Nanoscale Soft Wetting Observed in Co/Sapphire during Pulsed Laser Irradiation
Abstract
:1. Introduction
2. Materials and Methods
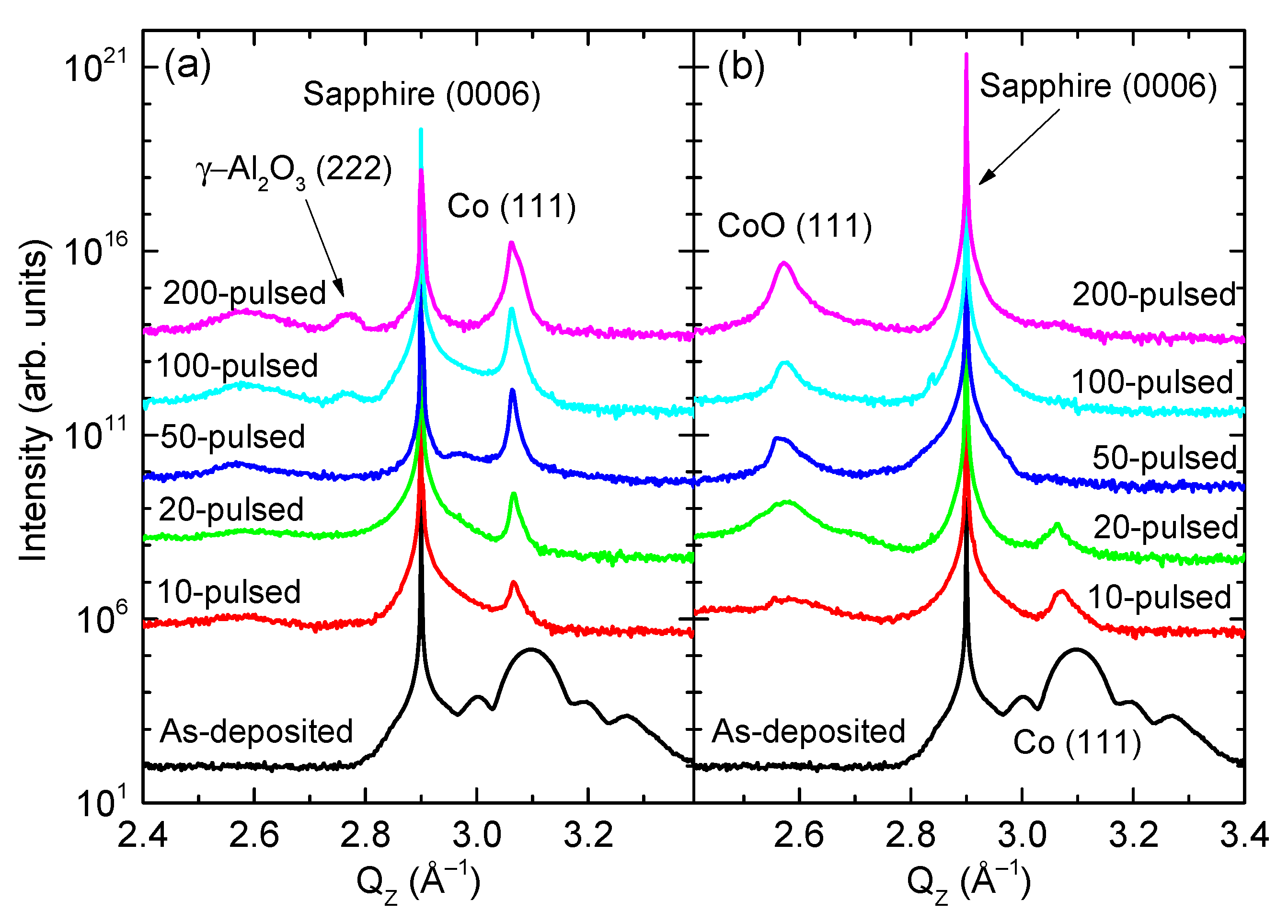
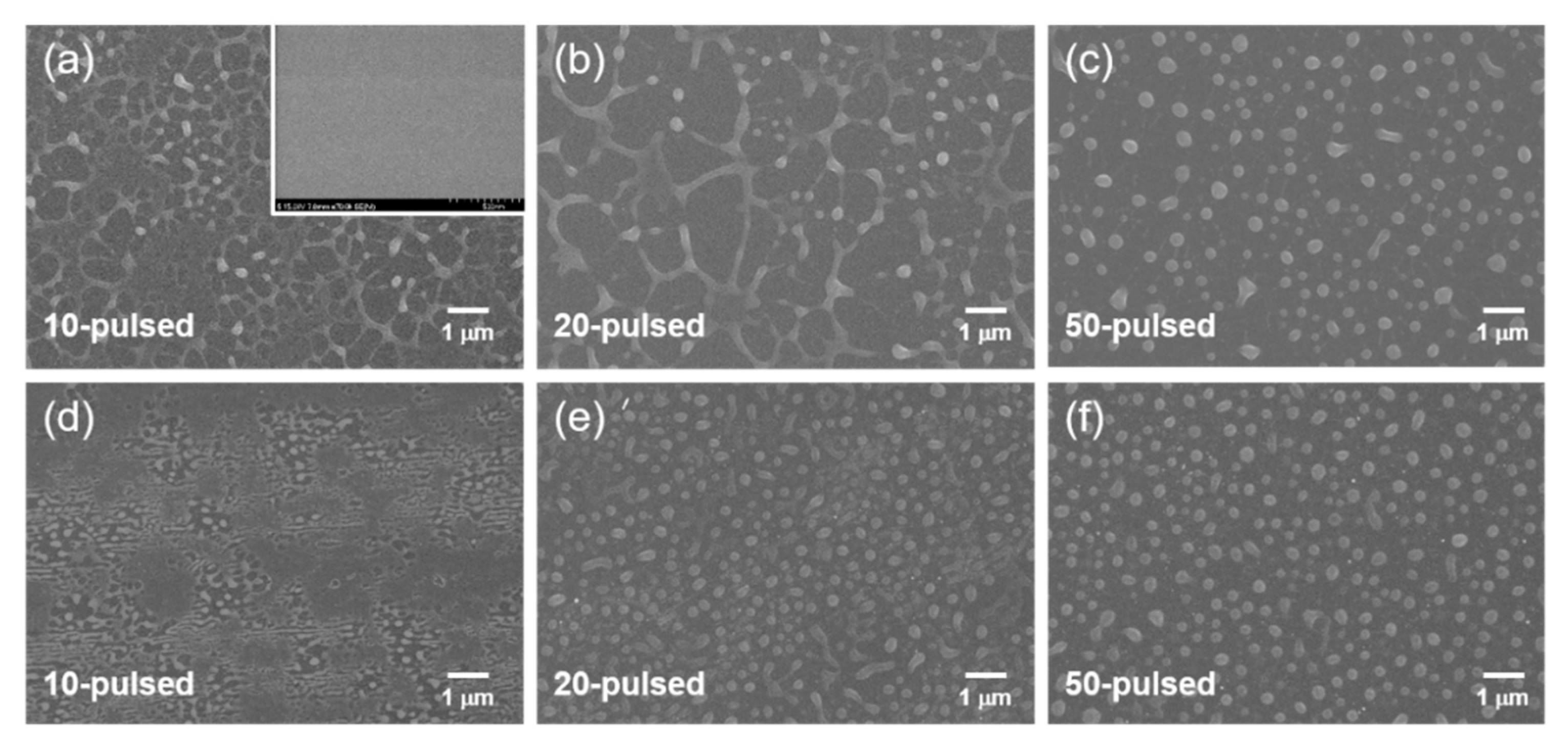
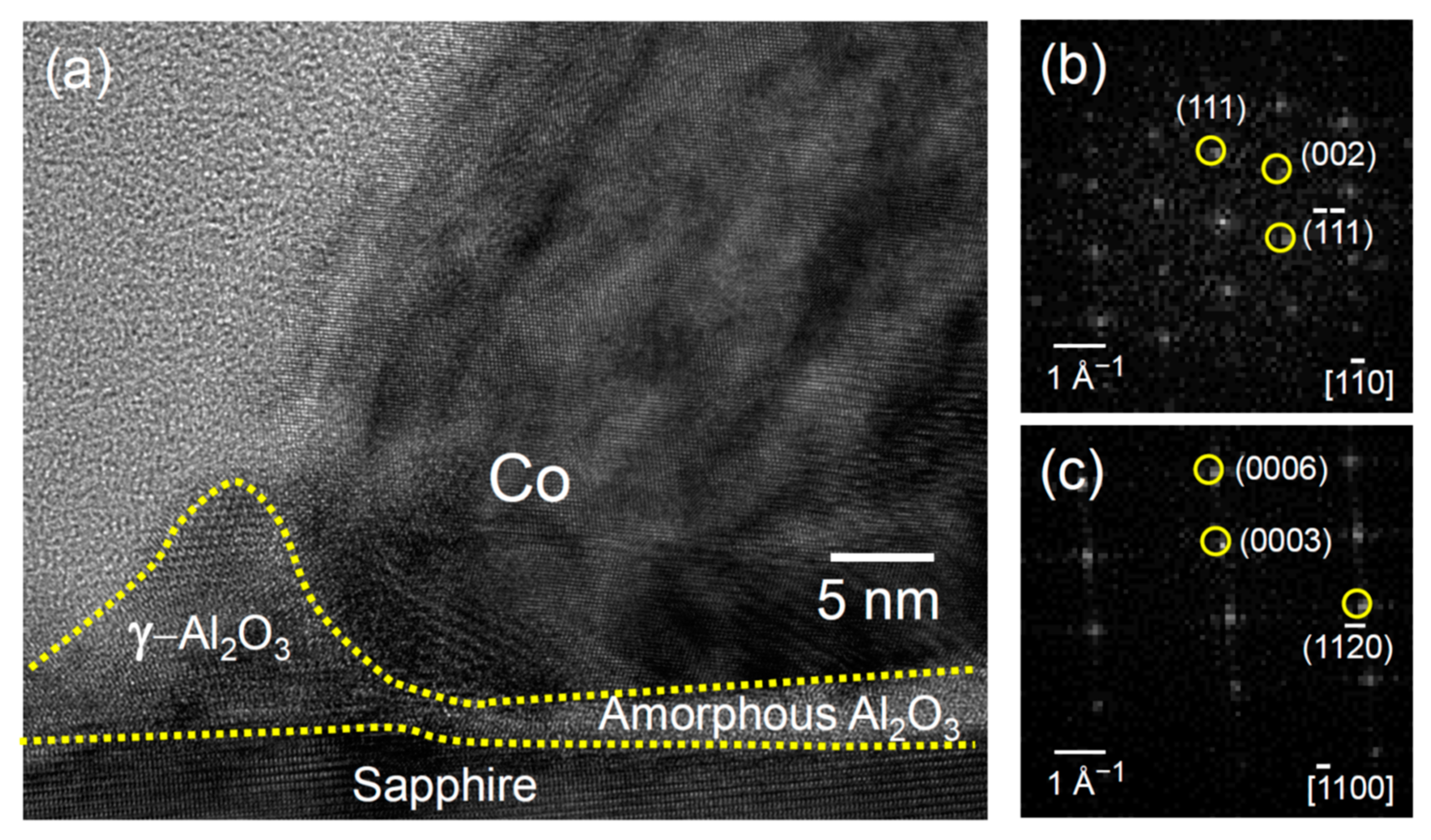
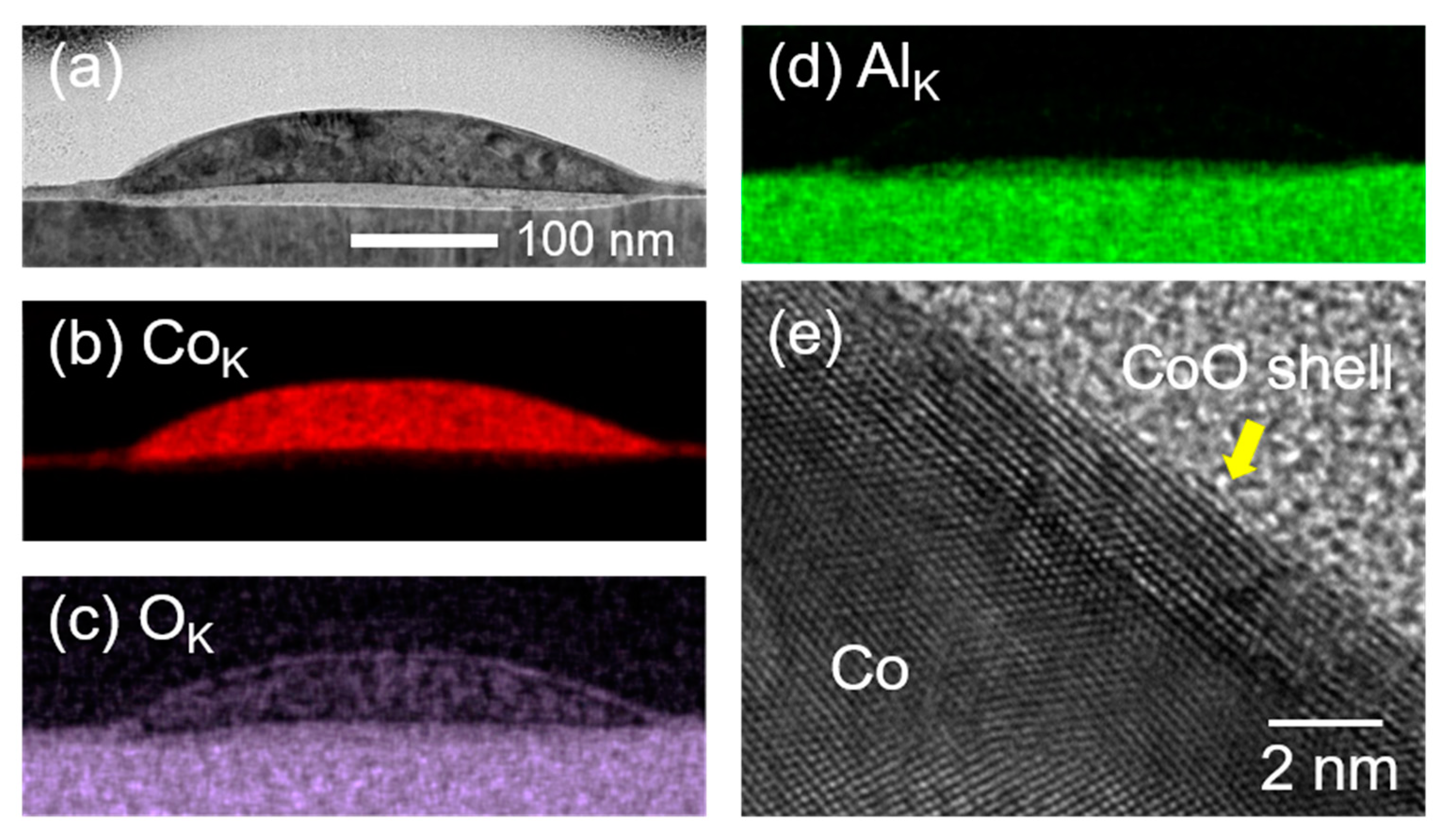
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Young, T. An essay on the cohesion of fluids. Phil. Trans. R. Soc. 1805, 95, 65–87. [Google Scholar]
- Makkonen, L. Young’s equation revisited. J. Phys. Condens. Matter 2016, 28, 135001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F. Vorlesungen Uber Die Theorie der Capillaritt, 1st ed.; B.G. Teubner: Leipzig, Germany, 1894; pp. 59–72. [Google Scholar]
- De Gennes, P.-G.; Brochard-Wyart, F.; Quere, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: New York, NY, USA, 2010; pp. 1–31. [Google Scholar]
- Marchand, A.; Das, S.; Snoeijer, J.H.; Andreotti, B. Contact angles on a soft solid: From Young’s law to Neumann’s law. Phys. Rev. Lett. 2012, 109, 236101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Style, R.W.; Dufresne, E.R. Static wetting on deformable substrates, from liquids to soft solids. Soft Matter 2012, 8, 7177–7184. [Google Scholar] [CrossRef] [Green Version]
- Jerison, E.R.; Xu, Y.; Wilen, L.A.; Dufresne, E.R. Deformation of an elastic substrate by a three-phase contact line. Phys. Rev. Lett. 2011, 106, 186103. [Google Scholar] [CrossRef] [Green Version]
- Sadullah, M.S.; Semprebon, C.; Kusumaatmaja, H. Drop dynamics on liquid-infused surfaces: The role of the lubricant ridge. Langmuir 2018, 34, 8112–8118. [Google Scholar] [CrossRef] [Green Version]
- Leong, F.Y.; Le, D.-V. Droplet dynamics on viscoelastic soft substrate: Toward coalescence control. Phys. Fluids 2020, 32, 062102. [Google Scholar] [CrossRef]
- Van Gorcum, M.; Karpitschka, S.; Andreotti, B.; Snoeijer, J.H. Spreading on viscoelastic solids: Are contact angles selected by Neumann’s law? Soft Matter 2020, 16, 1306–1322. [Google Scholar] [CrossRef] [Green Version]
- Karpitschka, S.; Das, S.; Van Gorcum, M.; Perrin, H.; Andreotti, B.; Snoeijer, J.H. Droplets move over viscoelastic substrates by surfing a ridge. Nat. Commun. 2015, 6, 7891. [Google Scholar] [CrossRef] [Green Version]
- De Pascalis, R.; Dervaux, J.; Ionescu, I.; Limat, L. Numerical multiscale modelling of nonlinear elastowetting. Eur. J. Mech. A 2018, 71, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Seiler, R.L.; Weibel, J.A.; Garimella, S.V. Soft surface: Droplets on soft surfaces exhibit a reluctance to coalesce due to an intervening wetting ridge. Adv. Mater. Interfaces 2020, 7, 2000731. [Google Scholar] [CrossRef]
- Dey, R.; Daga, A.; DasGupta, S.; Chakraborty, S. Electrically modulated dynamic spreading of drops on soft surfaces. Appl. Phys. Lett. 2015, 107, 034101. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Weon, B.M.; Lee, J.S.; Lee, J.; Kim, J.; Je, J.H. Visualization of asymmetric wetting ridges on soft solids with X-ray microscopy. Nat. Commun. 2014, 5, 4369. [Google Scholar] [CrossRef]
- Schellenberger, F.; Xie, J.; Encinas, N.; Hardy, A.; Klapper, M.; Papadopoulos, P.; Butt, H.-J.; Vollmer, D. Direct observation of drops on slippery lubricant-infused surfaces. Soft Matter 2015, 11, 7617–7626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, G.; Severtson, S.J. Dependence of wetting behavior on the thickness of highly viscoelastic films. J. Phys. Chem. C 2011, 115, 18729–18735. [Google Scholar] [CrossRef]
- Karpitschka, S.; Pandey, A.; Lubbers, L.A.; Weijs, J.H.; Botto, L.; Das, S.; Andreotti, B.; Snoeijer, J.H. Liquid drops attract or repel by the inverted Cheerios effect. Proc. Natl. Acad. Sci. USA 2016, 113, 7403–7407. [Google Scholar] [CrossRef] [Green Version]
- Carre, A.; Gastel, J.-C.; Shanahan, M.E.R. Viscoelastic effects in the spreading of liquids. Nature 1996, 379, 432–434. [Google Scholar] [CrossRef]
- Bischof, J.; Scherer, D.; Herminghaus, S.; Leiderer, P. Dewetting modes of thin metallic films: Nucleation of holes and spinodal dewetting. Phys. Rev. Lett. 1996, 77, 1536–1539. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Ruckenstein, E. Energetic criteria for the breakup of liquid films on nonwetting solid surfaces. J. Colloid Interface Sci. 1990, 137, 433–445. [Google Scholar] [CrossRef]
- Trice, J.; Thomas, D.; Favazza, C.; Sureshkumar, R.; Kalyanaraman, R. Pulsed-laser-induced dewetting in nanoscopic metal films: Theory and experiments. Phys. Rev. B 2007, 75, 235439. [Google Scholar] [CrossRef] [Green Version]
- Peschka, D.; Haefner, S.; Marquant, L.; Jacobs, K.; Münch, A.; Wagner, B. Signatures of slip in dewetting polymer films. Proc. Natl. Acad. Sci. USA 2019, 116, 9275–9284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Pandey, P.; Sui, M.; Li, M.-Y.; Zhang, Q.; Kunwar, S. Evolution of self-assembled Au NPs by controlling annealing temperature and dwelling time on sapphire (0001). Nanoscale Res. Lett. 2015, 10, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahlavan, A.A.; Cueto-Felgueroso, L.; Hosoi, A.E.; McKinley, G.H.; Juanes, R. Thin films in partial wetting: Stability, dewetting and coarsening. J. Fluid Mech. 2018, 845, 642–681. [Google Scholar] [CrossRef] [Green Version]
- Gentili, D.; Foschi, G.; Valle, F.; Cavallini, M.; Biscarini, F. Applications of dewetting in micro and nanotechnology. Chem. Soc. Rev. 2012, 41, 4430–4443. [Google Scholar] [CrossRef]
- Krishna, H.; Sachan, R.; Strader, J.; Favazza, C.; Khenner, M.; Kalyanaraman, R. Thickness-dependent spontaneous dewetting morphology of ultrathin Ag films. Nanotechnology 2010, 21, 155601. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, J.D.; Kondic, L.; Diez, J.; Wu, Y.; Rack, P.D. Self-assembly versus directed assembly of nanoparticles via pulsed laser induced dewetting of patterned metal films. Nano Lett. 2011, 11, 2478–2485. [Google Scholar] [CrossRef]
- Naffouti, M.; Backofen, R.; Salvalaglio, M.; Bottein, T.; Lodari, M.; Voigt, A.; David, T.; Benkouider, A.; Fraj, I.; Favre, L.; et al. Complex dewetting scenarios of ultrathin silicon films for large-scale nanoarchitectures. Sci. Adv. 2017, 3, eaao1472. [Google Scholar] [CrossRef] [Green Version]
- Gazit, N.; Richter, G.; Sharma, A.; Klinger, L.; Rabkin, E. Engineering of hollow AlAu2 nanoparticles on sapphire by solid state dewetting and oxidation of Al. Mater. Des. 2019, 165, 107557. [Google Scholar] [CrossRef]
- Herz, A.; Franz, A.; Theska, F.; Hentschel, M.; Kups, T.; Wang, D.; Schaff, P. Solid-state dewetting of single- and bilayer Au-W thin films: Unraveling the role of individual layer thickness, stacking sequence and oxidation on morphology evolution. AIP Adv. 2016, 6, 035109. [Google Scholar] [CrossRef] [Green Version]
- Monestrol, H.; Schmirgeld-Mignot, L.; Poissonnet, S.; Lebourgeois, C.; Martin, G. Reactive solid state dewetting: Interfacial cavitation in the system Ag-Ni-O. Interface Sci. 2003, 11, 379–390. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Chen, J.L.; Chen, C.; Lin, H.C.; Yang, S.S.; Hwang, C.C. Reactive wetting behaviors of Sn/Cu systems: A molecular dynamics study. Nanomicro Lett. 2010, 2, 60–67. [Google Scholar] [CrossRef]
- Makarov, S.V.; Milichko, V.A.; Mukhin, I.S.; Shishkin, I.I.; Zuev, D.A.; Mozharov, A.M.; Krasnok, A.E.; Belov, P.A. Controllable femtosecond laser-induced dewetting for plasmonic applications. Laser Photonics Rev. 2016, 10, 91–99. [Google Scholar] [CrossRef]
- Kondic, L.; González, A.G.; Diez, J.A.; Fowlkes, J.D.; Rack, P. Liquid-state dewetting of pulsed-laser-heated nanoscale metal films and other geometries. Annu. Rev. Fluid Mech. 2020, 52, 235–262. [Google Scholar] [CrossRef] [Green Version]
- Letfullin, R.R.; Joenathan, C.; George, T.F.; Zharov, V.P. Laser-induced explosion of gold nanoparticles: Potential role for nanophotothermolysis of cancer. Nanomedicine 2006, 1, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Singer, J.P.; Lin, P.-T.; Kooi, S.E.; Kimerling, L.C.; Michel, J.; Thomas, E.L. Direct-write thermocapillary dewetting of polymer thin films by a laser-induced thermal gradient. Adv. Mater. 2013, 25, 6100–6105. [Google Scholar] [CrossRef]
- Bornemann, S.; Yulianto, N.; Spende, H.; Herbani, Y.; Prades, J.D.; Wasisto, H.S.; Waag, A. Femtosecond Laser Lift-Off with Sub-Bandgap Excitation for Production of Free-Standing GaN Light-Emitting Diode Chips. Adv. Eng. Mater. 2020, 22, 1901192. [Google Scholar] [CrossRef] [Green Version]
- Rack, P.D.; Guan, Y.; Fowlkes, J.D.; Melechko, A.V.; Simpson, M.L. Pulsed laser dewetting of patterned thin metal films: A means of directed assembly. Appl. Phys. Lett. 2008, 92, 223108. [Google Scholar] [CrossRef] [Green Version]
- Yadavali, S.; Khenner, M.; Kalyanaraman, R. Pulsed laser dewetting of Au films: Experiments and modeling of nanoscale behavior. J. Mater. Res. 2013, 28, 1715–1723. [Google Scholar] [CrossRef]
- Duff, W.H.; Zhigilei, L.V. Computational study of cooling rates and recrystallization kinetics in short pulse laser quenching of metal targets. J. Phys. Conf. Ser. 2007, 59, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Ruffino, F.; Pugliara, A.; Carria, E.; Bongiorno, C.; Spinella, C.; Grimaldi, M.G. Formation of nanoparticles from laser irradiated Au thin film on SiO2/Si: Elucidating the rayleigh instability role. Mater. Lett. 2012, 84, 27–30. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction, 1st ed.; Dover: New York, NY, USA, 1990; pp. 15–40. [Google Scholar]
- Werner, D.; Furube, A.; Okamoto, T.; Hashimoto, S. Femtosecond laser-induced size reduction of aqueous gold nanoparticles: In situ and pump−probe spectroscopy investigations revealing Coulomb explosion. J. Phys. Chem. C 2011, 115, 8503–8512. [Google Scholar] [CrossRef]
- Ha, D.-H.; Moreau, L.M.; Honrao, S.; Hennig, R.G.; Robinson, R.D. The oxidation of cobalt nanoparticles into Kirkendall-hollowed CoO and Co3O4: The diffusion mechanisms and atomic structural transformations. J. Phys. Chem. C 2013, 117, 14303–14312. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, C.; Li, Z.Y.; Zhang, Z.; Li, J. Oxidation behavior of cobalt nanoparticles studied by in situ environmental transmission electron microscopy. Sci. Bull. 2017, 62, 775–778. [Google Scholar] [CrossRef] [Green Version]
- Chatain, D.; Galy, D. Interfaces between Pb grains and Cu surfaces. J. Mater. Sci. 2006, 41, 7769–7774. [Google Scholar] [CrossRef]
- Saiz, E.; Tomsia, A.P.; Cannon, R.M. Ridging effects on wetting and spreading of liquids and solids. Acta Mater. 1998, 46, 2349–2361. [Google Scholar] [CrossRef]
- Ghetta, V.; Chatain, D. Morphologies adopted by Al2O3 single-crystal surfaces in contact with Cu droplets. J. Am. Ceram. Soc. 2002, 85, 961–964. [Google Scholar] [CrossRef]
- Klinger, L.; Rabkin, E. Sintering of spherical particles of two immiscible phases controlled by surface and interphase boundary diffusion. Acta Mater. 2013, 61, 2607–2616. [Google Scholar] [CrossRef]
- Ahn, K.; Lee, S.Y.; Cho, I.H.; Kim, Y.; Kang, H.C.; Noh, D.Y. Phase separated bi-metallic nnaoparticles formed by pulsed laser dewetting. Nanotechnology 2020, 32, 085708. [Google Scholar] [CrossRef]
- Klinger, L.; Rabkin, E. On the nucleation of pores during the nanoscale Kirkendall effect. Mater. Lett. 2015, 161, 508–510. [Google Scholar] [CrossRef]
- Cao, P.; Bai, P.; Omrani, A.A.; Xiao, Y.; Meaker, K.L.; Tsai, H.-Z.; Yan, A.; Jung, H.S.; Khajeh, R.; Rodgers, G.F.; et al. Preventing thin film dewetting via graphene capping. Adv. Mater. 2017, 29, 1701536. [Google Scholar] [CrossRef]
- Su, D.; Yu, M.; Zhang, G.; Jiang, S.; Qin, Y.; Li, M. Highly thermally stable Au–Al bimetallic conductive thin films with a broadband transmittance between UV and NIR regions. J. Mater. Chem. C 2020, 8, 2852–2860. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.W.; Ham, D.; Han, S.; Noh, D.Y.; Kang, H.C. Nanoscale Soft Wetting Observed in Co/Sapphire during Pulsed Laser Irradiation. Nanomaterials 2021, 11, 268. https://doi.org/10.3390/nano11020268
Choi JW, Ham D, Han S, Noh DY, Kang HC. Nanoscale Soft Wetting Observed in Co/Sapphire during Pulsed Laser Irradiation. Nanomaterials. 2021; 11(2):268. https://doi.org/10.3390/nano11020268
Chicago/Turabian StyleChoi, Jung Won, Daseul Ham, Seonghyun Han, Do Young Noh, and Hyon Chol Kang. 2021. "Nanoscale Soft Wetting Observed in Co/Sapphire during Pulsed Laser Irradiation" Nanomaterials 11, no. 2: 268. https://doi.org/10.3390/nano11020268



