High Sensitivity Surface Plasmon Resonance Sensor Based on Periodic Multilayer Thin Films
Abstract
:1. Introduction
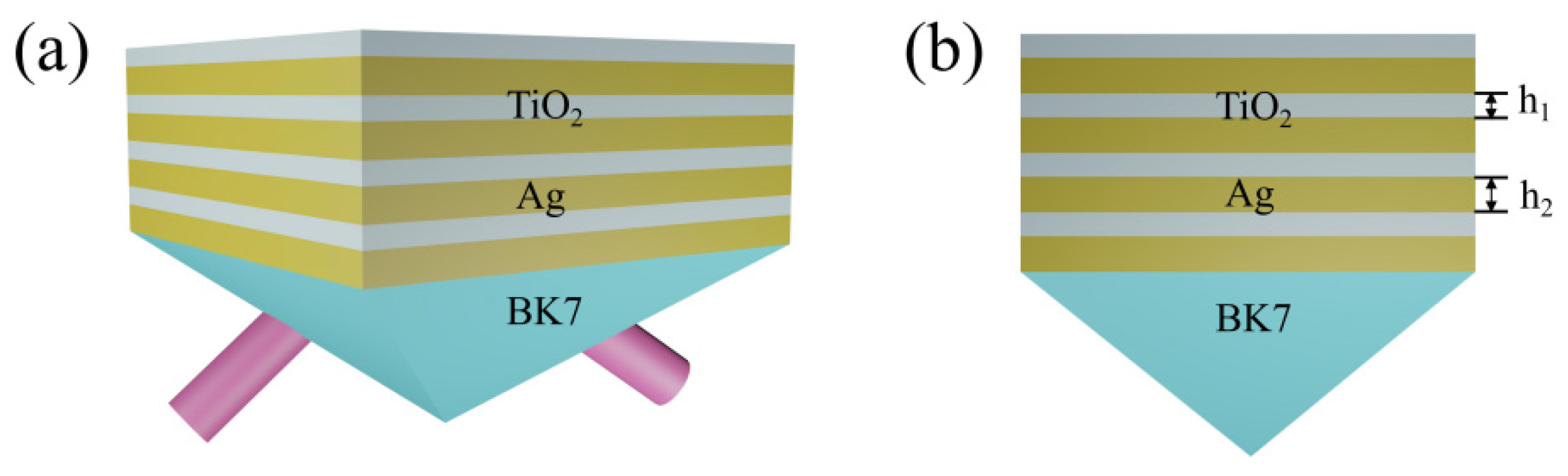
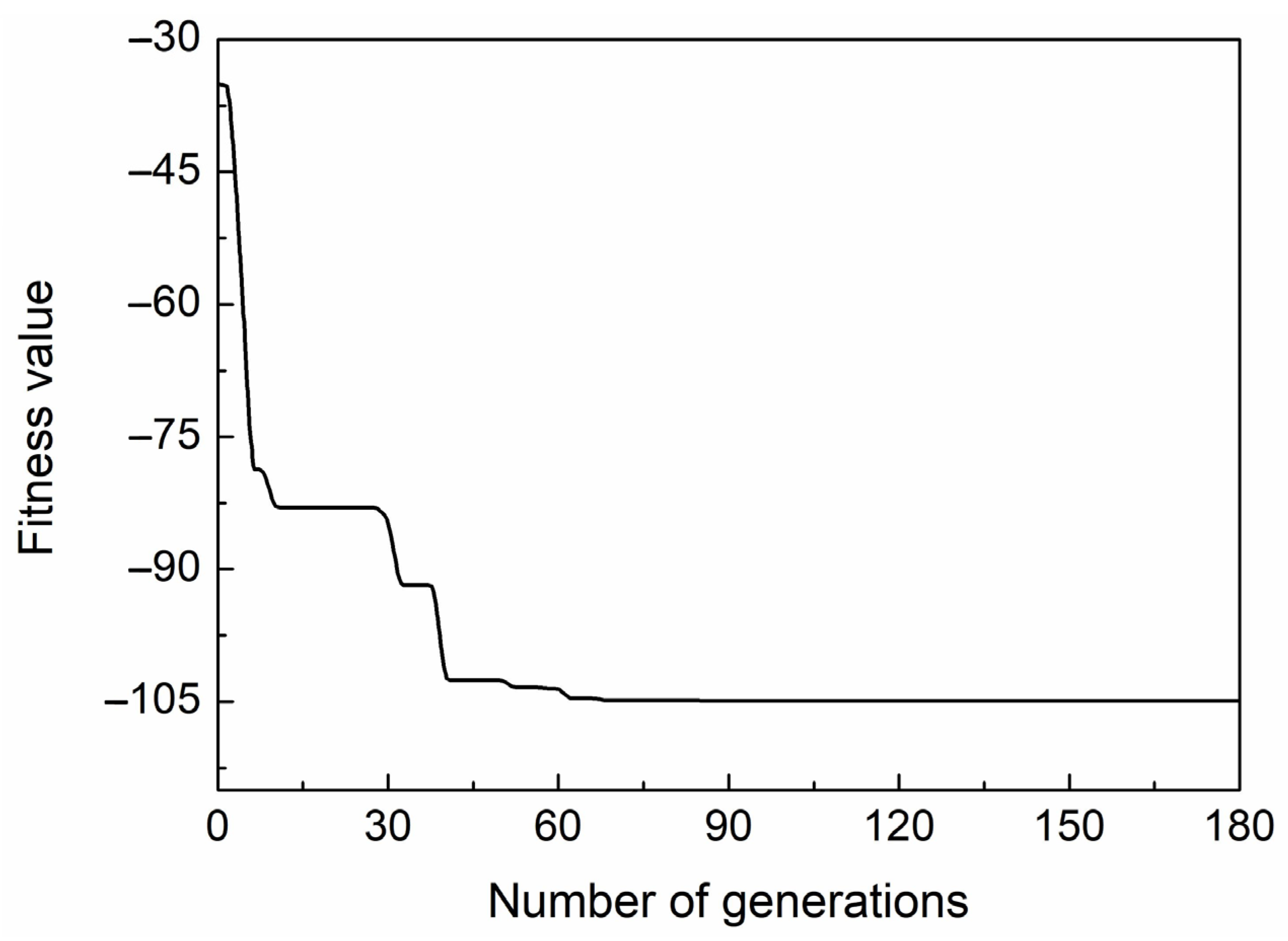
2. Structure Design and Optimization
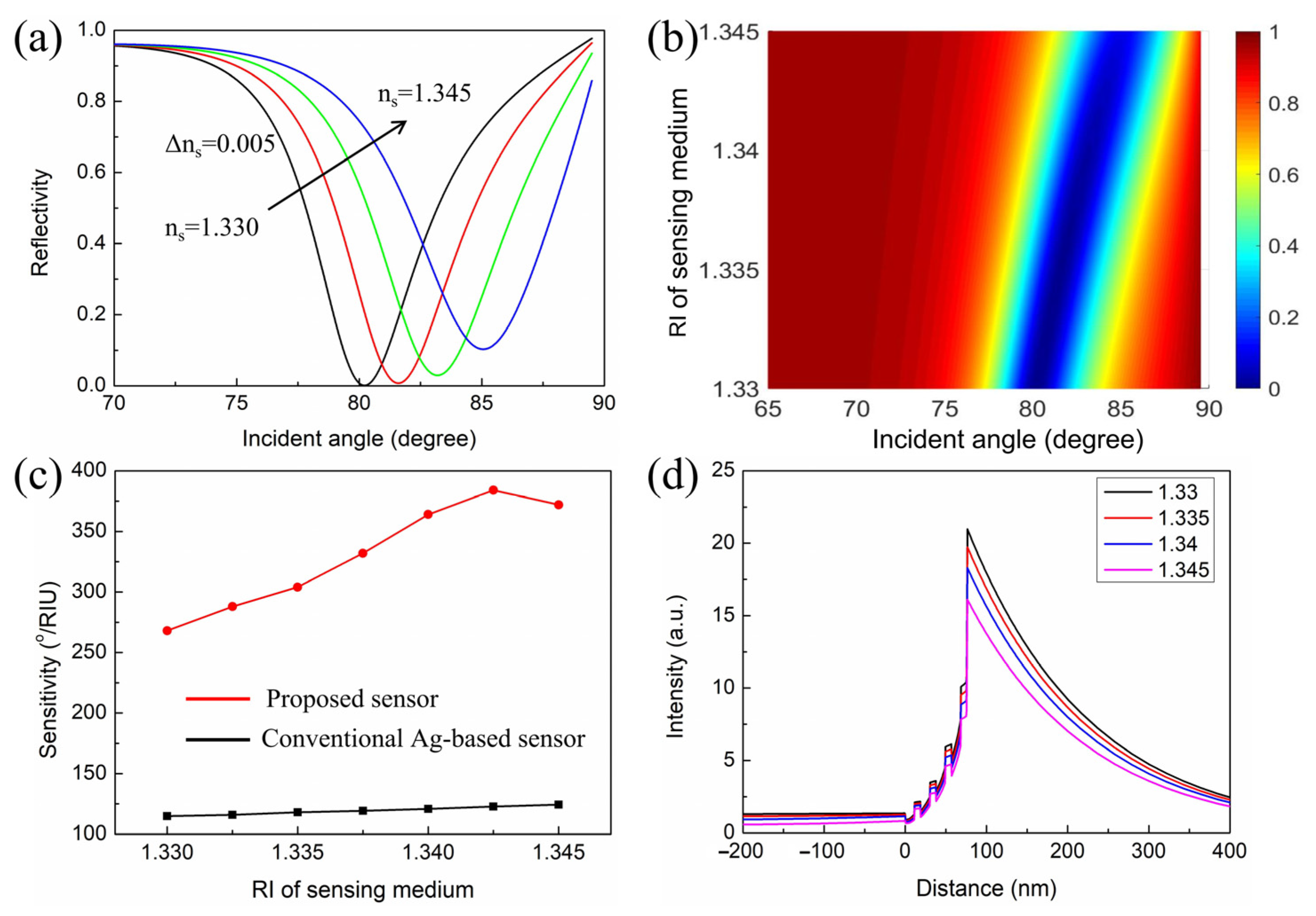
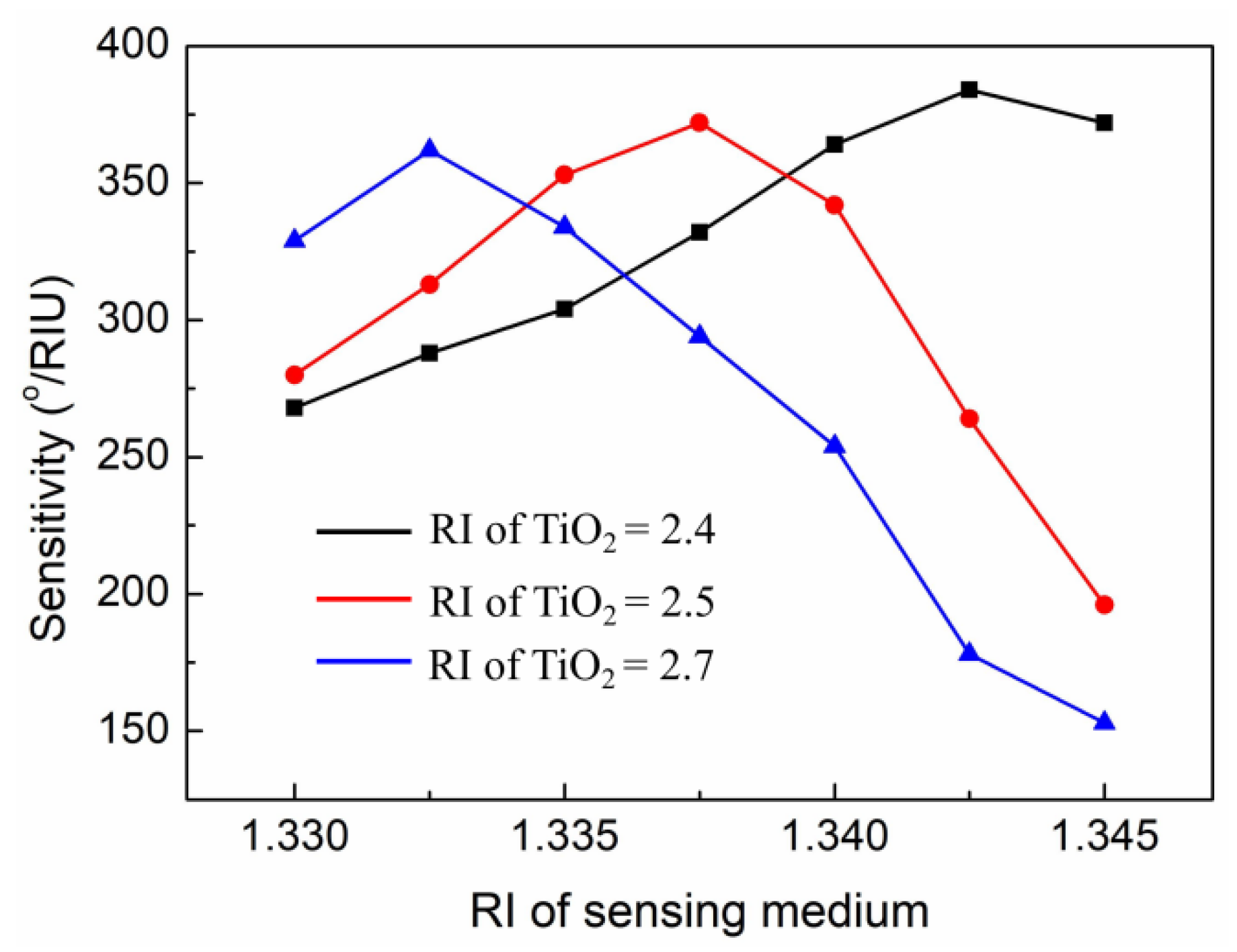
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Cai, H.; Wang, X.; Zhan, S. Layer analysis of axial spatial distribution of surface plasmon resonance sensing. Anal. Chim. Acta 2020, 1136, 141–150. [Google Scholar] [CrossRef]
- Daware, K.; Kasture, M.; Kalubarme, R.; Shinde, R.; Patil, K.; Suzuki, N.; Terashima, C.; Gosavi, S.; Fujishima, A. Detection of toxic metal ions Pb2+ in water using SiO2@Au core-shell nanostructures: A simple technique for water quality monitoring. Chem. Phys. Lett. 2019, 732, 136635. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens. Bioelectron. 2019, 142, 111449. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Qian, Y.; Zhang, T.; Zheng, L.; Fu, L. Quantification of shellfish major allergen tropomyosin by SPR biosensor with gold patterned Biochips. Food Control 2020, 107, 106547. [Google Scholar] [CrossRef]
- Chiu, N.; Yang, H. High-Sensitivity Detection of the Lung Cancer Biomarker CYFRA21-1 in Serum Samples Using a Carboxyl-MoS2 Functional Film for SPR-Based Immunosensors. Front. Bioeng. Biotechnol. 2020, 8, 234. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kim, Y.L.; Byun, K.M. Graphene-on-silver substrates for sensitive surface plasmon resonance imaging biosensors. Opt. Express 2011, 19, 458–466. [Google Scholar] [CrossRef]
- Maurya, J.B.; Prajapati, Y.K.; Singh, V.; Saini, J.P.; Tripathi, R. Performance of graphene–MoS2 based surface plasmon resonance sensor using Silicon layer. Opt. Quant. Electron. 2015, 47, 3599–3611. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, S.; Verma, A.; Prajapati, Y.K.; Saini, J.P. Effect of silicon on sensitivity of SPR biosensor using hybrid nanostructure of black phosphorus and MXene. Superlattice. Microst. 2020, 145, 106591. [Google Scholar] [CrossRef]
- Homola, J.; Koudela, I.; Yee, S.S. Surface plasmon resonance sensors based on diffraction gratings and prism couplers: Sensitivity comparison. Sens. Actuators. B Chem. 1999, 54, 16–24. [Google Scholar] [CrossRef]
- Lin, C.; Chen, S. Design of highly sensitive guided-wave surface plasmon resonance biosensor with deep dip using genetic algorithm. Opt. Commun. 2019, 445, 155–160. [Google Scholar] [CrossRef]
- Altintas, Z.; Uludag, Y.; Gurbuz, Y.; Tothill, I. Development of surface chemistry for surface plasmon resonance based sensors for the detection of proteins and DNA molecules. Anal. Chim. Acta 2012, 712, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Ahijado-Guzmán, R.; Prasad, J.; Rosman, C.; Henkel, A.; Tome, L.; Schneider, D.; Rivas, G.; Sönnichsen, C. Plasmonic Nanosensors for Simultaneous Quantification of Multiple Protein–Protein Binding Affinities. Nano Lett. 2014, 14, 5528–5532. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Tan, X.; Liu, L.; Zhou, F. Covalent affixation of histidine-tagged proteins tethered onto Ni-nitrilotriacetic acid sensors for enhanced surface plasmon resonance detection of small molecule drugs and kinetic studies of antibody/antigen interactions. Analyst 2019, 144, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Liu, Y.; Chang, S.; Chen, H.; Chen, J. Surface Plasmonic Sensors: Sensing Mechanism and Recent Applications. Sensors 2021, 21, 5262. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR biosensing technology: An overview of recent trends in smart layers design, multiplexing concepts, continuous monitoring and in vivo sensing. Anal. Chim. Acta 2020, 1104, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Holzinger, M.; Tabrizian, M.; Winters, S.; Berner, N.C.; Cosnier, S.; Duesberg, G.S. Noncovalently Functionalized Monolayer Graphene for Sensitivity Enhancement of Surface Plasmon Resonance Immunosensors. J. Am. Chem. Soc. 2015, 137, 2800–2803. [Google Scholar] [CrossRef]
- Lee, K.L.; Lee, C.W.; Wang, W.S.; Wei, P.K. Sensitive biosensor array using surface plasmon resonance on metallic nanoslits. J. Biomed. Opt. 2007, 12, 044023. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured Plasmonic Sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Sherry, L.J.; Jin, R.; Mirkin, C.A.; Schatz, G.C.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy of Single Silver Triangular Nanoprisms. Nano Lett. 2006, 6, 2060–2065. [Google Scholar] [CrossRef]
- Poddubny, A.; Iorsh, I.; Belov, P.; Kivshar, Y. Hyperbolic metamaterials. Nat. Photonics 2013, 7, 948–957. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Garoli, D.; Calandrini, E.; Giovannini, G.; Hubarevich, A.; Caligiuri, V.; De Angelis, F. Nanoporous gold metamaterials for high sensitivity plasmonic sensing. Nanoscale Horiz. 2019, 4, 1153–1157. [Google Scholar] [CrossRef]
- Bhatia, P.; Gupta, B.D. Surface-plasmon-resonance-based fiber-optic refractive index sensor: Sensitivity enhancement. Appl. Opt. 2011, 50, 2032–2036. [Google Scholar] [CrossRef]
- Lahav, A.; Auslender, M.; Abdulhalim, I. Sensitivity enhancement of guided-wave surface-plasmon resonance sensors. Opt. Lett. 2008, 33, 2539–2541. [Google Scholar] [CrossRef]
- Su, M.; Chen, X.; Tang, L.; Yang, B.; Zou, H.; Liu, J.; Li, Y.; Chen, S.; Fan, D. Black phosphorus (BP)–graphene guided-wave surface plasmon resonance (GWSPR) biosensor. Nanophotonics 2020, 9, 4265–4272. [Google Scholar] [CrossRef]
- Wu, L.; Jia, Y.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity Improved SPR Biosensor Based on the MoS2/Graphene–Aluminum Hybrid Structure. J. Lightwave Technol. 2017, 35, 82–87. [Google Scholar] [CrossRef]
- Kundu, P.K.; Elkamel, A.; Vargas, F.M.; Farooq, M.U. Genetic algorithm for multi-parameter estimation in sorption and phase equilibria problems. Chem. Eng. Commun. 2018, 205, 338–349. [Google Scholar] [CrossRef]
- Lin, C.; Chen, S. Design of high-performance Au-Ag-dielectric-graphene based surface plasmon resonance biosensors using genetic algorithm. J. Appl. Phys. 2019, 125, 113101. [Google Scholar] [CrossRef]
- Zeng, S.; Hu, S.; Xia, J.; Anderson, T.; Dinh, X.; Meng, X.; Coquet, P.; Yong, K. Graphene–MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B Chem. 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Cai, H.; Sun, Y.; Wang, X.; Zhan, S. Design of an ultra-broadband near-perfect bilayer grating metamaterial absorber based on genetic algorithm. Opt. Express 2020, 28, 15347. [Google Scholar] [CrossRef]
- Gupta, B.D.; Sharma, A.K. Sensitivity evaluation of a multi-layered surface plasmon resonance-based fiber optic sensor: A theoretical study. Sens. Actuators B Chem. 2005, 107, 40–46. [Google Scholar] [CrossRef]
- Abbas, A.; Linman, M.J.; Cheng, Q. Sensitivity comparison of surface plasmon resonance and plasmon-waveguide resonance biosensors. Sens. Actuators B Chem. 2011, 156, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhang, P.; He, Y.; Xu, Z.; Liu, L.; Ji, Y.; Ma, H. Plasmon waveguide resonance sensor using an Au-MgF2 structure. Appl. Opt. 2014, 53, 6344–6350. [Google Scholar] [CrossRef] [PubMed]
- Haddouche, I.; Cherbi, L. Comparison of finite element and transfer matrix methods for numerical investigation of surface plasmon waveguides. Opt. Commun. 2017, 382, 132–137. [Google Scholar] [CrossRef]
- Nguyen-Huu, N.; Lo, Y.L.; Chen, Y.B.; Yang, T.Y. Realization of integrated polarizer and color filters based on subwavelength metallic gratings using a hybrid numerical scheme. Appl. Opt. 2011, 50, 415–426. [Google Scholar] [CrossRef]
- Das, C.M.; Ouyang, Q.; Kang, L.; Guo, Y.; Dinh, X.; Coquet, P.; Yong, K. Augmenting sensitivity of surface plasmon resonance (SPR) sensors with the aid of anti-reflective coatings (ARCs). Photonics Nanostructures Fundam. Appl. 2020, 38, 100760. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, S.; Zhang, G.; Dai, X. High sensitivity refractive index sensor based on surface plasmon resonance with topological insulator. Results Phys. 2019, 14, 102477. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Teng, J. Design of an ultrasensitive SPR biosensor based on a graphene-MoS2 hybrid structure with a MgF2 prism. Appl. Opt. 2018, 57, 3639. [Google Scholar] [CrossRef]
- Wu, L.; Guo, J.; Wang, Q.; Lu, S.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity enhancement by using few-layer black phosphorus-graphene/TMDCs heterostructure in surface plasmon resonance biochemical sensor. Sens. Actuators B Chem. 2017, 249, 542–548. [Google Scholar] [CrossRef]
- Sun, P.; Wang, M.; Liu, L.; Jiao, L.; Du, W.; Xia, F.; Liu, M.; Kong, W.; Dong, L.; Yun, M. Sensitivity enhancement of surface plasmon resonance biosensor based on graphene and barium titanate layers. Appl. Surf. Sci. 2019, 475, 342–347. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, S.K. Gas sensing in Kretschmann configuration utilizing bi-metallic layer of Rhodium-Silver in visible region. Sens. Actuators B Chem. 2016, 237, 969–973. [Google Scholar] [CrossRef]
- Xu, Y.; Ang, Y.; Wu, L.; Ang, L. High Sensitivity Surface Plasmon Resonance Sensor Based on Two-Dimensional MXene and Transition Metal Dichalcogenide: A Theoretical Study. Nanomaterials 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; You, Q.; Shan, Y.; Gan, S.; Zhao, Y.; Dai, X.; Xiang, Y. Few-layer Ti3C2Tx MXene: A promising surface plasmon resonance biosensing material to enhance the sensitivity. Sens. Actuators B Chem. 2018, 277, 210–215. [Google Scholar] [CrossRef]
- Wicaksana, D.; Kobayashi, A.; Kinbara, A. Process effects on structural properties of TiO2 thin films by reactive sputtering. J. Vac. Sci. Technol. A 1992, 10, 1479–1482. [Google Scholar] [CrossRef]


| Layer Thickness | Fabrication Errors | |
|---|---|---|
| −10 Variations | +10 Variations | |
| Ag | 372°/RIU | 353°/RIU |
| TiO2 | 323°/RIU | 284°/RIU |
| Reference | Publication Year | Operating Wavelength | Metal | Sensitivity (Degree/RIU) |
|---|---|---|---|---|
| [8] | 2020 | 633 nm | Ag | 264 |
| [25] | 2020 | 633 nm | Al | 148.2 |
| [37] | 2019 | 633 nm | Au | 175 |
| [39] | 2017 | 633 nm | Ag | 279 |
| [40] | 2019 | 633 nm | Ag | 257 |
| [41] | 2016 | 632 nm | Rh and Ag | 220 |
| [42] | 2019 | 633 nm | Au | 198 |
| [43] | 2018 | 532 nm | Au | 224.5 |
| This work | —— | 633 nm | Ag | 384 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Shan, S.; Wang, X. High Sensitivity Surface Plasmon Resonance Sensor Based on Periodic Multilayer Thin Films. Nanomaterials 2021, 11, 3399. https://doi.org/10.3390/nano11123399
Cai H, Shan S, Wang X. High Sensitivity Surface Plasmon Resonance Sensor Based on Periodic Multilayer Thin Films. Nanomaterials. 2021; 11(12):3399. https://doi.org/10.3390/nano11123399
Chicago/Turabian StyleCai, Haoyuan, Shihan Shan, and Xiaoping Wang. 2021. "High Sensitivity Surface Plasmon Resonance Sensor Based on Periodic Multilayer Thin Films" Nanomaterials 11, no. 12: 3399. https://doi.org/10.3390/nano11123399






