Single-Walled Carbon Nanotubes Inhibit TRPC4-Mediated Muscarinic Cation Current in Mouse Ileal Myocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of SWCNTs Water Suspension
2.2. Molecular Docking
2.3. Molecular Dynamics (MD) Simulation
2.4. Cell Isolation
2.5. Patch Clamp Recordings
2.6. Chemicals
2.7. Data and Statistical Analysis
3. Results
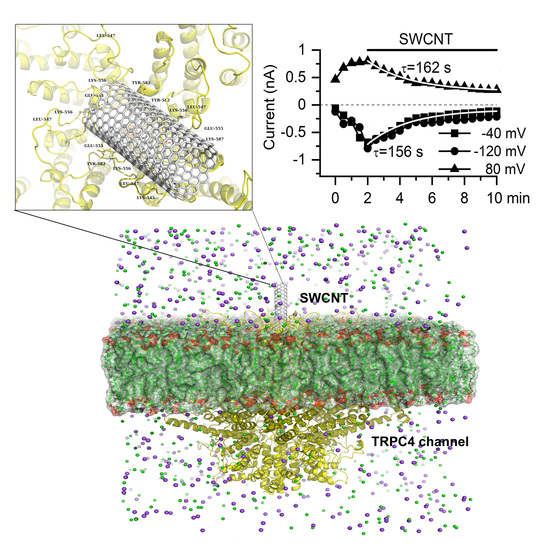
3.1. Modeling of SWCNT Binding to TRPC4 Channel
3.2. Inhibitory Action of SWCNT on mICAT in Ileal Myocytes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SWCNTs | single-walled carbon nanotubes |
| M receptors | muscarinic receptors |
| mICAT | muscarinic receptor cation current |
| GTPγS | guanosine 5′-O-[gamma-thio]triphosphate |
| TRPC4 | transient receptor potential cation channel subfamily C member 4 |
| SM | smooth muscles |
| HRTEM | High-resolution transmission electron microscopy |
| TGA | thermogravimetric analysis |
| AFM | atomic force microscopy |
| SDOCK | systematic docking algorithm |
| POPC | palmitoyl oleoyl phosphatidylcholine |
| MD | molecular dynamics |
| RMSD | root–mean–square deviation |
| RMSF | root–mean–square fluctuation |
| LJ-SR energy | Lennard–Jones short-range energy |
| EREV | reversal potential |
References
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef] [PubMed]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Heller, D.A.; Sharma, R.; Strano, M.S. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: Single particle tracking and a generic uptake model for nanoparticles. ACS Nano. 2009, 3, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Bilyy, R.; Shkandina, T.; Rotko, D.; Bychko, A.; Cherepanov, V.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N.; et al. Comparative study of membranotropic action of single- and multi-walled carbon nanotubes. J. Biosci. Bioeng. 2013, 115, 674–679. [Google Scholar] [CrossRef]
- Lacerda, L.; Russier, J.; Pastorin, G.; Herrero, M.A.; Venturelli, E.; Dumortier, H.; Al-Jamal, K.; Prato, M.; Kostarelos, K.; Bianco, A. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials 2012, 33, 3334–3343. [Google Scholar] [CrossRef]
- Vidu, R.; Rahman, M.; Mahmoudi, M.; Enachescu, M.; Poteca, T.D.; Opris, I. Nanostructures: A platform for brain repair and augmentation. Front. Syst. Neurosci. 2014, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Wang, Y.Y.; Lim, T.-S.; Pham, T.; Jain, D.; Burke, P.J. Detection of single ion channel activity with carbon nanotubes. Sci. Rep. 2015, 5, 9208. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.; Yoon, O.J.; Kim, H.W.; Lee, D.Y.; Kim, D.H.; Lee, D.H.; Kim, D.H.; Lee, B.W.; Lee, N.-E.; et al. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat. Nanotechnol. 2011, 6, 121–125. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano. Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. J. Cell Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Shapoval, L.M.; Prylutska, S.V.; Kotsyuruba, A.V.; Dmitrenko, O.V.; Prylutskyy, Y.I.; Sagach, V.F.; Ritter, U. Single-walled carbon nanotubes modulate cardiovascular control in rats. Mater. Werkst. 2016, 47, 208–215. [Google Scholar] [CrossRef]
- Bayat, N.; Lopes, V.; Schoelermann, J.; Jensen, L.; Cristobal, S. Vascular toxicity of ultra-small TiO2 nanoparticles and single walled carbon nanotubes in vitro and in vivo. Biomaterials 2015, 63, 1–13. [Google Scholar] [CrossRef]
- Minchenko, O.H.; Tsymbal, D.O.; Minchenko, D.; Prylutska, S.; Hnatiuk, O.S.; Prylutskyy, Y.; Tsierkezos, N.G.; Ritter, U. Single-walled carbon nanotubes affect the expression of genes associated with immune response in normal human astrocytes. Toxicol. Vitr. 2018, 52, 122–130. [Google Scholar] [CrossRef]
- Singla, R.; Sharma, C.; Shukla, A.K.; Acharya, A. Toxicity Concerns of Therapeutic Nanomaterials. J. Nanosci. Nanotechnol. 2019, 19, 1889–1907. [Google Scholar] [CrossRef]
- Yang, S.T.; Luo, J.; Zhou, Q.; Wang, H. Pharmacokinetics, metabolism and toxicity of carbon nanotubes for bio-medical purposes. Theranostics 2012, 2, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Harik, V.M. Geometry of carbon nanotubes and mechanisms of phagocytosis and toxic effects. Toxicol Lett. 2017, 273, 69–85. [Google Scholar] [CrossRef]
- Shapoval, L.M.; Dmytrenko, O.V.; Sagach, V.F.; Prylutska, S.V.; Khrapatiy, S.V.; Zavodovskyi, D.O.; Prylutskyy, Y.I.; Tsierkezos, N.; Ritter, U. Systemic Administrations of Water-Dispersible Single-Walled Carbon Nanotubes: Activation of NOS in Spontaneously Hypertensive Rats. Neurophysiology 2020, 52, 101–109. [Google Scholar] [CrossRef]
- Park, K.H.; Chhowalla, M.; Iqbal, Z.; Sesti, F. Single-walled Carbon Nanotubes Are a New Class of Ion Channel Blockers. J. Biol. Chem. 2003, 278, 50212–50216. [Google Scholar] [CrossRef] [Green Version]
- Amiri, H.; Shepard, K.L.; Nuckolls, C.; Hernández Sánchez, R. Single-Walled Carbon Nanotubes: Mimics of Biological Ion Channels. Nano. Lett. 2017, 17, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Yazda, K.; Tahir, S.; Michel, T.; Loubet, B.; Manghi, M.; Bentin, J.; Picaud, F.; Palmeri, J.; Henn, F.; Jourdain, V. Voltage-activated transport of ions through single-walled carbon nanotubes. Nanoscale 2017, 9, 11976–11986. [Google Scholar] [CrossRef]
- Melnyk, M.I.; Ivanova, I.V.; Dryn, D.O.; Prylutskyy, Y.I.; Hurmach, V.V.; Platonov, M.; Al Kury, L.T.; Ritter, U.; Soloviev, A.I.; Zholos, A.V. C60 fullerenes selectively inhibit BKCa but not Kv channels in pulmonary artery smooth muscle cells. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dryn, D.O.; Melnyk, M.I.; Al Kury, L.T.; Prylutskyy, Y.; Ritter, U.; Zholos, A.V. C 60 fullerenes disrupt cellular signalling leading to TRPC4 and TRPC6 channels opening by the activation of muscarinic receptors and G-proteins in small intestinal smooth muscles. Cell. Signal. 2018, 43, 40–46. [Google Scholar] [CrossRef]
- Tsvilovskyy, V.V.; Zholos, A.V.; Aberle, T.; Philipp, S.E.; Dietrich, A.; Zhu, M.X.; Birbaurmer, L.; Freichel, M.; Flockerzi, V. Deletion of TRPC4 and TRPC6 in Mice Impairs Smooth Muscle Contraction and Intestinal Motility In Vivo. Gastroenterology 2009, 137, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Bolton, T.B.; Prestwich, S.A.; Zholos, A.V.; Gordienko, D.V. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 1999, 61, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.V. Regulation of TRP-like muscarinic cation current in gastrointestinal smooth muscle with special reference to PLC/InsP3/Ca2+ system. Acta Pharmacol. Sin. 2006, 27, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.; Shi, J.; Zhu, Y.; Kustov, M.; Tian, J.B.; Stevens, A.; Wu, M.; Xu, J.; Long, S.; Yang, P.; et al. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J. Biol. Chem. 2011, 286, 33436–33446. [Google Scholar] [CrossRef] [Green Version]
- Tsvilovskyy, V.V.; Zholos, A.V.; Bolton, T.B. Effects of polyamines on the muscarinic receptor-operated catin curent in guinea-pig ileal smooth muscle myocytes. Br. J. Pharmacol. 2004, 143, 968–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zholos, A.; Tsytsyura, Y.D.; Philyppov, I.B.; Shuba, M.F.; Bolton, T.B. Voltage-dependent inhibition of the muscarinic cationic current in guinea-pig ileal cells by SK&F 96365. Br. J. Pharmacol. 2000, 129, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter, U.; Scharff, P.; Dmytrenko, O.; Kulish, N.; Prylutskyy, Y.; Belyi, N.; Gubanov, V.; Komarova, L.; Lizunova, S.; Shlapatskaya, V.; et al. Radiation damage and Raman vibrational modes of single-walled carbon nanotubes. Chem. Phys. Lett. 2007, 447, 252–256. [Google Scholar] [CrossRef]
- Himmerlich, M.; Krischok, S.; Lebedev, V.; Ambacher, O.; Schaefer, J. Morphology and surface electronic structure of MBE grown InN. J. Cryst. Growth 2007, 306, 6–11. [Google Scholar] [CrossRef]
- Korolovych, V.; Bulavin, L.; Prylutskyy, Y.; Khrapatiy, S.V.; Tsierkezos, N.G.; Ritter, U. Influence of Single-Walled Carbon Nanotubes on Thermal Expansion of Water. Int. J. Thermophys. 2014, 35, 19–31. [Google Scholar] [CrossRef]
- Emami, F.S.; Puddu, V.; Berry, R.J.; Varshney, V.; Patwardhan, S.V.; Perry, C.C.; Heintz, H. Force field and a surface model database for silica to simulate interfacial properties in atomic resolution. Chem. Mater. 2014, 26, 2647–2658. [Google Scholar] [CrossRef]
- Heinz, H.; Lin, T.-J.; Mishra, R.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef]
- Lin, T.J.; Heinz, H. Accurate Force Field Parameters and pH Resolved Surface Models for Hydroxyapatite to Understand Structure, Mechanics, Hydration, and Biological Interfaces. J. Phys. Chem. C 2016, 120, 4975–4992. [Google Scholar] [CrossRef] [Green Version]
- Heinz, H.; Vaia, R.A.; Farmer, B.L.; Naik, R.R. Accurate simulation of surfaces and interfaces of face-centered cubic metals using 12-6 and 9-6 lennard-jones potentials. J. Phys. Chem. C 2008, 112, 17281–17290. [Google Scholar] [CrossRef]
- Kozma, D.; Simon, I.; Tusnády, G.E. PDBTM: Protein Data Bank of transmembrane proteins after 8 years. Nucleic Acids Res. 2012, 41, D524–D529. [Google Scholar] [CrossRef] [Green Version]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucl. Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef]
- Warren, G.L.; Andrews, C.W.; Capelli, A.M.; Clarke, B.; LaLonde, J.; Lambert, M.H.; Lidvall, M.; Nevins, N.; Semus, S.F.; Senger, S.; et al. A critical assessment of docking programs and scoring functions. J. Med. Chem. 2006, 49, 5912–5931. [Google Scholar] [CrossRef]
- McMartin, C.; Bohacek, R.S. QXP: Powerful, rapid computer algorithms for structure-based drug design. J. Comput Aided Mol. Des. 1997, 11, 333–344. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Im, W. Automated Builder and Database of Protein/Membrane Complexes for Molecular Dynamics Simulations. PLoS ONE 2007, 2, e880. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for Mixed Bilayers and Its Application to Yeast Membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, F.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theor. Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI Membrane Builder for Complex Biological Membrane Simulations with Glycolipids and Lipoglycans. J. Chem. Theor. Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Mackerell, A.D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Skivka, L.M.; Prylutska, S.V.; Rudyk, M.P.; Khranovska, N.M.; Opeida, I.V.; Hurmach, V.V.; Prylutskyy, Y.I.; Sukhobud, L.F.; Ritter, U. C60 fullerene and its nanocomplexes with anticancer drugs modulate circulating phagocyte functions and dramatically increase ROS generation in transformed monocytes. Cancer Nanotechnol. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zholos, A.V.; Bolton, T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997, 122, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Zholos, A.V.; Tsytsyura, Y.D.; Gordienko, D.; Tsvilovskyy, V.V.; Bolton, T.B. Phospholipase C, but not InsP3or DAG, -dependent activation of the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 2004, 141, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Zholos, A.V.; Bolton, T.B. A novel GTP-dependent mechanism of ileal muscarinic metabotropic channel desensitization. Br. J. Pharmacol. 1996, 119, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Gordienko, D.V.; Zholos, A.V.; Bolton, T.B. Membrane ion channels as physiological targets for local Ca2+ signalling. J. Microsc. 1999, 196, 305–316. [Google Scholar] [CrossRef]
- Zholos, A.V.; Zholos, A.A.; Bolton, T.B. G-protein-gated TRP-like cationic channel activated by muscarinic receptors: Effect of potential on single-channel gating. J. Gen. Physiol. 2004, 123, 581–598. [Google Scholar] [CrossRef] [Green Version]
- Clapham, D.E.; Julius, D.; Montell, C.; Schultz, G. International Union of Pharmacology. XLIX. Nomenclature and Structure-Function Relationships of Transient Receptor Potential Channels. Pharmacol. Rev. 2005, 57, 427–450. [Google Scholar] [CrossRef] [Green Version]
- Freichel, M.; Tsvilovskyy, V.; Camacho-Londoño, J.E. TRPC4- and TRPC4-Containing Channels. Handb. Exp. Pharmacol. 2014, 222, 85–128. [Google Scholar] [CrossRef]
- Rubaiy, H.N.; Ludlow, M.J.; Henrot, M.; Gaunt, H.J.; Miteva, K.; Cheung, S.Y.; Tanahasi, N.; Hamzah, N.; Musialowsky, K.E.; Blythe, N.M.; et al. Picomolar, selective, and subtype-specific small-molecule inhibition of TRPC1/4/5 channels. J. Biol. Chem. 2017, 292, 8158–8173. [Google Scholar] [CrossRef] [Green Version]
- Cheung, S.Y.; Henrot, M.; Al-Saad, M.; Baumann, M.; Muller, H.; Unger, A.; Rubaiy, H.N.; Mathar, I.; Dinkel, K.; Nussbaumer, N.; et al. TRPC4/TRPC5 channels mediate adverse reaction to the cancer cell cytotoxic agent (-)-Englerin A. Oncotarget 2018, 9, 29634–29643. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Kury, L.T.; Papandreou, D.; Hurmach, V.V.; Dryn, D.O.; Melnyk, M.I.; Platonov, M.O.; Prylutskyy, Y.I.; Ritter, U.; Scharff, P.; Zholos, A.V. Single-Walled Carbon Nanotubes Inhibit TRPC4-Mediated Muscarinic Cation Current in Mouse Ileal Myocytes. Nanomaterials 2021, 11, 3410. https://doi.org/10.3390/nano11123410
Al Kury LT, Papandreou D, Hurmach VV, Dryn DO, Melnyk MI, Platonov MO, Prylutskyy YI, Ritter U, Scharff P, Zholos AV. Single-Walled Carbon Nanotubes Inhibit TRPC4-Mediated Muscarinic Cation Current in Mouse Ileal Myocytes. Nanomaterials. 2021; 11(12):3410. https://doi.org/10.3390/nano11123410
Chicago/Turabian StyleAl Kury, Lina T., Dimitrios Papandreou, Vasyl V. Hurmach, Dariia O. Dryn, Mariia I. Melnyk, Maxim O. Platonov, Yuriy I. Prylutskyy, Uwe Ritter, Peter Scharff, and Alexander V. Zholos. 2021. "Single-Walled Carbon Nanotubes Inhibit TRPC4-Mediated Muscarinic Cation Current in Mouse Ileal Myocytes" Nanomaterials 11, no. 12: 3410. https://doi.org/10.3390/nano11123410
APA StyleAl Kury, L. T., Papandreou, D., Hurmach, V. V., Dryn, D. O., Melnyk, M. I., Platonov, M. O., Prylutskyy, Y. I., Ritter, U., Scharff, P., & Zholos, A. V. (2021). Single-Walled Carbon Nanotubes Inhibit TRPC4-Mediated Muscarinic Cation Current in Mouse Ileal Myocytes. Nanomaterials, 11(12), 3410. https://doi.org/10.3390/nano11123410