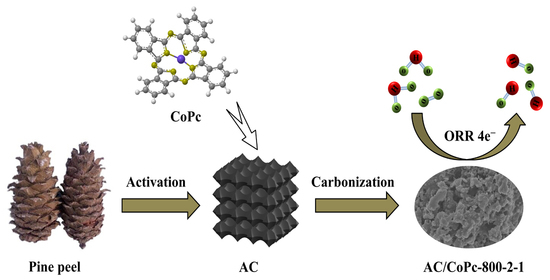
High-Level Oxygen Reduction Catalysts Derived from the Compounds of High-Specific-Surface-Area Pine Peel Activated Carbon and Phthalocyanine Cobalt
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of AC/CoPc Series Composite Catalysts
2.2.1. Preparation of AC
2.2.2. Preparation of AC/CoPc
2.3. Structure Characterizations
2.4. Electrochemical Characterizations
2.5. Calculation of Electron Transfer Number (n)
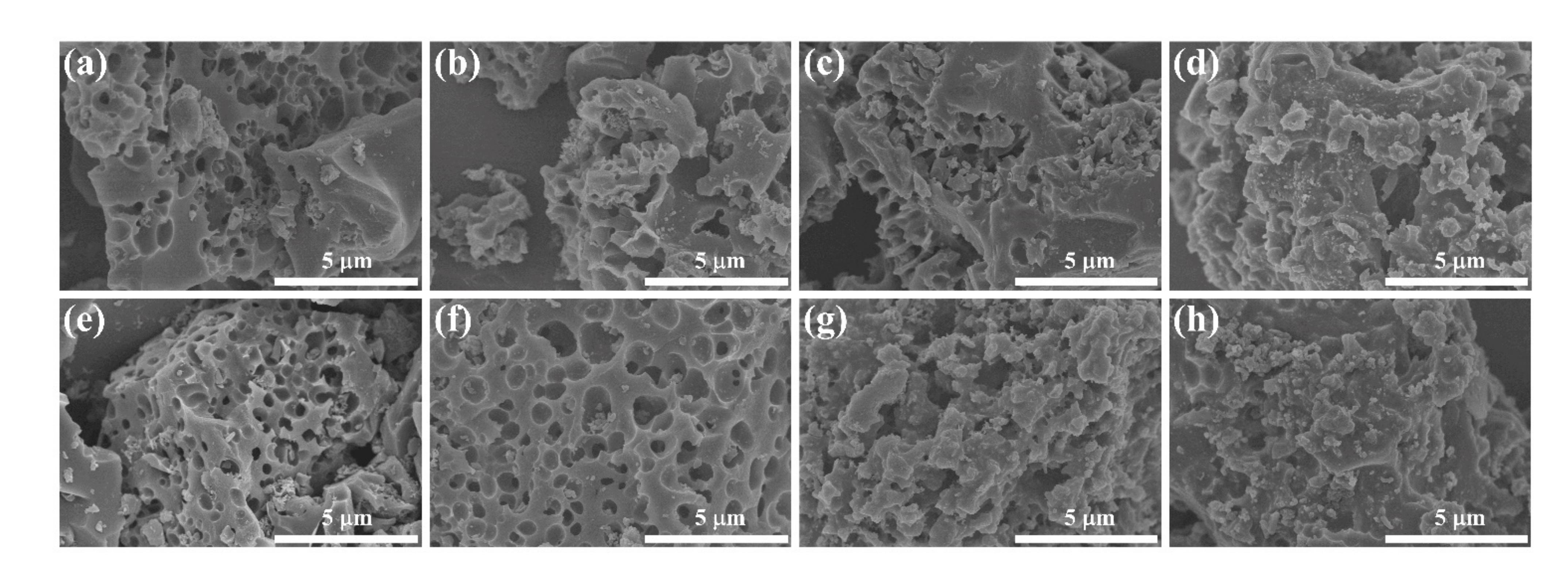
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Venegas, R.; Muñoz-Becerra, K.; Candia-Onfray, C.; Marco, J.; Zagal, J.H.; Recio, F.J. Experimental reactivity descriptors of M-N-C catalysts for the oxygen reduction reaction. Electrochimica Acta 2020, 332, 135340. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Hu, Z.; Chen, Y.; Yin, Y.; Tahini, H.A.; Lin, H.; Chen, C.; Zhang, X.; Shao, Z. Self-Assembled Ruddlesden-Popper/Perovskite Hybrid with Lattice-Oxygen Activation as a Superior Oxygen Evolution Electrocatalyst. Small 2020, 16, 2001204. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, C.; Su, P.; Shen, Z.; Liu, H.; Wang, G.; Liu, S.; Liu, J. Metal-organic-framework-derived formation of Co–N–doped carbon materials for efficient oxygen reduction reaction. J. Energy Chem. 2020, 40, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-W.; Zhang, W.-J.; Li, C.-X.; Gu, L.; Du, H.-M.; Ma, H.-Y.; Wang, S.-N.; Zhao, J.-S. A dinuclear cobalt cluster as electrocatalyst for oxygen reduction reaction. RSC Adv. 2019, 9, 42554–42560. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yu, H.-Y.; Zhang, T.-H.; Wang, W.-T.; Han, X.-F.; Yuan, Y.-X.; Yao, J.-L.; Yang, R.; Tian, J.-H. Honeycomb-like Self-Supported Co–N–C Catalysts with an Ultrastable Structure: Highly Efficient Electrocatalysts toward Oxygen Reduction Reaction in Alkaline and Acidic Solutions. ACS Appl. Energy Mater. 2021, 4, 2522–2530. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Tang, C.; Wang, J.; Zhang, Q.; Wang, Y.; Zhang, J. Atomic Modulation and Structure Design of Carbons for Bifunctional Electrocatalysis in Metal-Air Batteries. Adv. Mater. 2019, 31, e1803800. [Google Scholar] [CrossRef]
- Tan, J.; He, X.; Yin, F.; Chen, B.; Liang, X.; Li, G.; Yin, H. Bimetallic ZnCo zeolitic imidazolate framework/polypyrrole-polyaniline derived Co/N-doped carbon for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 15453–15464. [Google Scholar] [CrossRef]
- Li, Y.; Mo, C.; Li, J.; Yu, D. Pyrazine–nitrogen–rich exfoliated C4N nanosheets as efficient metal–free polymeric catalysts for oxygen reduction reaction. J. Energy Chem. 2020, 49, 243–247. [Google Scholar] [CrossRef]
- Zhang, B.; Le, M.; Chen, J.; Guo, H.; Wu, J.; Wang, L. Enhancing Defects of N-Doped Carbon Nanospheres Via Ultralow Co Atom Loading Engineering for a High-Efficiency Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2021, 4, 3439–3447. [Google Scholar] [CrossRef]
- Wan, H.; Chen, F.; Ma, W.; Liu, X.; Ma, R. Advanced electrocatalysts based on two-dimensional transition metal hydroxides and their composites for alkaline oxygen reduction reaction. Nanoscale 2020, 12, 21479–21496. [Google Scholar] [CrossRef]
- Kuznetsov, D.; Han, B.; Yu, Y.; Rao, R.R.; Hwang, J.; Román-Leshkov, Y.; Shao-Horn, Y. Tuning Redox Transitions via Inductive Effect in Metal Oxides and Complexes, and Implications in Oxygen Electrocatalysis. Joule 2018, 2, 225–244. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Sumboja, A.; Zong, Y.; Darr, J.A. Bifunctionally active nanosized spinel cobalt nickel sulfides for sustainable secondary zinc-air batteries: Examining the effects of compositional tuning on OER and ORR activity. Catal. Sci. Technol. 2020, 10, 2173–2182. [Google Scholar] [CrossRef]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Prasanna, K.; Jothi, V.R.; Vikraman, D.; Hussain, S.; Hwang, J.-H.; Kim, H.-S. Recent Advances in Nanostructured Transition Metal Carbide- and Nitride-Based Cathode Electrocatalysts for Li-O2 Batteries (LOBs): A Brief Review. Nanomaterials 2020, 10, 2106. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Hierarchical 3D Oxygenated Cobalt Molybdenum Selenide Nanosheets as Robust Trifunctional Catalyst for Water Splitting and Zinc-Air Batteries. Small 2020, 16, e2000797. [Google Scholar] [CrossRef]
- Meng, H.; Liu, Y.; Liu, H.; Pei, S.; Yuan, X.; Li, H.; Zhang, Y. ZIF67@MFC-Derived Co/N-C@CNFs Interconnected Frameworks with Graphitic Carbon-Encapsulated Co Nanoparticles as Highly Stable and Efficient Electrocatalysts for Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2020, 12, 41580–41589. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Cao, Y.; Yu, H.; Liang, H.; Peng, F. Facile Synthesis of Cobalt and Nitrogen Coordinated Carbon Nanotube as a High-Performance Electrocatalyst for Oxygen Reduction Reaction in Both Acidic and Alkaline Media. ACS Sustain. Chem. Eng. 2019, 7, 10951–10961. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, E.; Hu, C.; Zhao, Y.; Zhang, H.-M.; Zhang, Y.; Ji, M.; Yu, J.; Cong, G.; Liu, H.; et al. Controlled Synthesis of Co@N-Doped Carbon by Pyrolysis of ZIF with 2-Aminobenzimidazole Ligand for Enhancing Oxygen Reduction Reaction and the Application in Zn-Air Battery. ACS Appl. Mater. Interface. 2020, 12, 11693–11701. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Long, L.; Jia, J.; Liu, M. Carbon-nanotube-entangled Co,N-codoped carbon nanocomposite for oxygen reduction reaction. Nanotechnology 2021, 32, 205402. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, P.; Zuo, Z.; Gong, T.; Huang, N.; Lv, X.; Sun, Y.; Sun, X. N, P-co doped carbon nanotubes coupled with Co2P nanoparticles as bifunctional oxygen electrocatalyst. J. Electroanal. Chem. 2020, 871, 114327. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Qiao, M.; Wang, Y.; Mamat, X.; Chen, A.; Zou, G.; Li, L.; Hu, G.; Zhang, S.; Hu, X.; Voiry, D. Rational Design of Hierarchical, Porous, Co-Supported, N-Doped Carbon Architectures as Electrocatalyst for Oxygen Reduction. ChemSusChem 2020, 13, 741–748. [Google Scholar] [CrossRef]
- Gao, H.; Ma, Y.; Li, Y.; Cao, Y.; Yin, Z.; Luo, H.; Yan, J.; Zhang, Y. MOF-derived N-doped carbon coated Co/RGO composites with enhanced electrocatalytic activity for oxygen reduction reaction. Inorg. Chem. Commun. 2020, 123, 108330. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Zhang, G.; Sun, F.; Xing, G.; Tian, C.; Fu, H. Zinc Assisted Epitaxial Growth of N-Doped CNTs-Based Zeolitic Imidazole Frameworks Derivative for High Efficient Oxygen Reduction Reaction in Zn-Air Battery. Chem. Eng. J. 2020, 414, 127569. [Google Scholar] [CrossRef]
- Niu, H.J.; Zhang, L.; Feng, J.J.; Zhang, Q.L.; Huang, H.; Wang, A.J. Graphene-encapsulated cobalt nanoparticles embedded in porous nitrogen-doped graphitic carbon nanosheets as efficient electrocatalysts for oxygen reduction reaction. J. Colloid Interface Sci. 2019, 552, 744–751. [Google Scholar] [CrossRef]
- Praats, R.; Käärik, M.; Kikas, A.; Kisand, V.; Aruväli, J.; Paiste, P.; Merisalu, M.; Sarapuu, A.; Leis, J.; Sammelselg, V.; et al. Electroreduction of oxygen on cobalt phthalocyanine-modified carbide-derived carbon/carbon nanotube composite catalysts. J. Solid State Electrochem. 2020, 25, 57–71. [Google Scholar] [CrossRef]
- Kumar, A.; Gonçalves, J.M.; Lima, A.R.; Matias, T.A.; Nakamura, M.; Bernardes, J.S.; Araki, K.; Bertotti, M. Efficient and methanol resistant noble metal free electrocatalyst for tetraelectronic oxygen reduction reaction. Electrochimica Acta 2019, 326, 134984. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Fan, L.; Su, X.; Cong, T.; Wang, Y.; Liu, C.; Xiong, Y. Co and N co-doped porous carbon derived from corn stalk core as electrocatalyst for oxygen reduction reaction in alkaline medium. Int. J. Electrochem. Sci. 2020, 15, 11723–11731. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Li, O.L. Cobalt Nanoparticles on Plasma-Controlled Nitrogen-Doped Carbon as High-Performance ORR Electrocatalyst for Primary Zn-Air Battery. Nanomaterials 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Luo, J.; Tan, M.; Xiao, Y.; Gao, B.; Zheng, Y.; Lin, B. Co/CoNx decorated nitrogen-doped porous carbon derived from melamine sponge as highly active oxygen electrocatalysts for zinc-air batteries. J. Power Sources 2020, 453, 227900. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Li, Z. A cobalt-pyrrole coordination compound as high performance cathode catalyst for direct borohydride fuel cells. RSC Adv. 2020, 10, 29119–29127. [Google Scholar] [CrossRef]
- Huang, K.; Rong, C.; Zhang, W.; Yang, X.; Fan, Y.; Liu, L.; Yang, Z.; Chen, W.; Yang, J. MOF-assisted synthesis of Ni, Co, Zn, and N multidoped porous carbon as highly efficient oxygen reduction electrocatalysts in Zn-air batteries. Mater. Today Energy 2021, 19, 100579. [Google Scholar] [CrossRef]
- Gong, X.; Peng, L.; Wang, X.; Wu, L.; Liu, Y. Duckweed derived nitrogen self-doped porous carbon materials as cost-effective electrocatalysts for oxygen reduction reaction in microbial fuel cells. Int. J. Hydrogen Energy 2020, 45, 15336–15345. [Google Scholar] [CrossRef]
- Hao, X.; Chen, W.; Jiang, Z.; Tian, X.; Hao, X.; Maiyalagan, T.; Jiang, Z.-J. Conversion of maize straw into nitrogen-doped porous graphitized carbon with ultra-high surface area as excellent oxygen reduction electrocatalyst for flexible zinc-air batteries. Electrochim. Acta 2020, 362, 137143. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Chen, L.; Liu, Z.; Long, L.; Wang, S.; Liu, C.; Dong, S.; Jia, J. Graphitic Carbon Nitride (g-C3N4)-Derived Bamboo-Like Carbon Nanotubes/Co Nanoparticles Hybrids for Highly Efficient Electrocatalytic Oxygen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 4463–4472. [Google Scholar] [CrossRef]
- Peng, J.-D.; Wu, Y.-T.; Yeh, M.-H.; Kuo, F.-Y.; Vittal, R.; Ho, K.-C. Transparent Cobalt Selenide/Graphene Counter Electrode for Efficient Dye-Sensitized Solar Cells with Co2+/3+-Based Redox Couple. ACS Appl. Mater. Interfaces 2020, 12, 44597–44607. [Google Scholar] [CrossRef]
- Li, X.; Guan, B.Y.; Gao, S.; Lou, X.W. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci. 2019, 12, 648–655. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Gao, M.; Shang, H.; Liu, X. Co-Zn-MOFs Derived N-Doped Carbon Nanotubes with Crystalline Co Nanoparticles Embedded as Effective Oxygen Electrocatalysts. Nanomaterials 2021, 11, 261. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Zhang, B.; Zhang, J. Bifunctional Oxygen Electrocatalysis of N, S-Codoped Porous Carbon with Interspersed Hollow CoO Nanoparticles for Rechargeable Zn-Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 16720–16728. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.-M.; Du, Y.; et al. Nitrogen-Superdoped 3D Graphene Networks for High-Performance Supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef]
- Jaouen, F.; Herranz, J.; Lefèvre, M.; Dodelet, J.-P.; Kramm, U.; Herrmann, I.; Bogdanoff, P.; Maruyama, J.; Nagaoka, T.; Garsuch, A.; et al. Cross-Laboratory Experimental Study of Non-Noble-Metal Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2009, 1, 1623–1639. [Google Scholar] [CrossRef]
- Artyushkova, K.; Levendosky, S.; Atanassov, P.; Fulghum, J. XPS Structural Studies of Nano-composite Non-platinum Electrocatalysts for Polymer Electrolyte Fuel Cells. Top. Catal. 2007, 46, 263–275. [Google Scholar] [CrossRef]
- Arechederra, R.L.; Artyushkova, K.; Atanassov, P.; Minteer, S.D. Growth of Phthalocyanine Doped and Undoped Nanotubes Using Mild Synthesis Conditions for Development of Novel Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2010, 2, 3295–3302. [Google Scholar] [CrossRef]
- Morozan, A.; Jégou, P.; Jousselme, B.; Palacin, S. Electrochemical performance of annealed cobalt-benzotriazole/CNTs catalysts towards the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2011, 13, 21600–21607. [Google Scholar] [CrossRef]
- Yan, J.; Meng, H.; Xie, F.; Yuan, X.; Yu, W.; Lin, W.; Ouyang, W.; Yuan, D. Metal free nitrogen doped hollow mesoporous graphene-analogous spheres as effective electrocatalyst for oxygen reduction reaction. J. Power Sources 2014, 245, 772–778. [Google Scholar] [CrossRef]
- Huang, H.; Lan, Z.; Li, W.; Mo, W.; Zhao, L.; Zhang, J. A novel and low-cost CuPc@C catalyst derived from the compounds of sunflower straw and copper phthalocyanine pigment for oxygen reduction reaction. RSC Adv. 2021, 11, 15590–15597. [Google Scholar] [CrossRef]

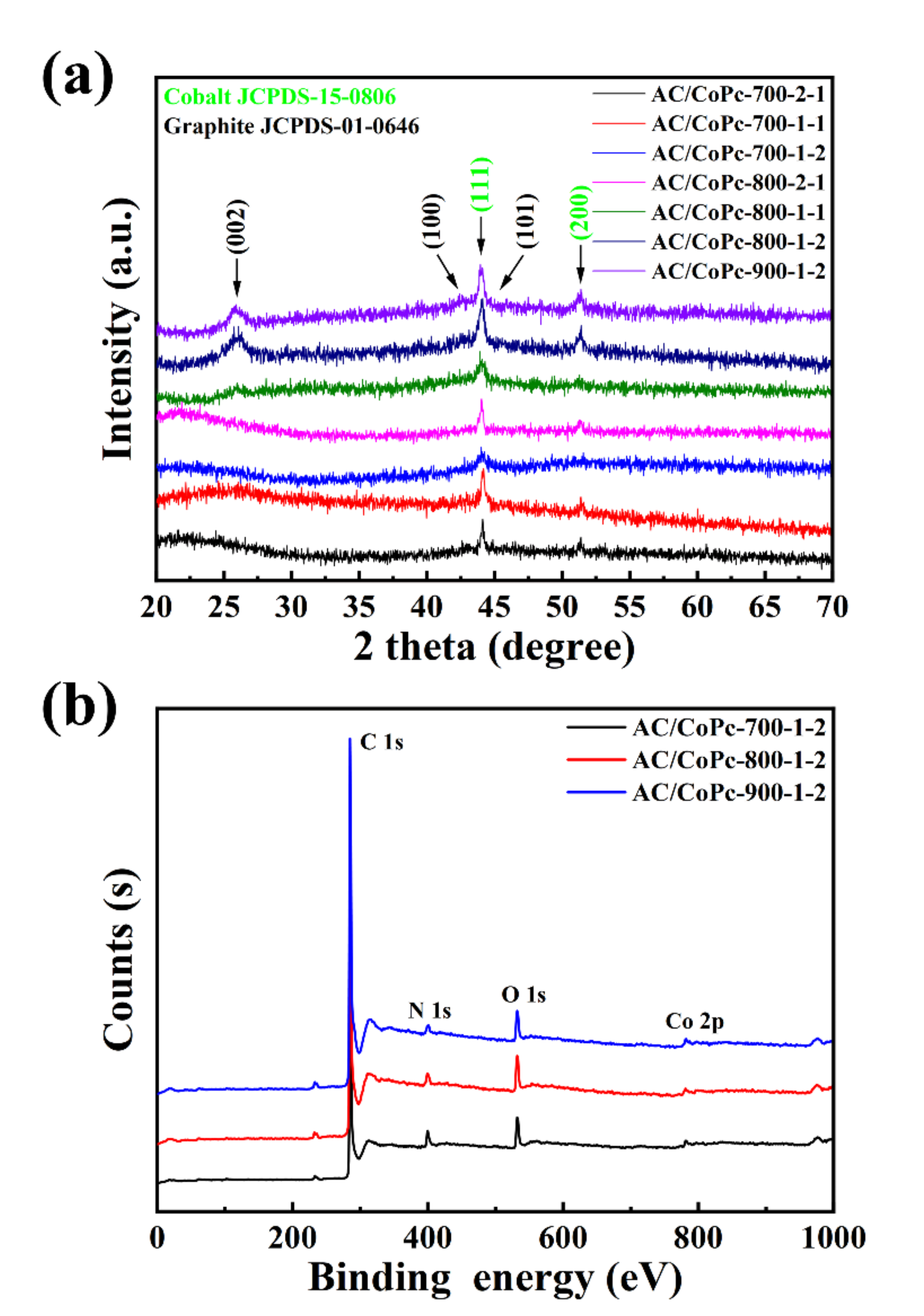



| Element contents (at%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Catalysts | C | O | Co | N total | N1 | N2 | N3 | N4 |
| AC/CoPc-700-1-2 | 85.19 | 7.45 | 0.73 | 6.63 | 58.47 | 5.64 | 24.31 | 11.58 |
| AC/CoPc-800-1-2 | 90.04 | 6.09 | 0.52 | 3.35 | 54.29 | 12.61 | 20.33 | 12.76 |
| AC/CoPc-900-1-2 | 92.02 | 4.56 | 0.51 | 2.91 | 53.04 | 7.79 | 1.07 | 38.10 |
| Catalyst | Eonset (V vs. RHE) | E1/2 (V vs. RHE) | Current Density at 0.95V (mA cm−2) | Current Density at 0.90V (mA cm−2) | Current Density at 0.85V (mA cm−2) | Current Density at 0.80V (mA cm−2) | Limited Current Density (mA cm−2) |
|---|---|---|---|---|---|---|---|
| AC/CoPc-800-1-2 | 1.006 | 0.860 | 0.869 | 1.688 | 2.264 | 2.658 | 4.50 |
| Pt/C (20 wt%) | 0.989 | 0.858 | 0.2322 | 1.060 | 2.524 | 3.722 | 4.70 |
| Catalysts 1 | The Content and Source of Co and N (at.%)2 | Eonset (V vs. RHE) | E1/2 (V vs. RHE) | Limited Current Density (mA cm−2) | Average Transferred Electron Number (n) | Durability | Ref. |
|---|---|---|---|---|---|---|---|
| AC/CoPc-800-1-2 | 0.52, 3.35 CoPc pine peel | 1.006 | 0.860 | 4.50 | 3.69 | 20,000 s/87.8% | This work |
| Co-N-C-800 | 0.83, 2.94 Co(NO3)2·6H2O 2-methylimidazole | 1.030 | 0.870 | 5.52 | 3.97 | 72,000 s/92% | [3] |
| Co-NC-700 | 1.07, 4.46 Co(Ac)2·4H2O 1,10-phenanthroline | 0.940 | 0.840 | 6.30 | 3.89 | ΔE1/2 −5 mV (10,000 cycles) | [5] |
| ZIF/ppy-pani-750 | 1.23, 11.21 Co(NO3)2·6H2O ppy-pani3 | 0.930 | 0.860 | 4.99 | 3.82 | 64,800 s/88.73% | [7] |
| ECo@D | 0.041, 5.23 EDTA-Co4 DA5 | 1.050 | 0.790 | 4.74 | 3.90 | 36,000 s/93.8% | [9] |
| Co/N-C@CNFs | 4.3, 3.2 Co(NO3)2·6H2O 2-methylimidazole | 0.952 | 0.852 | NR | 4.20 | 70,000 s/92% | [16] |
| Co@N-C-1 | NR6, 12.12 Co(OAc)2urea | 1.023 | 0.938 | 4.12 | 3.98 | 1000 s/80% | [17] |
| Co-N-CNTs | 0.59, 11.93 Co(NO3)2·6H2O 2-methylimidazole | 0.974 | 0.853 | NR | 4.00 | ΔE1/2 0 mV (10,000 cycles) | [18] |
| Co@NC-ZM-900 | 0.61, 1.94 CoPc melamine | 0.960 | 0.830 | NR | 4.18 | 20,000 s/94.8% | [19] |
| Co-NOPC-600 | 8.74, 4.67 Co(NO3)2·6H2O 2-methylimidazole | 0.950 | 0.860 | 5.20 | 3.93 | 86,400 s/85% | [23] |
| Co@NC/RGO-2.6 | NR, NR Co(NO3)2·6H2O 2-methylimidazole | 0.960 | 0.820 | 5.60 | 3.90 | ΔE1/2 −2 mV (5000 cycles) | [24] |
| Co/N-C | 0.68, 5.79 Co(NO3)2·6H2O 2-methylimidazole | 0.877 | 0.817 | 5.11 | 3.65 | 36,000 s/87.1% | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Lan, Z.; Mo, W.; Su, J.; Liang, H.; Yao, J.; Yang, W. High-Level Oxygen Reduction Catalysts Derived from the Compounds of High-Specific-Surface-Area Pine Peel Activated Carbon and Phthalocyanine Cobalt. Nanomaterials 2021, 11, 3429. https://doi.org/10.3390/nano11123429
Zhao L, Lan Z, Mo W, Su J, Liang H, Yao J, Yang W. High-Level Oxygen Reduction Catalysts Derived from the Compounds of High-Specific-Surface-Area Pine Peel Activated Carbon and Phthalocyanine Cobalt. Nanomaterials. 2021; 11(12):3429. https://doi.org/10.3390/nano11123429
Chicago/Turabian StyleZhao, Lei, Ziwei Lan, Wenhao Mo, Junyu Su, Huazhu Liang, Jiayu Yao, and Wenhu Yang. 2021. "High-Level Oxygen Reduction Catalysts Derived from the Compounds of High-Specific-Surface-Area Pine Peel Activated Carbon and Phthalocyanine Cobalt" Nanomaterials 11, no. 12: 3429. https://doi.org/10.3390/nano11123429
APA StyleZhao, L., Lan, Z., Mo, W., Su, J., Liang, H., Yao, J., & Yang, W. (2021). High-Level Oxygen Reduction Catalysts Derived from the Compounds of High-Specific-Surface-Area Pine Peel Activated Carbon and Phthalocyanine Cobalt. Nanomaterials, 11(12), 3429. https://doi.org/10.3390/nano11123429