO2 intercalation electrodes: The effect of cycling, storage, temperature, and surface film forming additives. Electrochim. Acta 2002, 47, 4291–4306. [Google Scholar] [CrossRef]
- Lin, J.; Lim, J.-M.; Youn, D.H.; Liu, Y.; Cai, Y.; Kawashima, K.; Kim, J.-H.; Peng, D.-L.; Guo, H.; Henkelman, G.; et al. Cu4SnS4-rich nanomaterials for thin-film lithium batteries with enhanced conversion reaction. ACS Nano 2019, 13, 10671–10681. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Qu, L.; Yang, J.; Xie, W.; Zhu, X. Exploring the critical role of grinding modification on the flotation recovery of electrode materials from spent lithium ion batteries. J. Clean. Prod. 2020, 274, 123066. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, B.B.; Ma, J.; Cui, G.L.; Chen, L.Q. Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem. Soc. Rev. 2018, 47, 6505–6602. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.; Wang, C.; Yu, X.; Liu, R.; Jiang, F.; Sun, X.; Du, A.; Zhou, X.; Cui, G. A novel bifunctional self-stabilized strategy enabling 4.6 V LiCoO2 with excellent long-term cyclability and high-rate capability. Adv. Sci. 2019, 6, 1900355. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yang, W.; Jiang, Z.; Huang, Z.; Wang, Y.; Wang, S. Improving diffusion kinetics and phase stability of LiCoO2 via surface modification at elevated voltage. Electrochim. Acta 2021, 380, 138227. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, S.; Wu, W.; Wu, Y.; Zhuang, Y.; Lin, J.; Wang, L.; Wei, Q.; Xie, Q.; Peng, D.-L. Sputtering Coating of Lithium Fluoride Film on Lithium Cobalt Oxide Electrodes for Reducing the Polarization of Lithium-Ion Batteries. Nanomaterials 2021, 11, 3393. https://doi.org/10.3390/nano11123393
Qu S, Wu W, Wu Y, Zhuang Y, Lin J, Wang L, Wei Q, Xie Q, Peng D-L. Sputtering Coating of Lithium Fluoride Film on Lithium Cobalt Oxide Electrodes for Reducing the Polarization of Lithium-Ion Batteries. Nanomaterials. 2021; 11(12):3393. https://doi.org/10.3390/nano11123393
Chicago/Turabian StyleQu, Shasha, Wenbin Wu, Yunfan Wu, Yanping Zhuang, Jie Lin, Laisen Wang, Qiulong Wei, Qingshui Xie, and Dong-Liang Peng. 2021. "Sputtering Coating of Lithium Fluoride Film on Lithium Cobalt Oxide Electrodes for Reducing the Polarization of Lithium-Ion Batteries" Nanomaterials 11, no. 12: 3393. https://doi.org/10.3390/nano11123393





