Lignin–Cellulose Nanocrystals from Hemp Hurd as Light-Coloured Ultraviolet (UV) Functional Filler for Enhanced Performance of Polyvinyl Alcohol Nanocomposite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
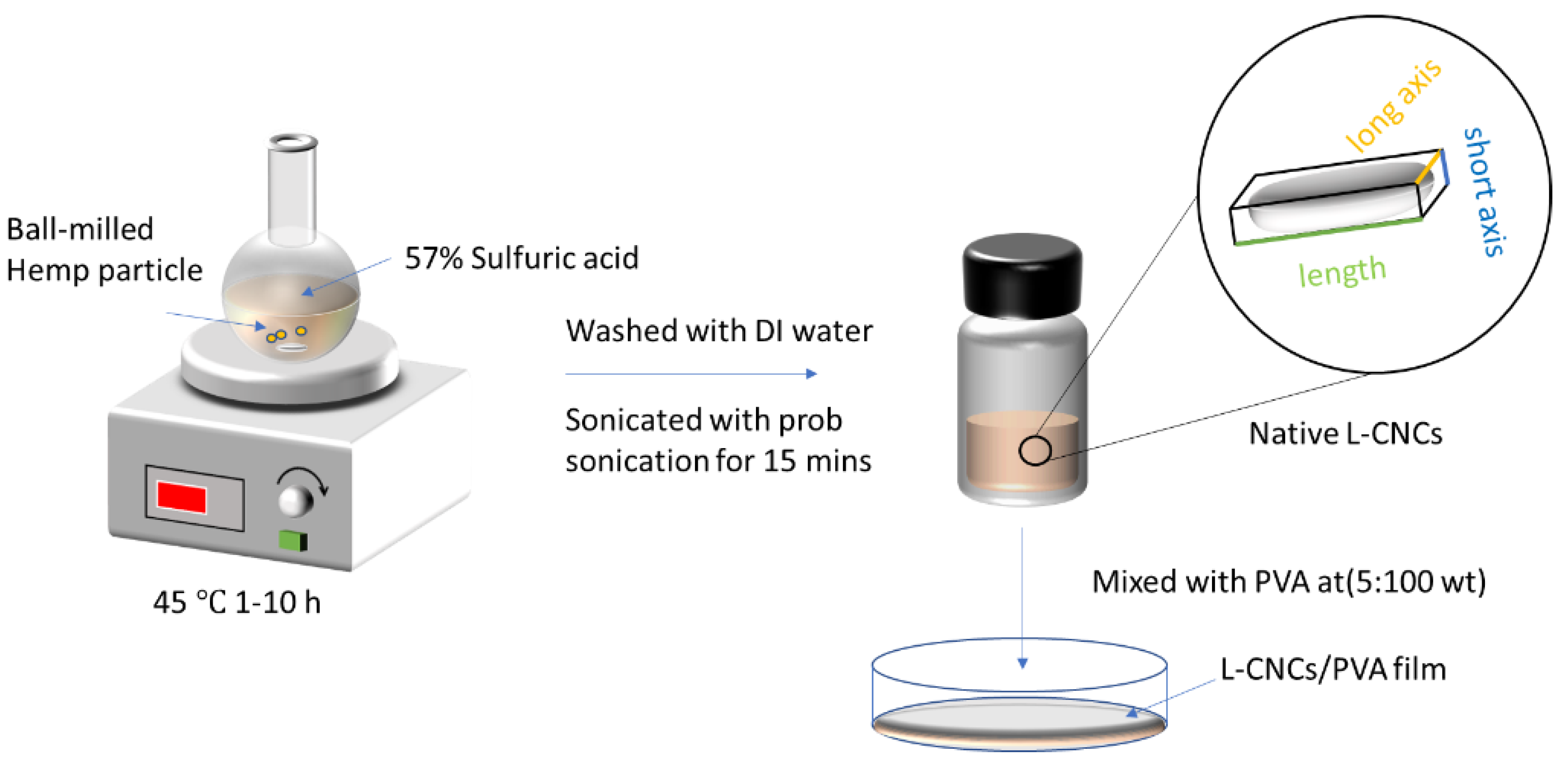
2.2. Fabrication of Native Lignin–Cellulose Nanocrystal (L-CNCs)
2.3. Preparation of L-CNCs/PVA Film
2.4. Material Characterisation
2.4.1. Lignin Content
2.4.2. Zeta Potential
2.4.3. The Sulphur Content and Yield of L-CNCs
2.4.4. Atomic Force Microscope (AFM)
2.4.5. Transmission Electron Microscopy (TEM)
2.4.6. Fourier Transform Infrared (FTIR)
2.4.7. UV-Shielding Performance of the L-CNCs and the Films
2.4.8. X-ray Diffraction (XRD)
2.4.9. Tensile Properties
2.4.10. Water Vapour Permeability
2.4.11. The Statistical Analysis
3. Results and Discussion
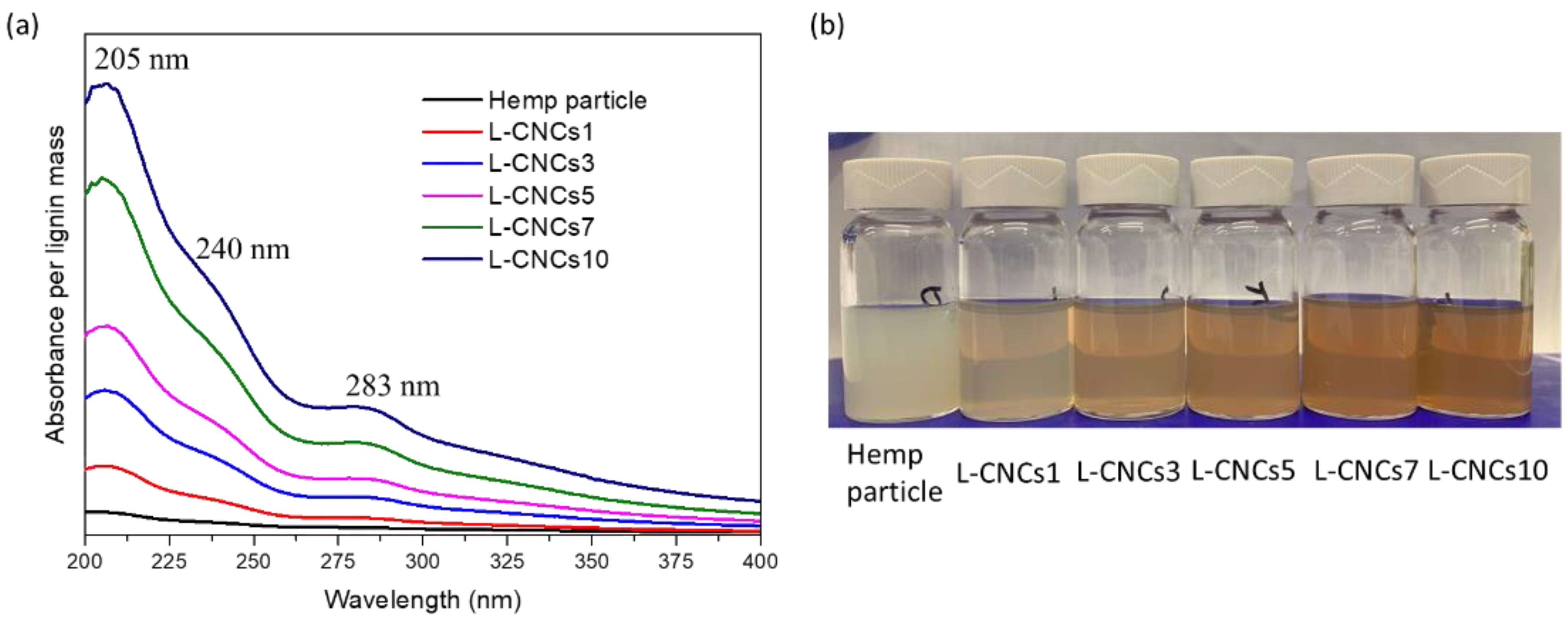
3.1. Lignin Content and Stability of L-CNCs
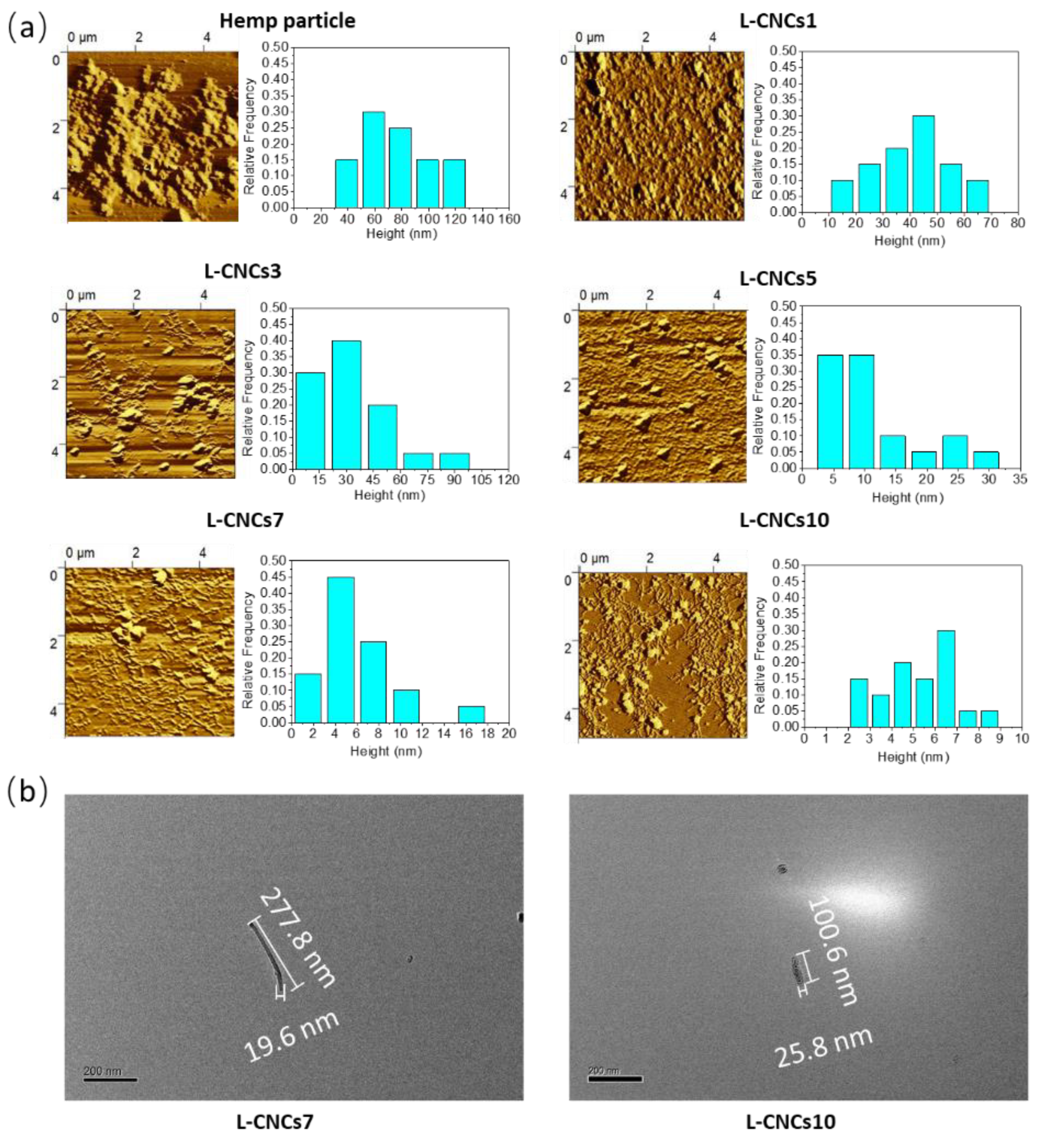
3.2. Morphology and Size Analysis of L-CNCs
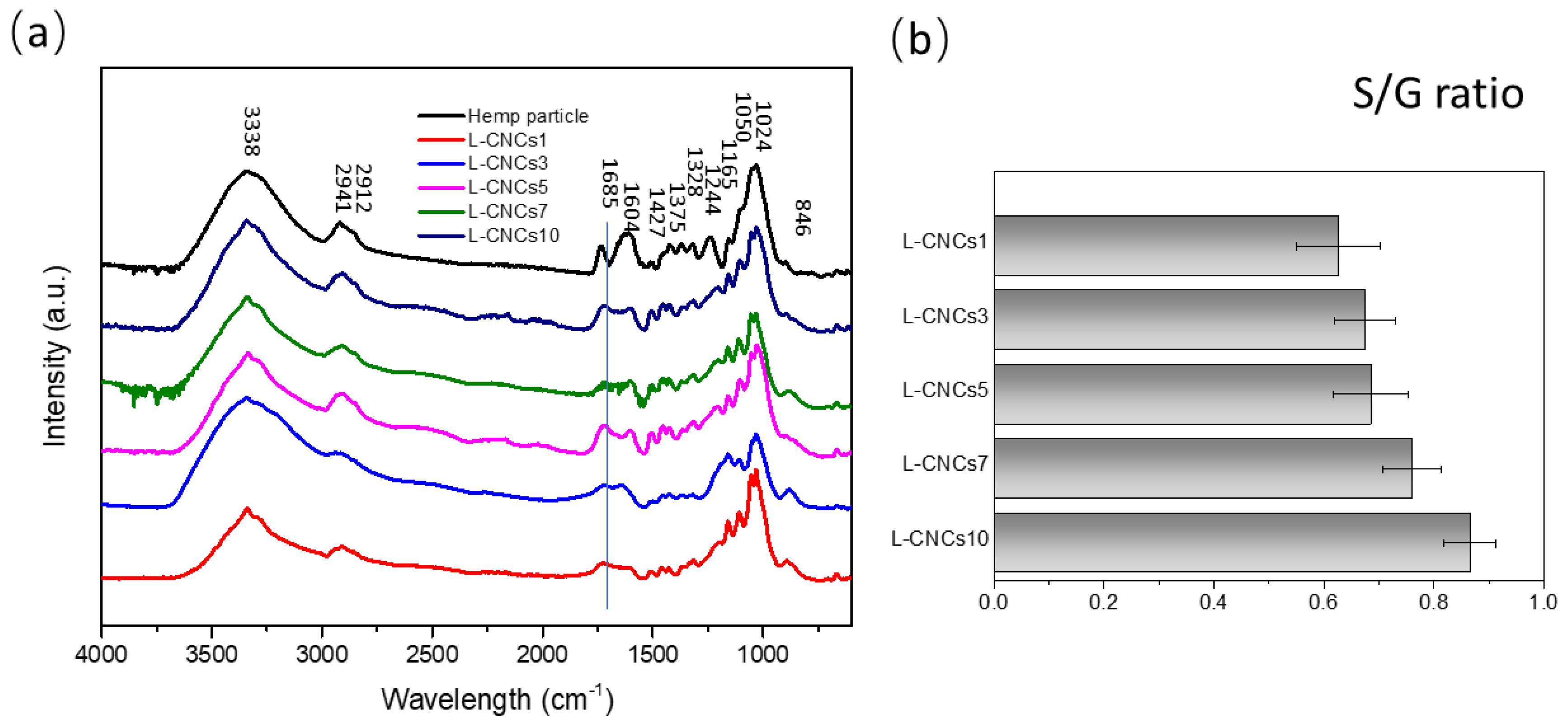
3.3. FTIR Analysis
3.3.1. FTIR Analysis of the L-CNCs Particles
3.3.2. FTIR Analysis of the L-CNCs/PVA Films
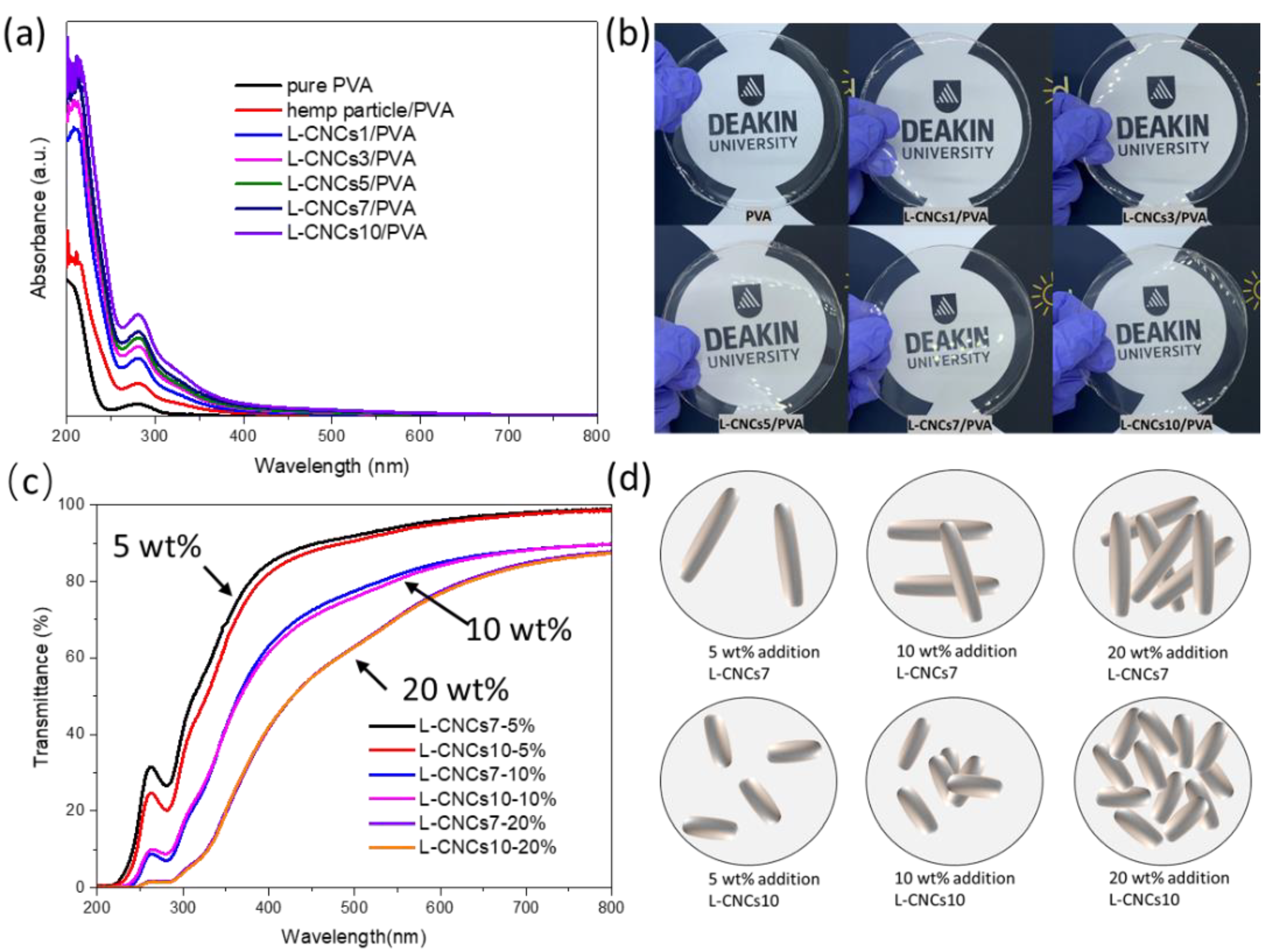
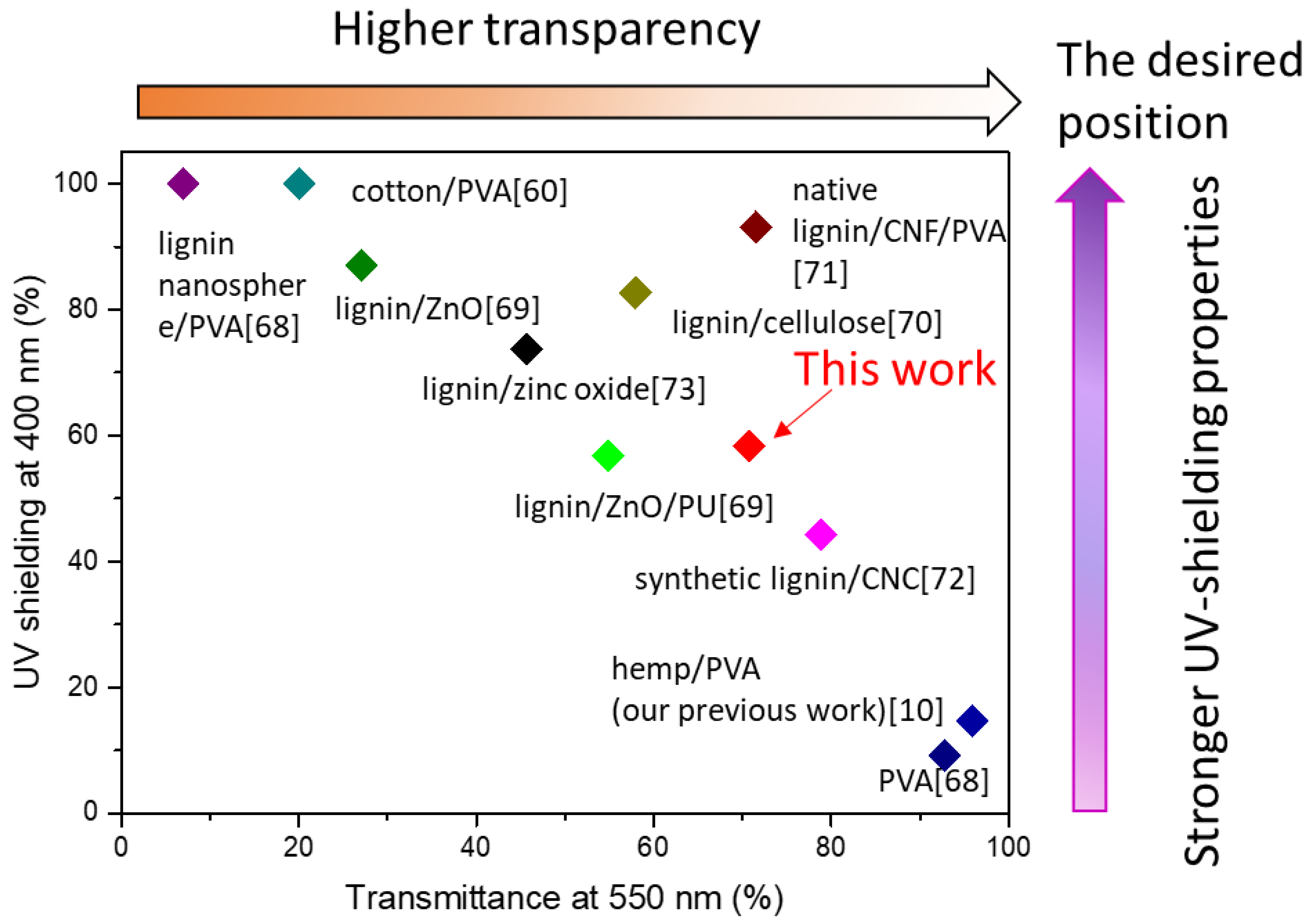
3.4. UV-Shielding Analysis
3.4.1. UV-Shielding Performance of Hemp Particle and L-CNCs
3.4.2. UV-Shielding Performance of the L-CNCs/PVA Films
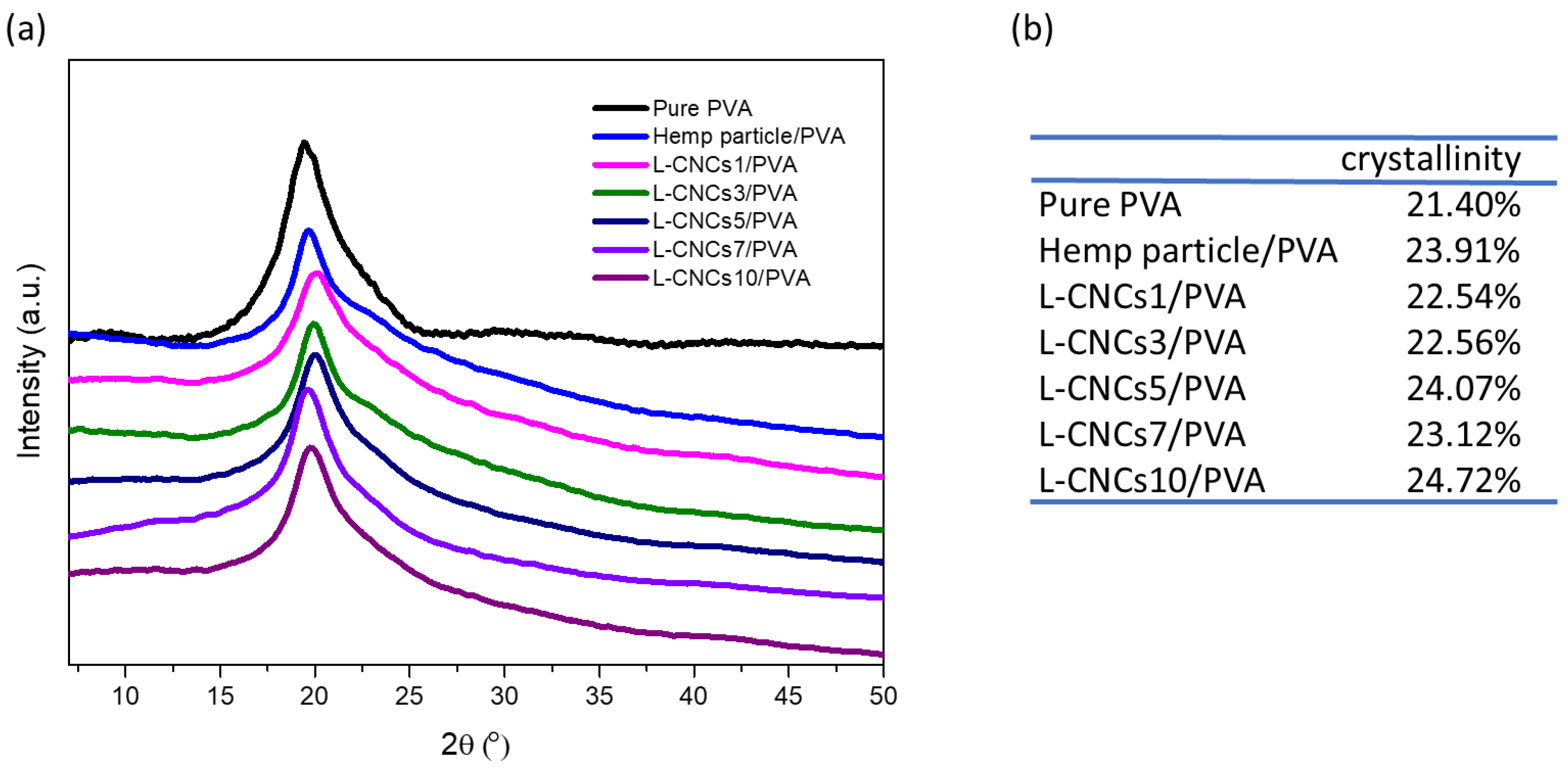
3.5. Crystallinity Analysis of the L-CNCs/PVA Films
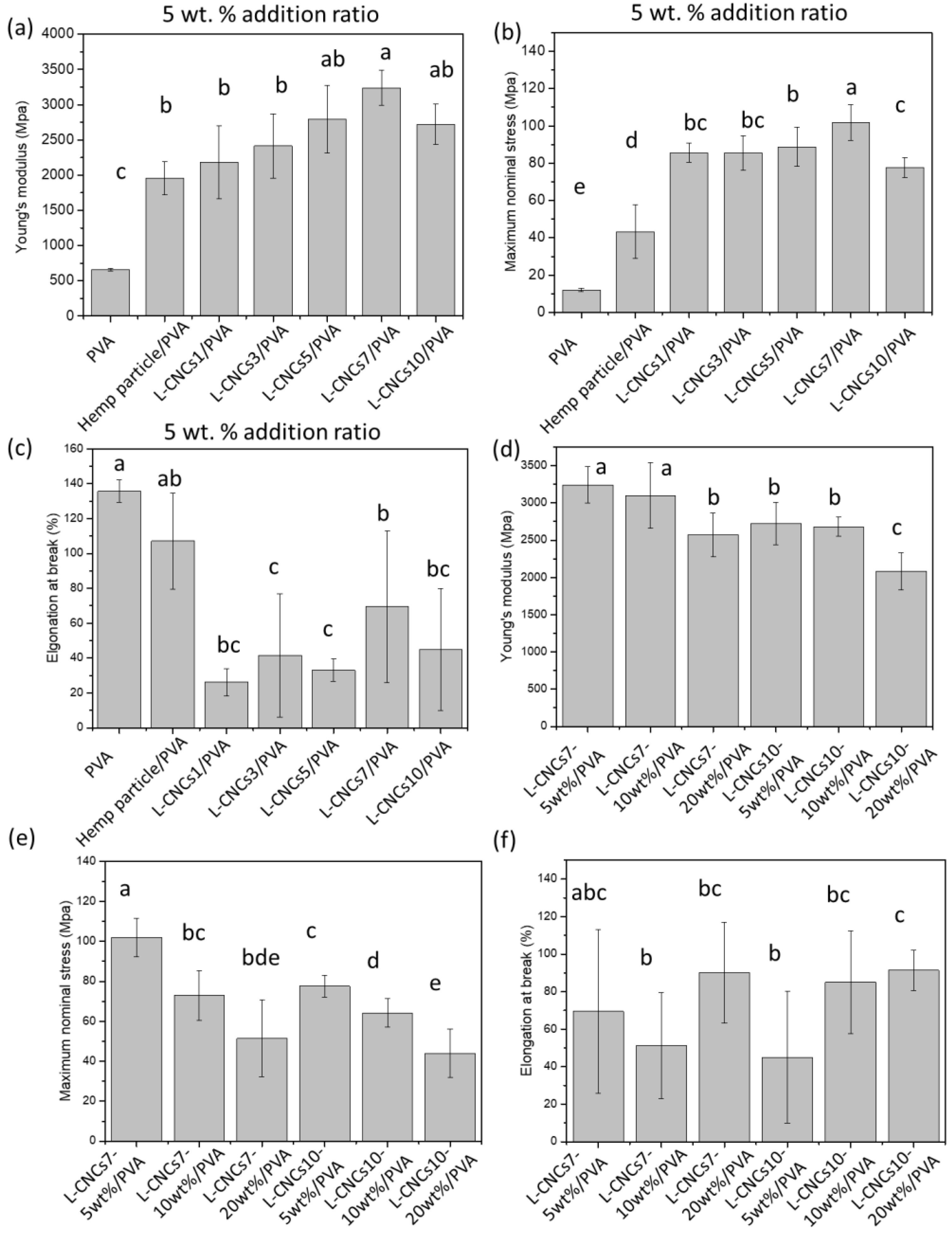
3.6. Tensile Properties of the L-CNCs/PVA Films
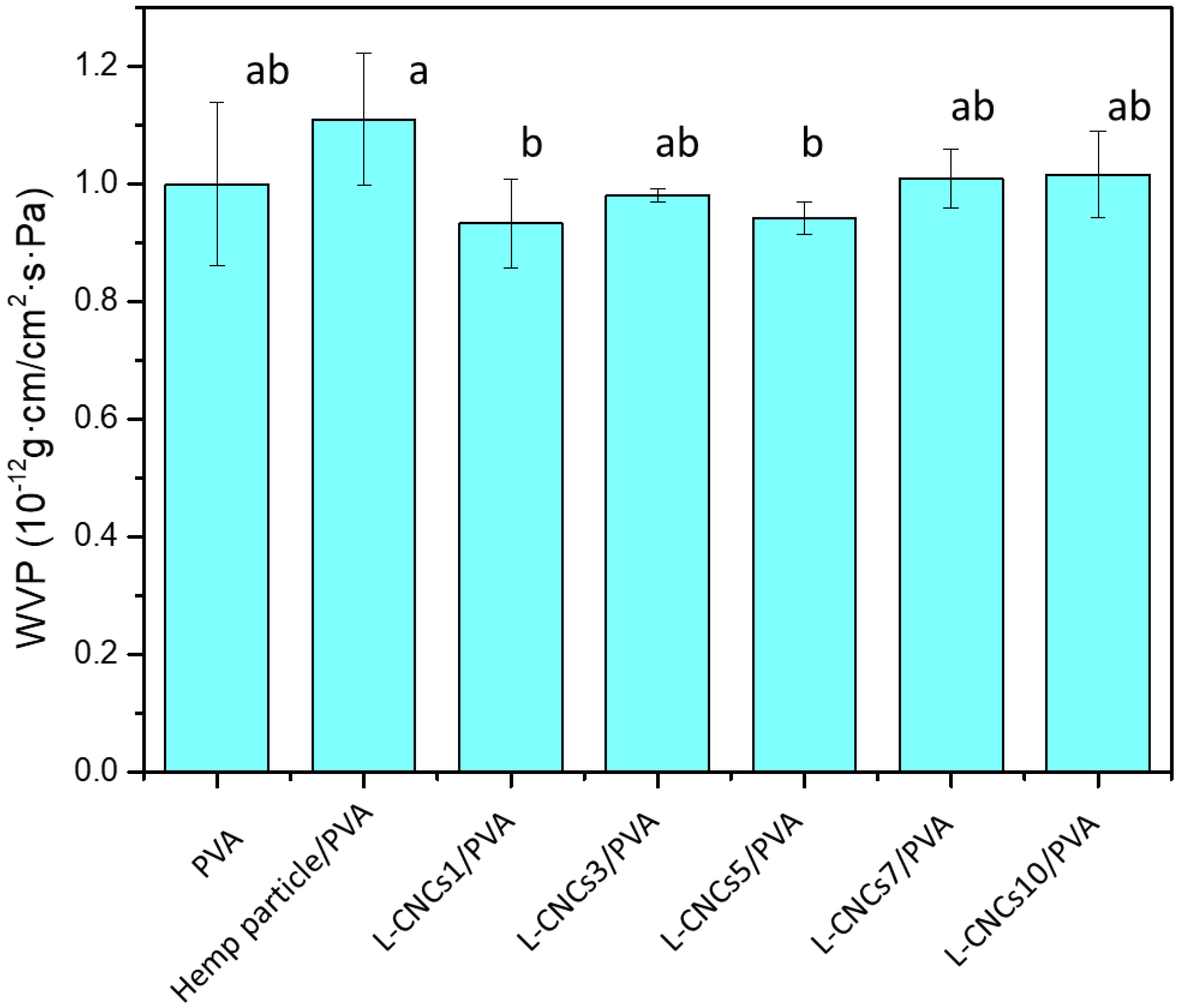
3.7. Water Vapour Permeability of the L-CNCs/PVA Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; Is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, A.N.M.A.; Zhang, Y.; Naebe, M. A review on lignocellulose/poly (vinyl alcohol) composites: Cleaner approaches for greener materials. Cellulose 2021, 1–24. [Google Scholar] [CrossRef]
- Dou, J.; Vuorinen, T.; Koivula, H.; Forsman, N.; Sipponen, M.; Hietala, S. Self-standing lignin-containing willow bark nanocellulose films for oxygen blocking and UV shielding. ACS Appl. Nano Mater. 2021, 4, 2921–2929. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Widsten, P. Lignin-based sunscreens—State-of-the-art, prospects and challenges. Cosmetics 2020, 7, 85. [Google Scholar] [CrossRef]
- Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and application of light-colored lignin nanoparticles for broad-spectrum sunscreens. Polymers 2020, 12, 699. [Google Scholar] [CrossRef] [Green Version]
- Baurhoo, B.; Ruiz-Feria, C.A.; Zhao, X. Purified lignin: Nutritional and health impacts on farm animals—A review. Anim. Feed Sci. Technol. 2008, 144, 175–184. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Catalytic conversion of structural carbohydrates and lignin to chemicals. Adv. Catal. 2017, 60, 59–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Remadevi, R.; Hinestroza, J.P.; Wang, X.; Naebe, M. Transparent ultraviolet (UV)-shielding films made from waste hemp hurd and polyvinyl alcohol (PVA). Polymers 2020, 12, 1190. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Jiang, J.; Carrillo-Enríquez, N.C.; Oguzlu, H.; Han, X.; Bi, R.; Song, M.; Saddler, J.N.; Sun, R.-C.; Jiang, F. High production yield and more thermally stable lignin-containing cellulose nanocrystals isolated using a ternary acidic deep eutectic solvent. ACS Sustain. Chem. Eng. 2020, 8, 7182–7191. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dai, H.; Zhu, J.Y. Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr. Polym. 2017, 167, 167–176. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Reiner, R.S.; Hunt, C.G.; Baez, C.; Ibach, R.; Hirth, K.C. Production of high lignin-containing and lignin-free cellulose nanocrystals from wood. Cellulose 2018, 25, 5791–5805. [Google Scholar] [CrossRef]
- Lowry, P.P.; Wagner, K.A. Hydrothermal Liquefaction Treatment Hazard Analysis Report; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2016. [Google Scholar]
- Hong, S.; Song, Y.; Yuan, Y.; Lian, H.; Liimatainen, H. Production and characterization of lignin containing nanocellulose from luffa through an acidic deep eutectic solvent treatment and systematic fractionation. Ind. Crop. Prod. 2020, 143, 111913. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, W.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation. Green Chem. 2016, 18, 1010–1018. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Physicochemical properties of film fabricated from cotton gin trash. Mater. Chem. Phys. 2020, 239, 122009. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Ewulonu, C.M.; Ralph, J.; Xu, F.; Zhang, Q.; Wu, M.; Huang, Y. Mild alkaline pretreatment for isolation of native-like lignin and lignin-containing cellulose nanofibers (LCNF) from crop waste. ACS Sustain. Chem. Eng. 2019, 7, 14135–14142. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. Cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Heitzmann, M.; Ali, A.; Legras, A.; Vandi, L.-J.; Milne, J. Hemp hurd flour as an alternative low cost filler in wood plastic composites. In Proceedings of the Conference on Performance-based and Lifecycle Structural Engineering, Brisbane, Australia, 9–11 December 2015; pp. 109–115. [Google Scholar] [CrossRef] [Green Version]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties characterization of chemically modified hemp hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Gutiérrez, A.; Ralph, J. Structural characterization of wheat straw lignin. evidence for a novel monomer in grasses. In Proceedings of the 17th International Symposium on Wood, Fibre and Pulping Chemistry, Vancouver, BC, Canada, 12–14 June 2013. [Google Scholar]
- Cai, Z.; Remadevi, R.; Al Faruque, M.A.; Setty, M.; Fan, L.; Haque, A.N.M.A.; Naebe, M. Fabrication of a cost-effective lemongrass (Cymbopogon citratus) membrane with antibacterial activity for dye removal. RSC Adv. 2019, 9, 34076–34085. [Google Scholar] [CrossRef] [Green Version]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of extractives in biomass. Lab. Anal. Proced. 2005, 1617, 1–9. [Google Scholar]
- Yao, L.; Chen, C.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Dong, C.; Yang, H. Insights of ethanol organosolv pretreatment on lignin properties of Broussonetia papyrifera. ACS Sustain. Chem. Eng. 2018, 6, 14767–14773. [Google Scholar] [CrossRef]
- Acid-Insoluble Lignin in Wood and Pulp (Reaffirmation of T 222 om-02). Available online: https://www.tappi.org/content/SARG/T222.pdf (accessed on 24 February 2020).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); (Revised July 2011); National Renewable Energy Laboratory: Golden, Colorado, 2008. [Google Scholar]
- Uskoković, V. Dynamic light scattering based microelectrophoresis: Main prospects and limitations. J. Dispers. Sci. Technol. 2012, 33, 1762–1786. [Google Scholar] [CrossRef] [Green Version]
- Seta, F.T.; An, X.; Liu, L.; Zhang, H.; Yang, J.; Zhang, W.; Nie, S.; Yao, S.; Cao, H.; Xu, Q.; et al. Preparation and characterization of high yield cellulose nanocrystals (CNC) derived from ball mill pretreatment and maleic acid hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Parot, J.; Mukherjee, A.; Couillard, M.; Zou, S.; Hackley, V.A.; Johnston, L.J. Characterization of size and aggregation for cellulose nanocrystal dispersions separated by asymmetrical-flow field-flow fractionation. Cellulose 2020, 27, 2015–2028. [Google Scholar] [CrossRef]
- Meng, Y.; Wu, Q.; Young, T.M.; Huang, B.; Wang, S.; Li, Y. Analyzing three-dimensional structure and geometrical shape of individual cellulose nanocrystal from switchgrass. Polym. Compos. 2017, 38, 2368–2377. [Google Scholar] [CrossRef]
- Bushell, M.; Meija, J.; Chen, M.; Batchelor, W.; Browne, C.; Cho, J.-Y.; Clifford, C.A.; Al-Rekabi, Z.; Vanderfleet, O.M.; Cranston, E.D.; et al. Particle size distributions for cellulose nanocrystals measured by atomic force microscopy: An interlaboratory comparison. Cellulose 2021, 28, 1387–1403. [Google Scholar] [CrossRef]
- Mariano, M.; Chirat, C.; El Kissi, N.; Dufresne, A. Impact of cellulose nanocrystal aspect ratio on crystallization and reinforcement of poly(butylene adipate-co-terephthalate). J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2284–2297. [Google Scholar] [CrossRef]
- Rana, R.; Langenfeld-Heyser, R.; Finkeldey, R.; Polle, A. FTIR spectroscopy, chemical and histochemical characterisation of wood and lignin of five tropical timber wood species of the family of Dipterocarpaceae. Wood Sci. Technol. 2010, 44, 225–242. [Google Scholar] [CrossRef] [Green Version]
- Saadiah, M.A.; Zhang, D.; Nagao, Y.; Muzakir, S.K.; Samsudin, A.S. Reducing crystallinity on thin film based CMC/PVA hybrid polymer for application as a host in polymer electrolytes. J. Non Cryst. Solids 2019, 511, 201–211. [Google Scholar] [CrossRef]
- ASTM D882-18—Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Available online: https://webstore.ansi.org/Standards/ASTM/ASTMD88218?gclid=CjwKCAjw_o-HBhAsEiwANqYhp8DjWRxVKfxJ4R_ODl23ahpp4C9wbhs0AGHQldrILpIVQ0z2Zr0KKBoCL8wQAvD_BwE (accessed on 6 July 2021).
- TAPPI T 464 om-2012 (R2018)—Water Vapor Transmission Rate of Paper and Paperboard at High Temperature and Humidity. Available online: https://webstore.ansi.org/standards/tappi/tappi464om2012r2018 (accessed on 6 July 2021).
- Bunzel, M.; Schüßler, A.; Tchetseubu Saha, G. Chemical characterization of Klason lignin preparations from plant-based foods. J. Agric. Food Chem. 2011, 59, 12506–12513. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: A review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Kristiani, A.; Abimanyu, H.; Setiawan, A.H.; Aulia, F. Effect of pretreatment process by using diluted acid to characteristic of oil palm’s frond. Energy Procedia 2013, 32, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Matsakas, L.; Gerber, M.; Yu, L.; Rova, U.; Christakopoulos, P. Preparation of low carbon impact lignin nanoparticles with controllable size by using different strategies for particles recovery. Ind. Crop. Prod. 2020, 147, 112243. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Dickwella Widanage, M.C.; Mentink-Vigier, F.; Cosgrove, D.J.; Wang, T. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun. 2019, 10, 347. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Naebe, M. Flexible water-resistant semi-transparent cotton gin trash/poly (vinyl alcohol) bio-plastic for packaging application: Effect of plasticisers on physicochemical properties. J. Clean. Prod. 2021, 303, 126983. [Google Scholar] [CrossRef]
- Lionetto, F.; Del Sole, R.; Cannoletta, D.; Vasapollo, G.; Maffezzoli, A. Monitoring Wood degradation during weathering by cellulose crystallinity. Materials 2012, 5, 1910–1922. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Xiao, L.; Meng, L.; Zhang, X.; Sun, R. Isolation and Structural Characterization of Lignin from Cotton Stalk Treated in an Ammonia Hydrothermal System. Int. J. Mol. Sci. 2012, 13, 15209–15226. [Google Scholar] [CrossRef]
- Spiridon, I.; Teaca, C.A.; Bodîrlău, R. Structural changes evidenced by FTIR spectroscopy in cellulosic materials after pre-treatment with ionic liquid and enzymatic hydrolysis. Bioresources 2011, 6, 400–413. [Google Scholar] [CrossRef]
- Chandel, A.K.; Antunes, F.F.A.; Anjos, V.; Bell, M.J.V.; Rodrigues, L.N.; Singh, O.V.; Rosa, C.A.; Pagnocca, F.C.; Da Silva, S.S. Ultra-structural mapping of sugarcane bagasse after oxalic acid fiber expansion (OAFEX) and ethanol production by Candida shehatae and Saccharomyces cerevisiae. Biotechnol. Biofuels 2013, 6, 4. [Google Scholar] [CrossRef]
- Infrared Spectroscopy Absorption Table—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Parameters/Infrared_Spectroscopy_Absorption_Table (accessed on 28 November 2021).
- Zhang, L.; Yan, L.; Wang, Z.; Laskar, D.D.; Swita, M.S.; Cort, J.R.; Yang, B. Characterization of lignin derived from water-only and dilute acid flowthrough pretreatment of poplar wood at elevated temperatures. Biotechnol. Biofuels 2015, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, T.; Chiba, W.; Saito, K.; Fukushima, K.; Uraki, Y.; Ubukata, M. Influence of syringyl to guaiacyl ratio on the structure of natural and synthetic lignins. J. Agric. Food Chem. 2009, 58, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from micro- to nanosize: Production methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasiukaitytė-Grojzdek, E.; Huš, M.; Grilc, M.; Likozar, B. Acid-catalysed α-O-4 aryl-ether bond cleavage in methanol/(aqueous) ethanol: Understanding depolymerisation of a lignin model compound during organosolv pretreatment. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Sammons, R.J.; Harper, D.P.; Labbé, N.; Bozell, J.J.; Elder, T.; Rials, T.G. Characterization of Organosolv lignins using thermal and FT-IR spectroscopic analysis. BioResources 2013, 8, 2752–2767. [Google Scholar] [CrossRef]
- Awungacha Lekelefac, C.; Busse, N.; Herrenbauer, M.; Czermak, P. Photocatalytic based degradation processes of lignin derivatives. Int. J. Photoenergy 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Nuruddin, M.; Chowdhury, R.A.; Szeto, R.; Howarter, J.A.; Erk, K.A.; Szczepanski, C.R.; Youngblood, J.P. Structure-Property Relationship of cellulose nanocrystal-polyvinyl alcohol thin films for high barrier coating applications. ACS Appl. Mater. Interfaces 2021, 13, 12472–12482. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaramudu, T.; Zhai, L.; Kim, H.C.; Agumba, D.O. Preparation of Cellulose Nanocrystal-Reinforced Physical Hydrogels for Actuator Application. Crystals 2020, 10, 969. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Biodegradable cotton gin trash/poly(vinyl alcohol) composite plastic: Effect of particle size on physicochemical properties. Powder Technol. 2020, 375, 1–10. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv. Polym. Technol. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- You, T.; Xu, F. Applications of Molecular Spectroscopic Methods to the Elucidation of Lignin Structure. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; IntechOpen: London, UK, 2016; pp. 235–260. [Google Scholar]
- Jančič, U.; Bračič, M.; Ojstršek, A.; Božič, M.; Mohan, T.; Gorgieva, S. Consolidation of cellulose nanofibrils with lignosulphonate bio-waste into excellent flame retardant and UV blocking membranes. Carbohydr. Polym. 2021, 251, 117126. [Google Scholar] [CrossRef]
- Nakano, J.; Meshitsuka, G. The Detection of Lignin; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Zhang, H.; Chen, F.; Liu, X.; Fu, S. Micromorphology influence on the colour performance of lignin and its application in guiding the preparation of light-coloured lignin sunscreen. ACS Sustain. Chem. Eng. 2018, 6, 12532–12540. [Google Scholar] [CrossRef]
- Zhang, Y.; Naebe, M. Lignin: A review on structure, properties, and applications as a light-colored UV absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, X.; Li, D.; Dong, Z.; Wang, J.; Ren, L.; Xue, B.; Li, F. Enhanced ultraviolet shielding performances of TiO2; nanorods in different crystalline structures and illite-loaded composites. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 608, 125532. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, Y.; Li, G.; Han, Y.; Chu, F. Transparent nanocomposite films of lignin nanospheres and poly(vinyl alcohol) for UV-Absorbing. Ind. Eng. Chem. Res. 2018, 57, 1207–1212. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Liu, W.; Fu, F.; Yang, D. A novel lignin/ZnO hybrid nanocomposite with excellent UV-absorption ability and its application in transparent polyurethane coating. Ind. Eng. Chem. Res. 2017, 56, 11133–11141. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-lignin biodegradable and flexible UV protection film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Ismail, M.Y.; Zhang, K.; Tejesvi, M.V.; Ämmälä, A. Transparent lignin-containing wood nanofiber films with UV-blocking, oxygen barrier, and anti-microbial properties. J. Mater. Chem. A 2020, 8, 7935–7946. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V. Natural organic UV-absorbent coatings based on cellulose and lignin: Designed effects on spectroscopic properties. Biomacromolecules 2012, 13, 4081–4088. [Google Scholar] [CrossRef]
- Kaur, R.; Thakur, N.S.; Chandna, S.; Bhaumik, J. Development of agri-biomass based lignin derived zinc oxide nanocomposites as promising UV protectant-cum-antimicrobial agents. J. Mater. Chem. B 2020, 8, 260–269. [Google Scholar] [CrossRef]
- Abdel Bary, E.M.; Soliman, Y.A.; Fekri, A.; Harmal, A.N. Aging of novel membranes made of PVA and cellulose nanocrystals extracted from Egyptian rice husk manufactured by compression moulding process. Int. J. Environ. Stud. 2018, 75, 750–762. [Google Scholar] [CrossRef]
- Marchi, B.C.; Keten, S. Microstructure and size effects on the mechanics of two dimensional, high aspect ratio nanoparticle assemblies. Front. Mater. 2019, 6, 174. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]


| Sample Abbreviation | Acid Insoluble Lignin (%) | Acid Soluble Lignin (%) | Zeta Potential Value (mV) | Sulphur Element Content (wt. %) | Yield (%) |
|---|---|---|---|---|---|
| Hemp particle | 11.3% | 1.4% | −11.36 ± 0.64 d | 0.6 | 86.8 |
| L−CNCs1 | 10.4% | 1.8% | −28.63 ± 0.67 c | 4.77 | 48 |
| L−CNCs3 | 12.7% | 1.6% | −35.43 ± 0.76 ab | 6.25 | 25.5 |
| L−CNCs5 | 11.3% | 2.2% | −34.26 ± 1.33 b | 7.01 | 19.8 |
| L−CNCs7 | 10.6% | 2.9% | −34.3 ± 0.88 b | 9.74 | 13.8 |
| L−CNCs10 | 13.2% | 3.4% | −36.43 ± 0.72 a | 10.41 | 11.8 |
| Sample Abbreviation | Average Length (L, nm) | Average Height (H, nm) | Aspect Ratios (L/H) |
|---|---|---|---|
| Hemp particle | 654.8 ± 339.6 a | 73.5 ± 27.3 a | 8.9 |
| L-CNCs1 | 517.3 ± 184.4 b | 40.5 ± 14.1 b | 12.8 |
| L-CNCs3 | 498.3 ± 147 b | 30.5 ± 20.3 c | 16.3 |
| L-CNCs5 | 486.5 ± 137.4 b | 10.5 ± 7.3 d | 46.3 |
| L-CNCs7 | 485.3 ± 172.3 b | 5.7 ± 3.3 d | 85.1 |
| L-CNCs10 | 195.8 ± 63.5 c | 4.9 ± 1.7 d | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Haque, A.N.M.A.; Naebe, M. Lignin–Cellulose Nanocrystals from Hemp Hurd as Light-Coloured Ultraviolet (UV) Functional Filler for Enhanced Performance of Polyvinyl Alcohol Nanocomposite Films. Nanomaterials 2021, 11, 3425. https://doi.org/10.3390/nano11123425
Zhang Y, Haque ANMA, Naebe M. Lignin–Cellulose Nanocrystals from Hemp Hurd as Light-Coloured Ultraviolet (UV) Functional Filler for Enhanced Performance of Polyvinyl Alcohol Nanocomposite Films. Nanomaterials. 2021; 11(12):3425. https://doi.org/10.3390/nano11123425
Chicago/Turabian StyleZhang, Yi, Abu Naser Md Ahsanul Haque, and Maryam Naebe. 2021. "Lignin–Cellulose Nanocrystals from Hemp Hurd as Light-Coloured Ultraviolet (UV) Functional Filler for Enhanced Performance of Polyvinyl Alcohol Nanocomposite Films" Nanomaterials 11, no. 12: 3425. https://doi.org/10.3390/nano11123425
APA StyleZhang, Y., Haque, A. N. M. A., & Naebe, M. (2021). Lignin–Cellulose Nanocrystals from Hemp Hurd as Light-Coloured Ultraviolet (UV) Functional Filler for Enhanced Performance of Polyvinyl Alcohol Nanocomposite Films. Nanomaterials, 11(12), 3425. https://doi.org/10.3390/nano11123425









