Metal Nanostructures for Environmental Pollutant Detection Based on Fluorescence
Abstract
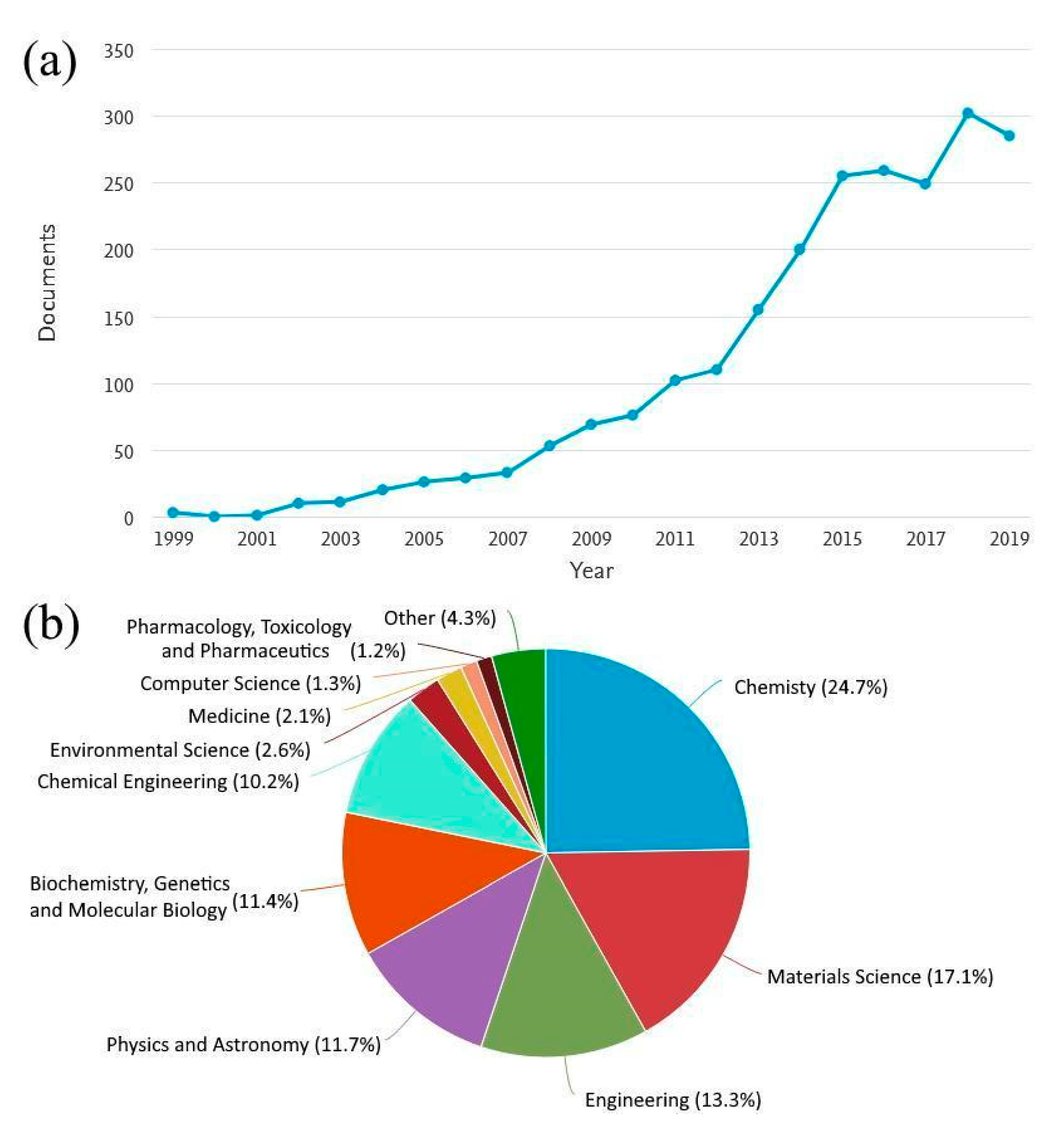
:1. Introduction
| Pesticide | * WHO | ** USA | New Zeeland | Australia | Canada | *** EU |
|---|---|---|---|---|---|---|
| Alachlor | 20 | 2 | 20 | - | - | N. a. |
| Aldicarb | 10 | 7 | 10 | 1 | 9 | N. a. |
| Aldicarb sulfone | - | 7 | - | - | - | N. a. |
| Aldicarb sulfoxide | - | 7 | - | - | - | N. a. |
| Aldrin/diedrin | 0.03 | - | 0.03 | 0.01 | 0.7 | N. a. |
| Atrazine | 2 | 3 | 2 | 0.5 | 5 | N. a. |
| Bentazone | 300 | - | 4 | 2 | - | 0.1 |
| Carbaryl | - | - | - | 5 | 90 | N. a. |
| Carbofuran | 7 | 40 | 8 | 5 | 90 | N. a. |
| Chlordane | 0.2 | 2 | 0.2 | 0.01 | - | N. a. |
| Cyanazine | 0.6 | 0.7 | - | 10 | N. a. | |
| 2,4-D | 30 | 70 | 40 | 0.1 | 100 | 0.1 |
| DDT | 2 | - | - | - | - | N. a. |
| Diazinon | - | - | 10 | 1 | 20 | 0.1 |
| 1,2-Dibromo-3-chloropropane | 1 | 0.2 | 1 | - | - | N. a. |
| Diquat | 10 | 20 | 10 | 0.5 | 70 | 0.1 |
| EDB | 0.4–15 | 0.05 | - | 1 | - | N. a. |
| Fenoprop(2,4,5-TP) | 9 | 50 | 10 | - | - | N. a. |
| Glyphosate | - | 700 | - | 10 | 280 | 0.1 |
| Heptachlor | - | 0.4 | - | - | - | 0.1 |
| Heptachlor epoxide | - | 0.2 | - | - | - | N. a. |
| Hexachlorobenzene | 1 | 1 | 1 | - | - | 0.1 |
| Lindane | 2 | 0.2 | 2 | 0.05 | - | 0.1 |
| Malathion | - | - | - | 50 | 190 | 0.1 |
| Methoxychlor | 20 | 40 | 20 | 0.2 | 900 | 0.1 |
| Pentachlorophenol | 9 | 1 | 10 | 0.01 | 60 | 0.1 |
| Picloram | - | 500 | 20 | - | 190 | 0.1 |
| Propazine | - | - | 70 | 0.5 | - | 0.1 |
| Simazine | 2 | 4 | 2 | 0.5 | 10 | 0.1 |
| 2,4,5-T | 9 | - | 10 | 0.05 | - | N. a. |
| Trifluralin | 20 | - | 30 | 0.1 | 45 | 0.1 |
| Metal ions | EU | WHO | EPA |
|---|---|---|---|
| Iron (Fe) | 0.2 | 0.3 | 0.3 |
| Manganese (Mn) | 0.05 | 0.05 | 0.05 |
| Copper (Cu) | 1 | 1 | 1.3 |
| Zinc (Zn) | 0.1 | 5 | 5 |
| Silver (Ag) | 0.01 | - | - |
| Arsenic (As) | 0.05 | 0.01 | 0.01 |
| Cadmium (Cd) | 0.005 | 0.005 | 0.005 |
| Chromium (Cr) | 0.05 | 0.05 | 0.1 |
| Mercury (Hg) | 0.001 | 0.001 | 0.002 |
| Nickel (Ni) | 0.05 | - | 0.1 |
| Lead (Pb) | 0.05 | 0.015 | 0.005 |
2. Physical Aspects behind Fluorescence Response to Environmental Pollutants
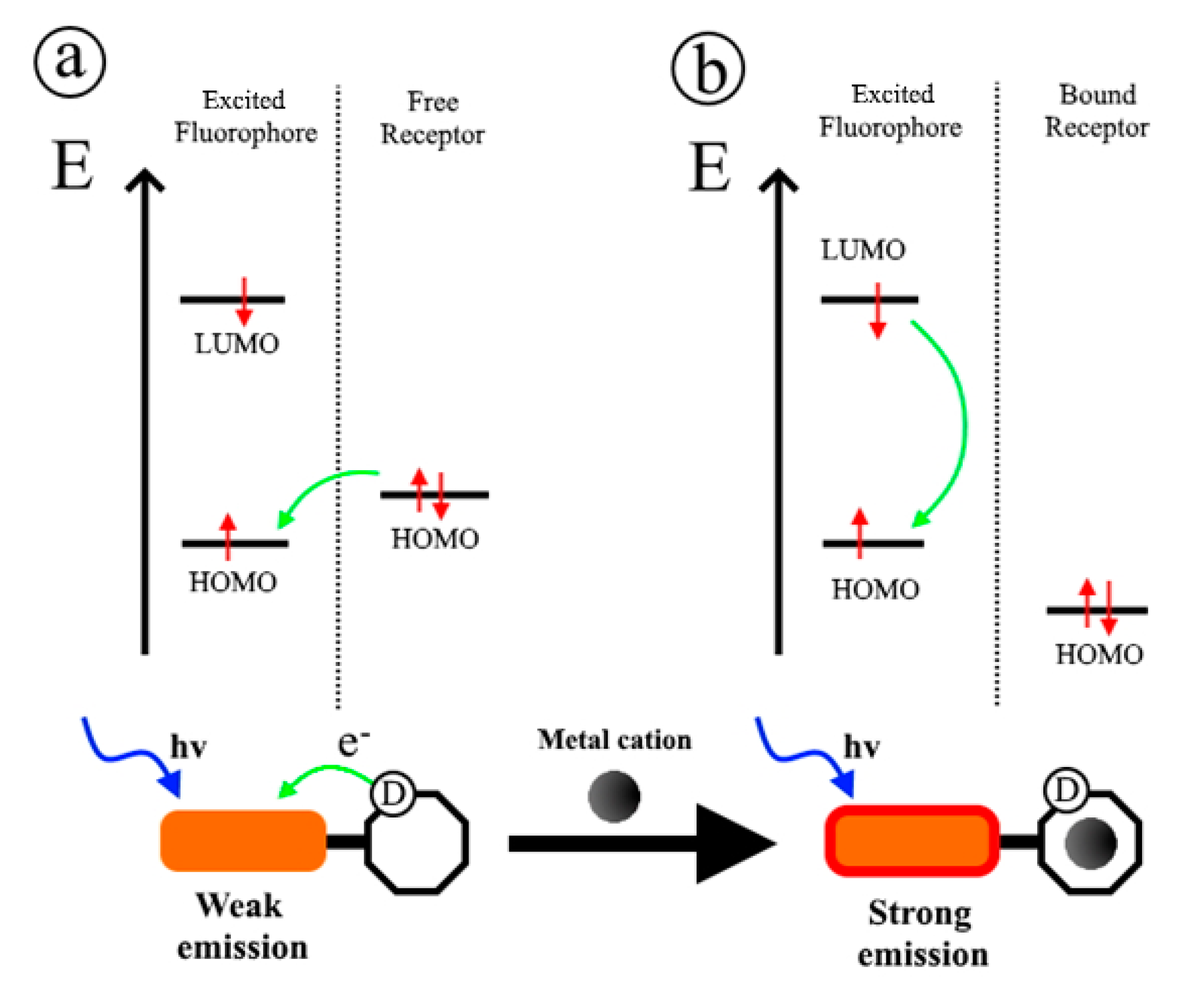
2.1. Photoinduced Electron Transfer and Photoinduced Charge Transfer Processes
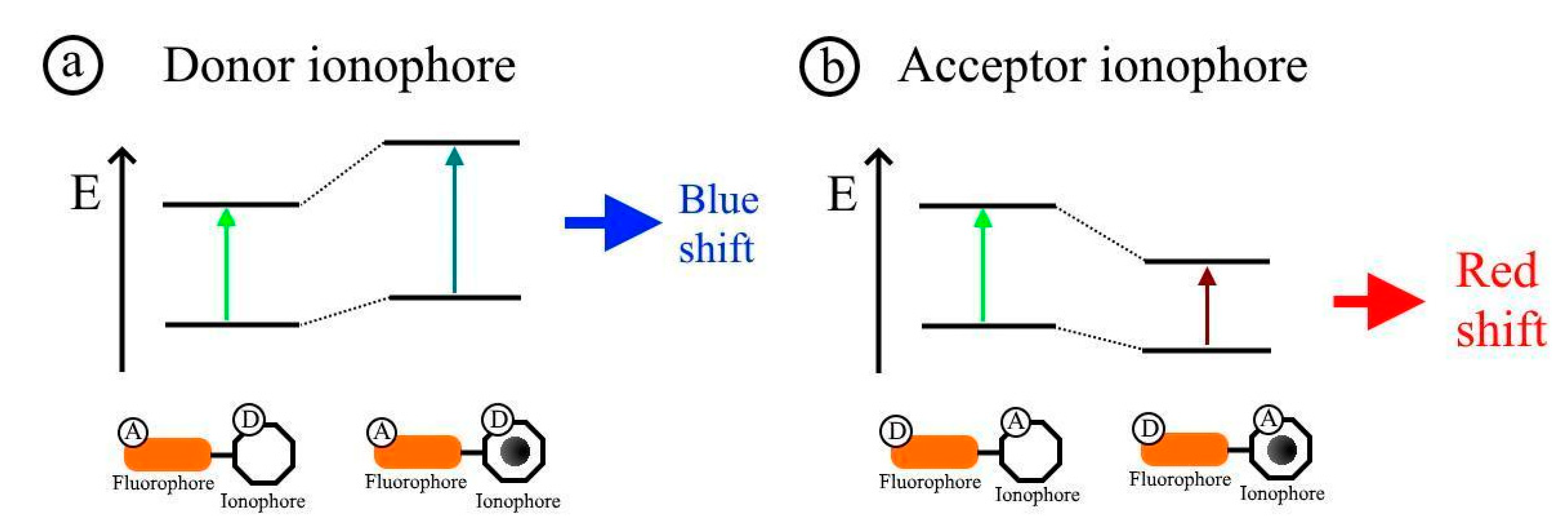
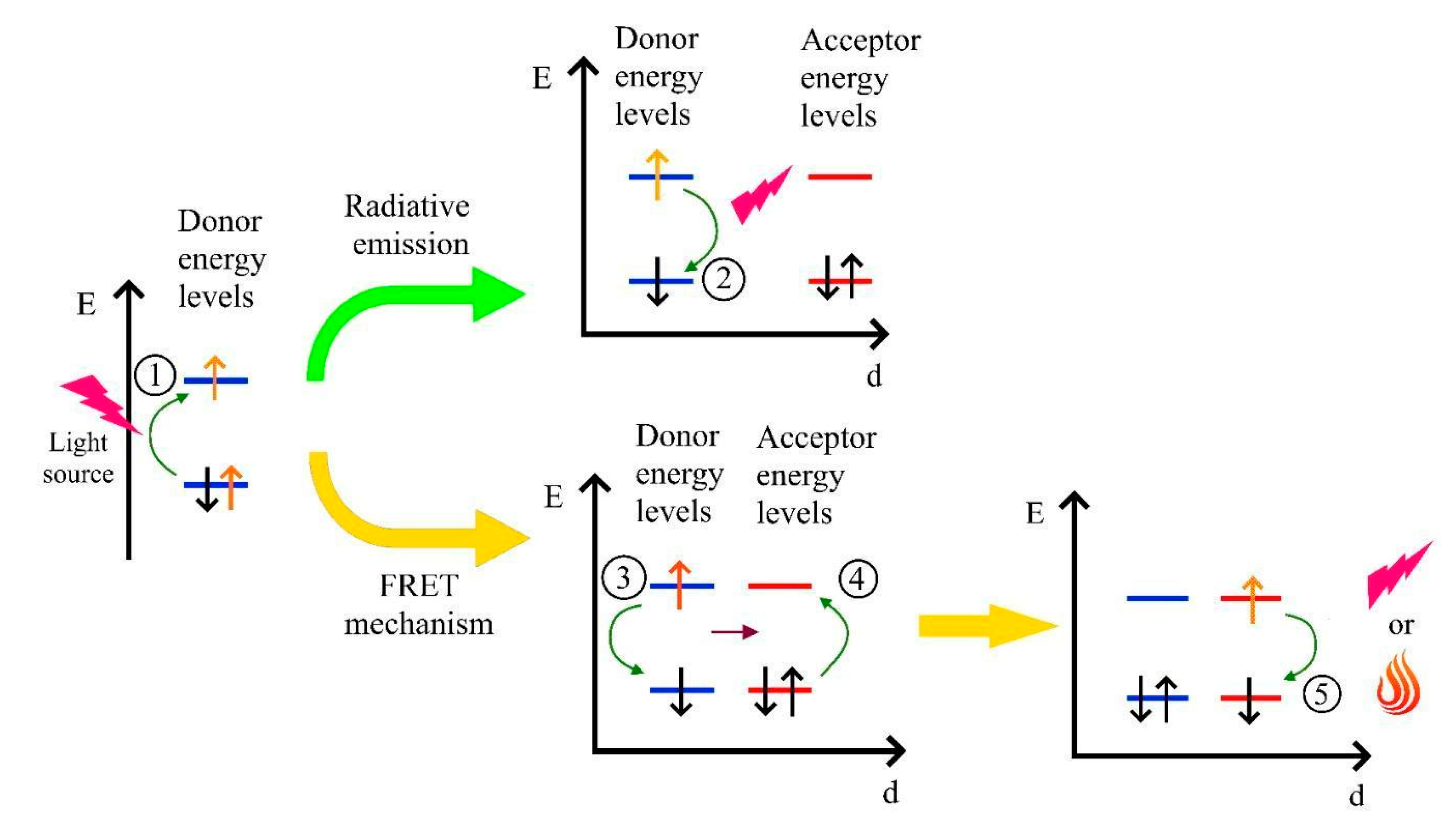
2.2. Fluorescence Resonance Energy Transfer Mechanism
2.3. Aggregation-Induced Emission Effect
2.4. Inner-Filter Effect
3. Recent Developments on the Heavy Metal Ions Detection
3.1. Silver
3.2. Chromium
3.3. Cadmium
3.4. Copper
3.5. Mercury
3.6. Manganese
3.7. Lead
4. Recent Developments on Pesticides Detection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils-To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Tsade, H. Atomic Absorption Spectroscopic Determination of Heavy Metal Concentrations in Kulufo River, Arbaminch, Gamo Gofa, Ethiopia. J. Environ. Anal. Chem. 2016, 3, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Zounr, R.A.; Tuzen, M.; Khuhawar, M.Y. A simple and green deep eutectic solvent based air assisted liquid phase microextraction for separation, preconcentration and determination of lead in water and food samples by graphite furnace atomic absorption spectrometry. J. Mol. Liq. 2018, 259, 220–226. [Google Scholar] [CrossRef]
- Azimi, S.; Es’haghi, Z. A Magnetized Nanoparticle Based Solid-Phase Extraction Procedure Followed by Inductively Coupled Plasma Atomic Emission Spectrometry to Determine Arsenic, Lead and Cadmium in Water, Milk, Indian Rice and Red Tea. Bull. Environ. Contam. Toxicol. 2017, 98, 830–836. [Google Scholar] [CrossRef]
- Samarina, T.O.; Volkov, D.S.; Mikheev, I.V.; Proskurnin, M.A. High-Sensitivity and High-Performance Determination of Trace Aluminum in Water for Pharmaceutical Purposes by Microwave Plasma and Inductively Coupled Plasma–Atomic Emission Spectrometry. Anal. Lett. 2018, 51, 659–672. [Google Scholar] [CrossRef]
- Hernández, F.; Sancho, J.V.; Pozo, O.J. Critical review of the application of liquid chromatography/mass spectrometry to the determination of pesticide residues in biological samples. Anal. Bioanal. Chem. 2005, 382, 934–946. [Google Scholar] [CrossRef]
- Su, S.; Chen, B.; He, M.; Hu, B. Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 2014, 123, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, J.S.; Malik, A.K.; Kaur, V.; Schmitt-Kopplin, P. A Review on Solid Phase Micro Extraction—High Performance Liquid Chromatography (SPME-HPLC) Analysis of Pesticides. Crit. Rev. Anal. Chem. 2005, 35, 71–85. [Google Scholar] [CrossRef]
- Sherma, J. Review of advances in the thin layer chromatography of pesticides: 2010–2012. J. Environ. Sci. Health Part B 2013, 48, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, M.A. Atomic Absorption Spectroscopy, 1st ed.; Akhyar Farrukh, M., Ed.; InTech: London, UK, 2012; ISBN 978-953-307-817-5. [Google Scholar]
- Ezoddin, M.; Shemirani, F.; Abdi, K.; Saghezchi, M.K.; Jamali, M.R. Application of modified nano-alumina as a solid phase extraction sorbent for the preconcentration of Cd and Pb in water and herbal samples prior to flame atomic absorption spectrometry determination. J. Hazard. Mater. 2010, 178, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Cadorim, H.R.; Schneider, M.; Hinz, J.; Luvizon, F.; Dias, A.N.; Carasek, E.; Welz, B. Effective and High-Throughput Analytical Methodology for the Determination of Lead and Cadmium in Water Samples by Disposable Pipette Extraction Coupled with High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry (HR-CS GF AAS). Anal. Lett. 2019, 52, 2133–2149. [Google Scholar] [CrossRef]
- Yavuz, E.; Tokalıoğlu, Ş.; Patat, Ş. Magnetic dispersive solid phase extraction with graphene/ZnFe2O4 nanocomposite adsorbent for the sensitive determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry. Microchem. J. 2018, 142, 85–93. [Google Scholar] [CrossRef]
- Arslan, Y.; Kabak, B.; Trak, D.; Kendüzler, E. Separation and Preconcentration of Nickel(II) from Drinking, Spring, and Lake Water Samples with Amberlite CG-120 Resin and Determination by Flame Atomic Absorption Spectrometry. Anal. Sci. 2018, 34, 1143–1147. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Mehata, M.S. Rapid sensing of lead metal ions in an aqueous medium by MoS2 quantum dots fluorescence turn-off. Mater. Res. Bull. 2020, 131, 110978. [Google Scholar] [CrossRef]
- Bala Subramaniyan, S.; Veerappan, A. Water soluble cadmium selenide quantum dots for ultrasensitive detection of organic, inorganic and elemental mercury in biological fluids and live cells. RSC Adv. 2019, 9, 22274–22281. [Google Scholar] [CrossRef] [Green Version]
- Fahimi-Kashani, N.; Rashti, A.; Hormozi-Nezhad, M.R.; Mahdavi, V. MoS2 quantum-dots as a label-free fluorescent nanoprobe for the highly selective detection of methyl parathion pesticide. Anal. Methods 2017, 9, 716–723. [Google Scholar] [CrossRef]
- Huang, S.; Guo, M.; Tan, J.; Geng, Y.; Wu, J.; Tang, Y.; Su, C.; Lin, C.C.; Liang, Y. Novel Fluorescence Sensor Based on All-Inorganic Perovskite Quantum Dots Coated with Molecularly Imprinted Polymers for Highly Selective and Sensitive Detection of Omethoate. ACS Appl. Mater. Interfaces 2018, 10, 39056–39063. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Guo, M.; Tan, J.; Geng, Y.; Huang, S.; Tang, Y.; Su, C.; Lin, C.; Liang, Y. Development of high-luminescence perovskite quantum dots coated with molecularly imprinted polymers for pesticide detection by slowly hydrolysing the organosilicon monomers in situ. Sens. Actuators B Chem. 2019, 291, 226–234. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.; Zhu, T.; Deng, M.; Ikechukwu, I.P.; Huang, W.; Yin, G.; Bai, Y.; Qu, D.; Huang, X.; et al. All-inorganic CsPbBr3 perovskite quantum dots as a photoluminescent probe for ultrasensitive Cu2+ detection. J. Mater. Chem. C 2018, 6, 4793–4799. [Google Scholar] [CrossRef]
- Chakraborti, H.; Sinha, S.; Ghosh, S.; Pal, S.K. Interfacing water soluble nanomaterials with fluorescence chemosensing: Graphene quantum dot to detect Hg2+ in 100% aqueous solution. Mater. Lett. 2013, 97, 78–80. [Google Scholar] [CrossRef]
- Raj, S.K.; Yadav, V.; Bhadu, G.R.; Patidar, R.; Kumar, M.; Kulshrestha, V. Synthesis of highly fluorescent and water soluble graphene quantum dots for detection of heavy metal ions in aqueous media. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ju, L.; Cui, H. Chemiluminescent and fluorescent dual-signal graphene quantum dots and their application in pesticide sensing arrays. J. Mater. Chem. C 2017, 5, 7753–7758. [Google Scholar] [CrossRef]
- Ciotta, E.; Paoloni, S.; Richetta, M.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Casciardi, S.; Pizzoferrato, R. Sensitivity to heavy-metal ions of unfolded fullerene quantum dots. Sensors 2017, 17, 2614. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.F.; Williard, P.G.; Wang, L.S. Polymorphism of phosphine-protected gold nanoclusters: Synthesis and characterization of a new 22-Gold-Atom cluster. Small 2016, 12, 2518–2525. [Google Scholar] [CrossRef]
- Burratti, L.; Bolli, E.; Casalboni, M.; de Matteis, F.; Mochi, F.; Francini, R.; Casciardi, S.; Prosposito, P. Synthesis of fluorescent ag nanoclusters for sensing and imaging applications. Mater. Sci. Forum 2018, 941, 2243–2248. [Google Scholar] [CrossRef]
- Burratti, L.; Ciotta, E.; Bolli, E.; Kaciulis, S.; Casalboni, M.; De Matteis, F.; Garzón-Manjón, A.; Scheu, C.; Pizzoferrato, R.; Prosposito, P. Fluorescence enhancement induced by the interaction of silver nanoclusters with lead ions in water. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123634. [Google Scholar] [CrossRef]
- Sokołowska, K.; Malola, S.; Lahtinen, M.; Saarnio, V.; Permi, P.; Koskinen, K.; Jalasvuori, M.; Häkkinen, H.; Lehtovaara, L.; Lahtinen, T. Towards Controlled Synthesis of Water-Soluble Gold Nanoclusters: Synthesis and Analysis. J. Phys. Chem. C 2019, 123, 2602–2612. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Yan, C.; Bai, G.; Liu, Y. DNA-functionalized gold nanoparticle-based fluorescence polarization for the sensitive detection of silver ions. Colloids Surf. B Biointerfaces 2018, 167, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Yang, Z.; Lee, K.H.; Chang, H.T. Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II). Angew. Chem. Int. Ed. 2007, 46, 6824–6828. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.H.; Hu, C.C.; Chiu, T.C. A fluorescence turn-on probe for sensing thiodicarb using rhodamine B functionalized gold nanoparticles. Dyes Pigments 2019, 171, 107674. [Google Scholar] [CrossRef]
- Roy, B.; Bairi, P.; Nandi, A.K. Selective colorimetric sensing of mercury(ii) using turn off–turn on mechanism from riboflavin stabilized silver nanoparticles in aqueous medium. Analyst 2011, 136, 3605. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lodeiro, J.; Núñez, C.; Oliveira, E.; Capelo, J.L.; Lodeiro, C. 1D chain fluorescein-functionalized gold and silver nanoparticles as new optical mercury chemosensor in aqueous media. J. Nanoparticle Res. 2013, 15, 1828. [Google Scholar] [CrossRef]
- Fratoddi, I.; Cartoni, A.; Venditti, I.; Catone, D.; O’Keeffe, P.; Paladini, A.; Toschi, F.; Turchini, S.; Sciubba, F.; Testa, G.; et al. Gold nanoparticles functionalized by rhodamine B isothiocyanate: A new tool to control plasmonic effects. J. Colloid Interface Sci. 2018, 513, 10–19. [Google Scholar] [CrossRef]
- Hamilton, D.J.; Ambrus, Á.; Dieterle, R.M.; Felsot, A.S.; Harris, C.A.; Holland, P.T.; Katayama, A.; Kurihara, N.; Linders, J.; Unsworth, J.; et al. Regulatory limits for pesticide residues in water (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 1123–1155. [Google Scholar] [CrossRef]
- European Environment Agency, Consultation on Pesticides in Surface Waters and Groundwater Report. Available online: https://forum.eionet.europa.eu/nrc-eionet-freshwater/library/pesticides-water/consultation-on-on-pesticides-in-surface-waters-and-groundwater-report (accessed on 2 July 2020).
- Council of European Communities. Relating to the Quality of Water Intended for Human Consumption; Council Directive of 15 July 1980 80/778/EEC; Official Journal of European Communities: Brussels, Belgium, 1980; No. 299/11-29.
- WHO. Chemical aspects. In Guideline for Drinking Water, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; pp. 155–201. [Google Scholar]
- United States Environmental Protection Agency Ground Water and Drinking Water. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulation-table (accessed on 2 July 2020).
- Valeur, B. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Mishra, D. Resorcinarene-Embedded Stable Silver Nanoparticles: A Fluorescent Nanoprobe for Pb(II) in Water. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 1360–1370. [Google Scholar] [CrossRef]
- Afaneh, A.T.; Schreckenbach, G. Fluorescence enhancement/quenching based on metal orbital control: Computational studies of a 6-thienyllumazine-based mercury sensor. J. Phys. Chem. A 2015, 119, 8106–8116. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, M.; Jana, J.; Pal, A.; Pal, T. Synergism of gold and silver invites enhanced fluorescence for practical applications. RSC Adv. 2016, 6, 17683–17703. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Bhopate, D.P.; Kolekar, G.B.; Patil, S.R. N-methyl isatin nanoparticles as a novel probe for selective detection of Cd2+ ion in aqueous medium based on chelation enhanced fluorescence and application to environmental sample. Sens. Actuators B Chem. 2015, 220, 864–872. [Google Scholar] [CrossRef]
- Guo, Z.; Niu, Q.; Li, T.; Sun, T.; Chi, H. A fast, highly selective and sensitive colorimetric and fluorescent sensor for Cu 2+ and its application in real water and food samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 97–103. [Google Scholar] [CrossRef]
- Ciotta, E.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Stella, L.; Kaciulis, S.; Soltani, P.; Placidi, E.; Pizzoferrato, R. Discriminating between different heavy metal ions with fullerene-derived nanoparticles. Sensors 2018, 18, 1496. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, K.D.; Vyas, D.J.; Makwana, B.A.; Darjee, S.M.; Jain, V.K.; Shah, H. Turn-on fluorescence probe for selective detection of Hg(II) by calixpyrrole hydrazide reduced silver nanoparticle: Application to real water sample. Chin. Chem. Lett. 2016, 27, 731–737. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, G.; Liu, M. Metal-enhanced fluorescence effect of Ag and Au nanoparticles modified with rhodamine derivative in detecting Hg2+. Sens. Actuators B Chem. 2015, 212, 495–504. [Google Scholar] [CrossRef]
- Gu, Y.; Li, N.; Gao, M.; Wang, Z.; Xiao, D.; Li, Y.; Jia, H.; He, H. Microwave-assisted synthesis of BSA-modified silver nanoparticles as a selective fluorescent probe for detection and cellular imaging of cadmium(II). Microchim. Acta 2015, 182, 1255–1261. [Google Scholar] [CrossRef]
- Kuscu, M.; Akan, O.B. A physical channel model and analysis for nanoscale molecular communications with frster resonance energy transfer (FRET). IEEE Trans. Nanotechnol. 2012, 11, 200–207. [Google Scholar] [CrossRef]
- Chen, G.; Song, F.; Xiong, X.; Peng, X. Fluorescent nanosensors based on Fluorescence Resonance Energy Transfer (FRET). Ind. Eng. Chem. Res. 2013, 52, 11228–11245. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for Fluorescence Resonance Energy Transfer Analysis: Beyond Traditional Donor–Acceptor Combinations. Angew. Chem. Int. Ed. 2006, 45, 4562–4589. [Google Scholar] [CrossRef] [PubMed]
- Clapp, A.R.; Medintz, I.L.; Mattoussi, H. Förster resonance energy transfer investigations using quantum-dot fluorophores. ChemPhysChem 2006, 7, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, B.M.; Siu, M.; Agarwal, H.; Alivisatos, A.P.; Liphardt, J. Calibration of Dynamic Molecular Rulers Based on Plasmon Coupling between Gold Nanoparticles. Nano Lett. 2005, 5, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Li, H.; Zhang, Y.; Yao, S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens. Bioelectron. 2015, 68, 168–174. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, J.; Guo, L.; Luo, F.; Qiu, B.; Hong, G.; Lin, Z. Targets regulated formation of boron nitride quantum dots–Gold nanoparticles nanocomposites for ultrasensitive detection of acetylcholinesterase activity and its inhibitors. Sens. Actuators B Chem. 2019, 279, 61–68. [Google Scholar] [CrossRef]
- Chen, Q.; Sheng, R.; Wang, P.; Ouyang, Q.; Wang, A.; Ali, S.; Zareef, M.; Hassan, M.M. Ultra-sensitive detection of malathion residues using FRET-based upconversion fluorescence sensor in food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 118654. [Google Scholar] [CrossRef]
- Nebu, J.; Anjali Devi, J.S.; Aparna, R.S.; Aswathy, B.; Lekha, G.M.; Sony, G. Fluorescence turn-on detection of fenitrothion using gold nanoparticle quenched fluorescein and its separation using superparamagnetic iron oxide nanoparticle. Sens. Actuators B Chem. 2018, 277, 271–280. [Google Scholar] [CrossRef]
- Huang, D.; Niu, C.; Ruan, M.; Wang, X.; Zeng, G.; Deng, C. Highly sensitive strategy for Hg2+ detection in environmental water samples using long lifetime fluorescence quantum dots and gold nanoparticles. Environ. Sci. Technol. 2013, 47, 4392–4398. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Y.; Lu, Y.; Yan, C. DNA-functionalization gold nanoparticles based fluorescence sensor for sensitive detection of Hg2+ in aqueous solution. Sens. Actuators B Chem. 2015, 211, 1–6. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.L.; Basu, H.; Saha, S.; Singhal, R.K.; Kailasa, S.K. One pot synthesis of fluorescent gold nanoclusters from Curcuma longa extract for independent detection of Cd2+, Zn2+ and Cu2+ ions with high sensitivity. J. Mol. Liq. 2020, 304, 112697. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, D.; Jin, R.; Li, H.; Yan, X.; Liu, F.; Sun, P.; Gao, Y.; Lu, G. On-site monitoring of thiram via aggregation-induced emission enhancement of gold nanoclusters based on electronic-eye platform. Sens. Actuators B Chem. 2019, 296, 126641. [Google Scholar] [CrossRef]
- Cai, Y.; Qiu, Z.; Lin, X.; Zeng, W.; Cao, Y.; Liu, W.; Liu, Y. Self-Assembled Nanomaterials Based on Aggregation-Induced Emission of AuNCs: Fluorescence and Colorimetric Dual-Mode Biosensing of Organophosphorus Pesticides. Sens. Actuators B Chem. 2020, 321, 128481. [Google Scholar] [CrossRef]
- Fonin, A.V.; Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Fluorescence of dyes in solutions with high absorbance. Inner filter effect correction. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Kubista, M.; Sjöback, R.; Eriksson, S.; Albinsson, B. Experimental correction for the inner-filter effect in fluorescence spectra. Analyst 1994, 119, 417–419. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zhou, P.; Yang, W.; Tao, H.; Qiu, S.; Feng, C. A novel fluorescent aptasensor for ultrasensitive and selective detection of acetamiprid pesticide based on the inner filter effect between gold nanoparticles and carbon dots. Analyst 2018, 143, 5151–5160. [Google Scholar] [CrossRef]
- Niu, W.J.; Shan, D.; Zhu, R.H.; Deng, S.Y.; Cosnier, S.; Zhang, X.J. Dumbbell-shaped carbon quantum dots/AuNCs nanohybrid as an efficient ratiometric fluorescent probe for sensing cadmium (II) ions and l-ascorbic acid. Carbon N. Y. 2016, 96, 1034–1042. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Han, X.; Su, X. A ratiometric fluorescent quantum dots based biosensor for organophosphorus pesticides detection by inner-filter effect. Biosens. Bioelectron. 2015, 74, 277–283. [Google Scholar] [CrossRef]
- Wang, G.; Shao, C.; Yan, C.; Li, D.; Liu, Y. Fluorescence polarization sensor platform based on gold nanoparticles for the efficient detection of Ag (I). J. Lumin. 2019, 210, 21–27. [Google Scholar] [CrossRef]
- Wang, N.; Ga, L.; Jia, M.; Ai, J. Synthesis of fluorescent copper nanoparticles and ultrasensitive free label detection of Ag+. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Ren, G.; Tang, M.; Chai, F.; Qu, F.; Wang, C.; Su, Z. Fluorescent silicon nanoparticles for sensing Hg2+ and Ag+ as well visualization of latent fingerprints. Dyes Pigments 2018, 149, 686–695. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Shen, X.; Zhu, W.; Xu, L.; Zhou, X. Aggregation-induced emission from gold nanoclusters for use as a luminescence-enhanced nanosensor to detect trace amounts of silver ions. J. Colloid Interface Sci. 2016, 467, 90–96. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, X.; Li, H.; Zhang, Y.; Yao, S. On–off–on fluorescent silicon nanoparticles for recognition of chromium(VI) and hydrogen sulfide based on the inner filter effect. Sens. Actuators B Chem. 2017, 238, 196–203. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Ren, G.; Li, S.; Zhu, B.; Chai, F.; Qu, F.; Wang, C.; Su, Z. Multicolorful fluorescent-nanoprobe composed of Au nanocluster and carbon dots for colorimetric and fluorescent sensing Hg2+ and Cr6+. Sens. Actuators B Chem. 2018, 262, 678–686. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Wang, T.; Yun, Z.; Li, G.; Liu, J.; Jiang, G. Facile preparation of glutathione-stabilized gold nanoclusters for selective determination of chromium (III) and chromium (VI) in environmental water samples. Anal. Chim. Acta 2013, 770, 140–146. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, M.; Wu, X.; Wang, F.; Liu, L. Methionine-capped gold nanoclusters as a fluorescence-enhanced probe for cadmium(II) sensing. Sensors 2018, 18, 658. [Google Scholar] [CrossRef] [Green Version]
- Akshath, U.S.; Bhatt, P.; Singh, S.A. Differential Interaction of Metal Ions with Gold Nanoclusters and Application in Detection of Cobalt and Cadmium. J. Fluoresc. 2020, 30, 537–545. [Google Scholar] [CrossRef]
- Makwana, B.A.; Vyas, D.J.; Bhatt, K.D.; Darji, S.; Jain, V.K. Novel fluorescent silver nanoparticles: Sensitive and selective turn off sensor for cadmium ions. Appl. Nanosci. 2016, 6, 555–566. [Google Scholar] [CrossRef] [Green Version]
- Contino, A.; Maccarrone, G.; Zimbone, M.; Reitano, R.; Musumeci, P.; Calcagno, L.; Oliveri, I. Pietro Tyrosine capped silver nanoparticles: A new fluorescent sensor for the quantitative determination of copper(II) and cobalt(II) ions. J. Colloid Interface Sci. 2016, 462, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hou, Y.; Wang, Y.; Wang, Y.; Li, T.; Song, C.; Wei, N.; Wang, Q. Sensitive and selective detection of Cu2+ ions based on fluorescent Ag nanoparticles synthesized by R-phycoerythrin from marine algae Porphyra yezoensis. Ecotoxicol. Environ. Saf. 2019, 168, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, Y.; Li, Y.; Liu, Q.; Xie, M. Fluorescent and colorimetric sensor for Cu2+ ion based on formaldehyde modified hyperbranched polyethylenimine capped gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 78–86. [Google Scholar] [CrossRef]
- Cao, D.; Fan, J.; Qiu, J.; Tu, Y.; Yan, J. Masking method for improving selectivity of gold nanoclusters in fluorescence determination of mercury and copper ions. Biosens. Bioelectron. 2013, 42, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Irudayaraj, J. Fluorescent Ag clusters via a protein-directed approach as a Hg(II) ion sensor. Anal. Chem. 2011, 83, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Banerjee, A. Facile synthesis of water-soluble fluorescent silver nanoclusters and HgII sensing. Chem. Mater. 2010, 22, 4364–4371. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+-Au+ interactions. Chem. Commun. 2010, 46, 961–963. [Google Scholar] [CrossRef]
- Wang, J.; Ma, S.; Ren, J.; Yang, J.; Qu, Y.; Ding, D.; Zhang, M.; Yang, G. Fluorescence enhancement of cysteine-rich protein-templated gold nanoclusters using silver(I) ions and its sensing application for mercury(II). Sens. Actuators B Chem. 2018, 267, 342–350. [Google Scholar] [CrossRef]
- Tan, D.; He, Y.; Xing, X.; Zhao, Y.; Tang, H.; Pang, D. Aptamer functionalized gold nanoparticles based fluorescent probe for the detection of mercury (II) ion in aqueous solution. Talanta 2013, 113, 26–30. [Google Scholar] [CrossRef]
- Daware, K.; Shinde, R.; Kalubarme, R.S.; Kasture, M.; Pandey, A.; Terashima, C.; Gosavi, S.W. Development of optical sensing probe for Hg(II) ions detection in ground water using Au, Hexanedithiol and Rhodamine B nanocomposite system. Sens. Actuators B Chem. 2018, 265, 547–555. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Celebioglu, A.; Uyar, T. Real-time selective visual monitoring of Hg 2+ detection at ppt level: An approach to lighting electrospun nanofibers using gold nanoclusters. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yuan, Y.; Pu, G.; Xu, N. An “on-off-on” fluorescence assay based on silicon nanoparticles for selective detection of manganese(II). Anal. Methods 2017, 9, 2553–2560. [Google Scholar] [CrossRef]
- Han, B.; Xiang, R.; Hou, X.; Yu, M.; Peng, T.; Li, Y.; He, G. One-step rapid synthesis of single thymine-templated fluorescent copper nanoclusters for “turn on” detection of Mn2+. Anal. Methods 2017, 9, 2590–2595. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, C. Highly sensitive and selective detection of Pb2+ using a turn-on fluorescent aptamer DNA silver nanoclusters sensor. Talanta 2018, 182, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Liu, Y.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 1577–1581. [Google Scholar] [CrossRef]
- Wang, X.F.; Xiang, L.P.; Wang, Y.S.; Xue, J.H.; Zhu, Y.F.; Huang, Y.Q.; Chen, S.H.; Tang, X. A “turn-on” fluorescence assay for lead(II) based on the suppression of the surface energy transfer between acridine orange and gold nanoparticles. Microchim. Acta 2016, 183, 1333–1339. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Chen, Z.; Ge, P.; Jia, R.; Xiao, E.; Zeng, W. A turn-on fluorescent nanoprobe for lead(II) based on the aggregation of weakly associated gold(I)-glutathione nanoparticles. Microchim. Acta 2017, 184, 4209–4215. [Google Scholar] [CrossRef]
- Singh, S.; Halder, A.; Sinha, O.; Sarkar, P.K.; Singh, P.; Banerjee, A.; Ahmed, S.A.; Alharbi, A.; Obaid, R.J.; Ghosh, S.K.; et al. Nanoparticle-based “turn-on” scattering and post-sample fluorescence for ultrasensitive detection of water pollution in wider window. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Polley, N.; Chakrabarti, S.; Lemmens, P.; Pal, S.K. Nanosurface Energy Transfer Based Highly Selective and Ultrasensitive “Turn on” Fluorescence Mercury Sensor. ACS Sens. 2016, 1, 789–797. [Google Scholar] [CrossRef]
- Schierow, L.J. Pesticide Law: A Summary of the Statutes; Congressional Research Service: Washington, DC, USA, 2011.
- Xiang, H.; Cai, Q.; Li, Y.; Zhang, Z.; Cao, L.; Li, K.; Yang, H. Sensors Applied for the Detection of Pesticides and Heavy Metals in Freshwaters. J. Sens. 2020, 2020, 1–22. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Kim, G.W.; Park, T.J. A facile and sensitive detection of organophosphorus chemicals by rapid aggregation of gold nanoparticles using organic compounds. Biosens. Bioelectron. 2015, 67, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, Y.; Li, X.; Dong, C.; Liu, H.; Miao, L.; Low, P.J.; Gao, Z.; Hosmane, N.S.; Wu, A. Rapid and sensitive colorimetric sensing of the insecticide pymetrozine using melamine-modified gold nanoparticles. Anal. Methods 2018, 10, 417–421. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, X.; Yuan, C.; Shi, R.; Wang, Y. Double responsive analysis of carbaryl pesticide based on carbon quantum dots and Au nanoparticles. Dyes Pigments 2020, 181, 108529. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Jiang, S.; Fu, Z. A facile luminescence resonance energy transfer method for detecting cyano-containing pesticides in herbal medicines. Microchem. J. 2020, 152, 104451. [Google Scholar] [CrossRef]
- Luo, Q.; Lai, J.; Qiu, P.; Wang, X. An ultrasensitive fluorescent sensor for organophosphorus pesticides detection based on RB-Ag/Au bimetallic nanoparticles. Sens. Actuators B Chem. 2018, 263, 517–523. [Google Scholar] [CrossRef]
- Rong, Y.; Li, H.; Ouyang, Q.; Ali, S.; Chen, Q. Rapid and sensitive detection of diazinon in food based on the FRET between rare-earth doped upconversion nanoparticles and graphene oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118500. [Google Scholar] [CrossRef]
- Su, L.; Wang, S.; Wang, L.; Yan, Z.; Yi, H.; Zhang, D.; Shen, G.; Ma, Y. Fluorescent aptasensor for carbendazim detection in aqueous samples based on gold nanoparticles quenching Rhodamine B. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117511. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.; Wang, X.; Yao, W.; Zhang, W.; Jiang, L. An aptamer based aggregation assay for the neonicotinoid insecticide acetamiprid using fluorescent upconversion nanoparticles and DNA functionalized gold nanoparticles. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Hu, T.; Lu, S.; Chen, C.; Sun, J.; Yang, X. Colorimetric sandwich immunosensor for Aβ(1-42) based on dual antibody-modified gold nanoparticles. Sens. Actuators B Chem. 2017, 243, 792–799. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Huo, J.; Zhang, A.; Pan, Y.; Bai, H.; Jiao, Z.; Fang, T.; Wang, X.; Cai, Y.; et al. Modified enzyme-linked immunosorbent assay strategy using graphene oxide sheets and gold nanoparticles functionalized with different antibody types. Anal. Chem. 2013, 85, 6228–6232. [Google Scholar] [CrossRef]
- Li, X.; Cui, H.; Zeng, Z. A simple colorimetric and fluorescent sensor to detect organophosphate pesticides based on adenosine triphosphate-modified gold nanoparticles. Sensors 2018, 18, 4302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Wang, Y.; Jia, J.; Xiang, Y. Colorimetric aptasensors for determination of tobramycin in milk and chicken eggs based on DNA and gold nanoparticles. Food Chem. 2018, 249, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-L.; Wang, Q.; Lei, H.-T.; Eremin, S.A.; Shen, Y.-D.; Wang, H.; Beier, R.C.; Yang, J.-Y.; Maksimova, K.A.; Sun, Y.-M. A simple, rapid and high-throughput fluorescence polarization immunoassay for simultaneous detection of organophosphorus pesticides in vegetable and environmental water samples. Anal. Chim. Acta 2011, 708, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Hall, C.K. Binding Preferences of Amino Acids for Gold Nanoparticles: A Molecular Simulation Study. Langmuir 2016, 32, 7888–7896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Hua, X.; Chen, H.; Gonzalez-Sapienza, G.; Barnych, B.; Liu, F.; Wang, M.; Hammock, B.D. A dual signal immunochromatographic strip for the detection of imidaclothiz using a recombinant fluorescent-peptide tracer and gold nanoparticles. Sens. Actuators B Chem. 2019, 297, 126714. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Z.; Jin, M.; Du, P.; Chen, G.; Cui, X.; Zhang, Y.; Qin, G.; Yan, F.; Abd El-Aty, A.M.; et al. Fluorescence immunoassay for multiplex detection of organophosphate pesticides in agro-products based on signal amplification of gold nanoparticles and oligonucleotides. Food Chem. 2020, 326, 126813. [Google Scholar] [CrossRef]
- Yu, J.; Guo, T.; Zhang, W.; Li, B.; Liu, L.; Hua, R. Green upconversion nanoparticles for 2,4-dichlorophenoxyacetic acid and fenitrothion detection. J. Alloys Compd. 2019, 771, 187–194. [Google Scholar] [CrossRef]
- Li, H.; Jin, R.; Kong, D.; Zhao, X.; Liu, F.; Yan, X.; Lin, Y.; Lu, G. Switchable fluorescence immunoassay using gold nanoclusters anchored cobalt oxyhydroxide composite for sensitive detection of imidacloprid. Sens. Actuators B Chem. 2019, 283, 207–214. [Google Scholar] [CrossRef]
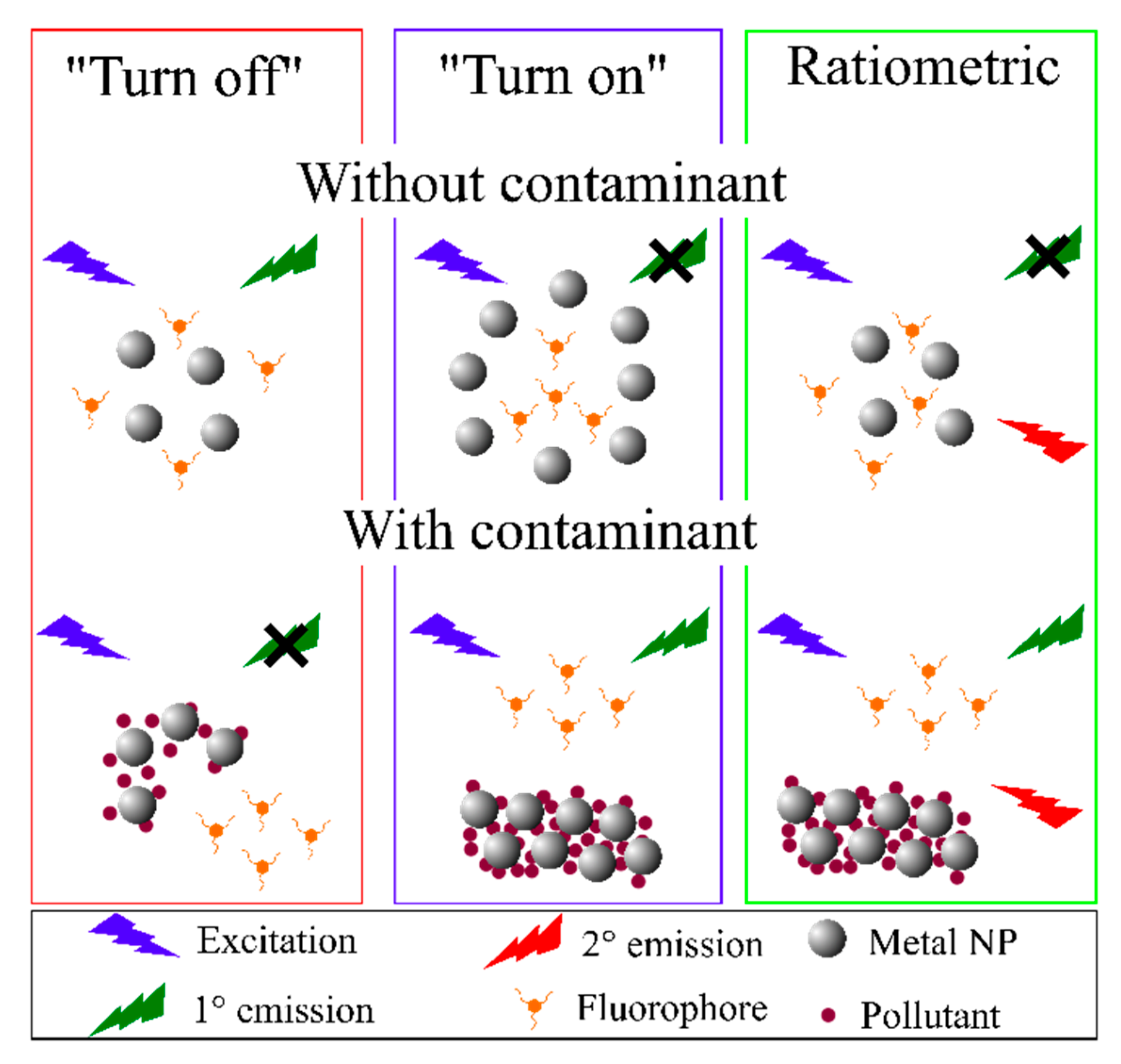
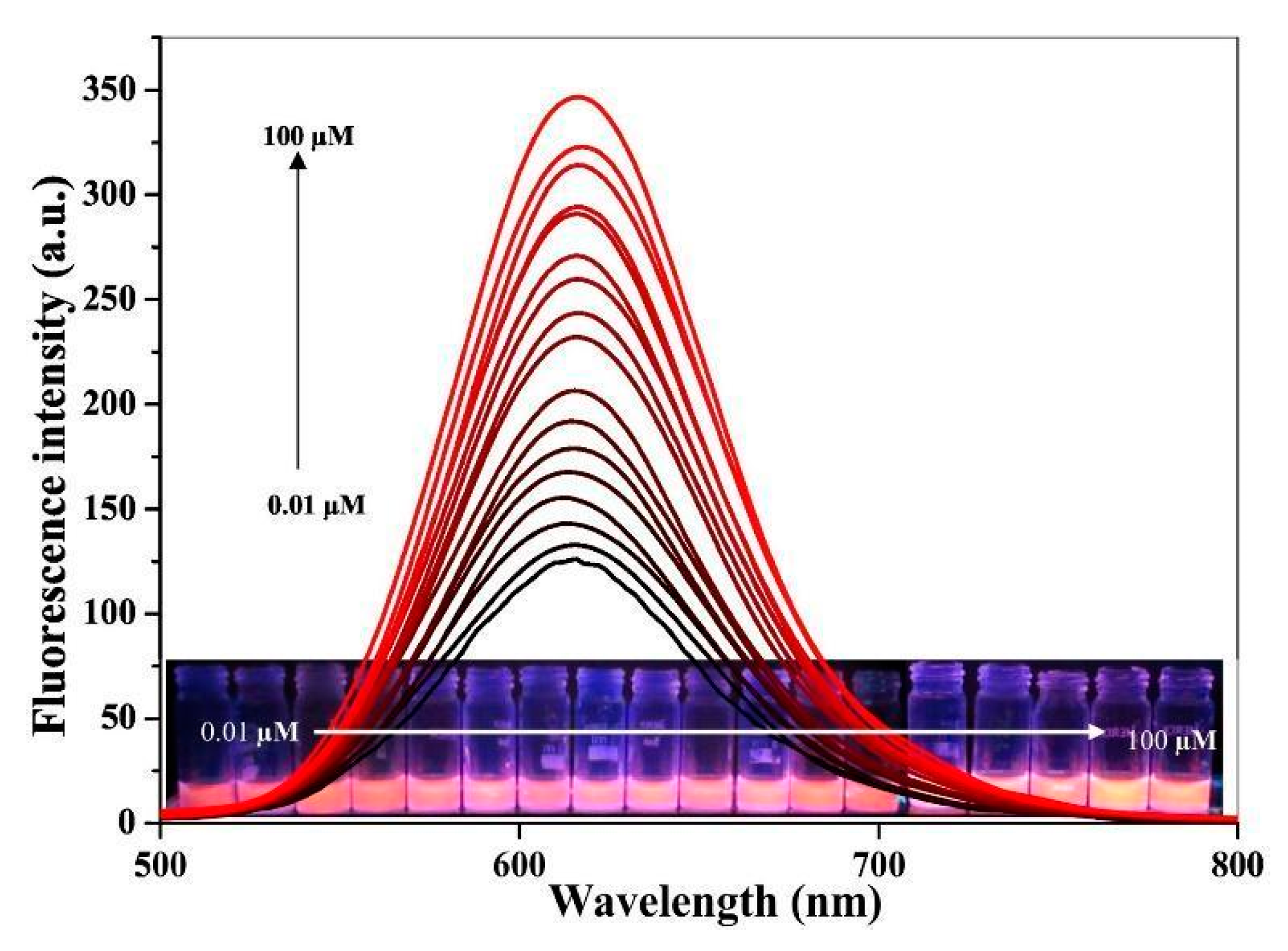


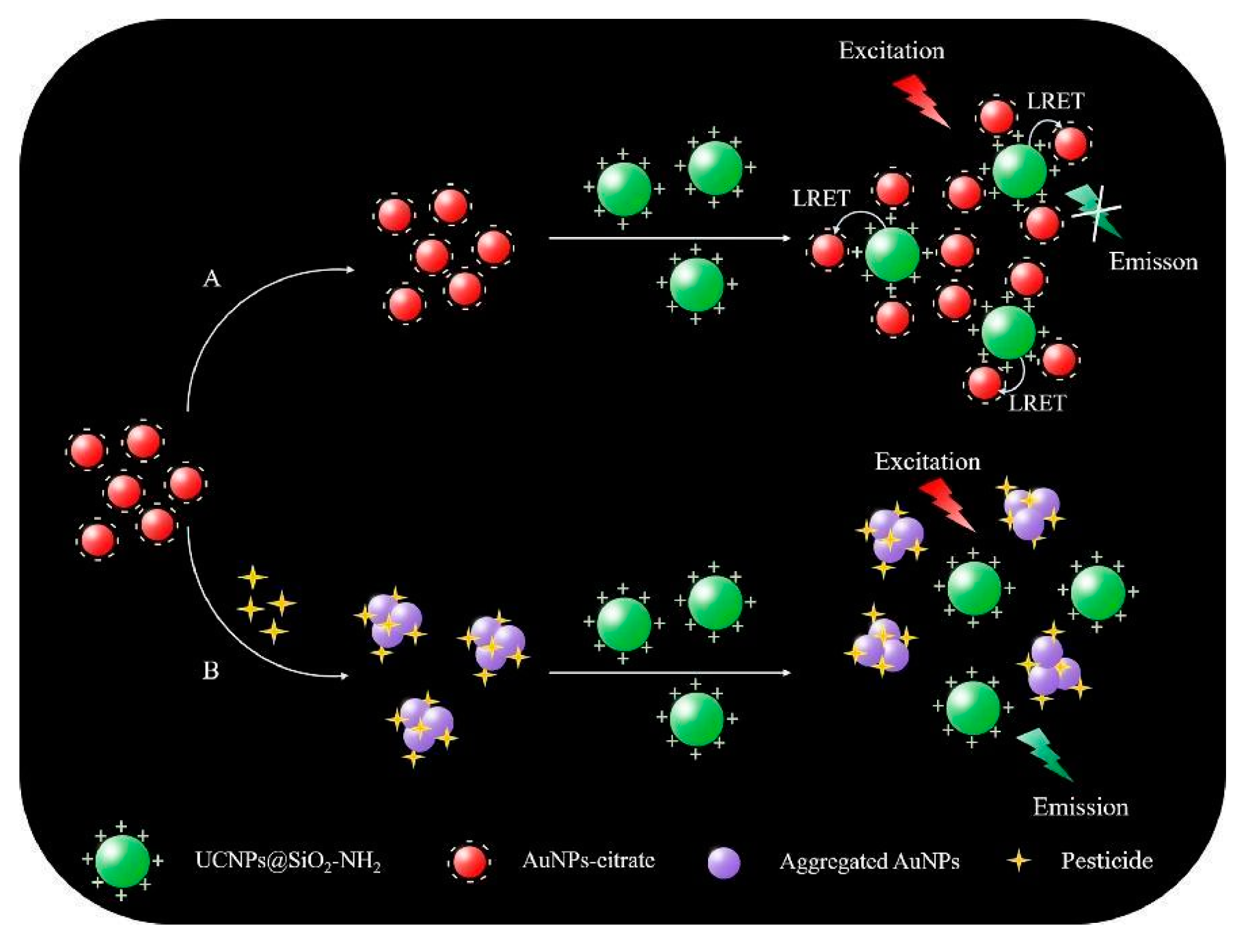


| Sensing mechanism | Analyte | LOD | Ref. |
|---|---|---|---|
| Thiol-DNA-functionalized gold nanoparticles (AuNPs) quenched by a formation of a complex induced by Ag(I) | Ag(I) | 9.5 nM | [33] |
| Glc-CuNPs quenched by aggregation induced by Ag(I) | Ag(I) | 100 µM | [77] |
| SiNPs quenched by aggregation induced by Ag(I) | Ag(I) | 0.457 µM | [78] |
| Hg(II) | 2.676 µM | ||
| AuNCs covered with glutathione enhanced the luminescence due to aggregation-induced emission of the functionalized gold nanoclusters by LMCT or LMMCT | Ag(I) | 0.2 nM | [79] |
| AgNCs-dBSA quenched by Hg-Ag metallophilic interaction | Hg(II) | 10 nM | [90] |
| AgNCs-DHLA quenched by aggregation induced by Hg(II) | Hg(II) | 10−10 M | [91] |
| AuNCs quenched by Hg-Au metallophilic interaction | Hg(II) | 0.5 nM | [92] |
| AuNCs-Ag@Keratin quenched by Hg-Au and Hg-Ag metallophilic interactions | Hg(II) | 2.31 nM | [93] |
| AuNPs-MUA aggregation-induced fluorescence quenching by Hg(II) | Hg(II) | 5 nM | [34] |
| AuNPs–MSD-FAM sensor based on NSET | Hg(II) | 16 nM | [94] |
| AuNPs-DNA-FAM sensor based on FRET | Hg(II) | 8 nM | [65] |
| AuNPs-QDs sensor based on time-gated FRET | Hg(II) | 0.87 nM | [64] |
| AuNPs-RB-HDT nanocomposites system, the FRET effect from RB to AuNPs, Hg(II) ions induce AuNPs aggregation as a consequence the RB is not quenched anymore | Hg(II) | 0.5 ppb | [95] |
| AuNCs -PCL nanofibers quenched by Au-Hg amalgam formation | Hg(II) | 50 ppt | [96] |
| SiNPs sensor based on IFE | Cr(VI) | 28 n M | [80] |
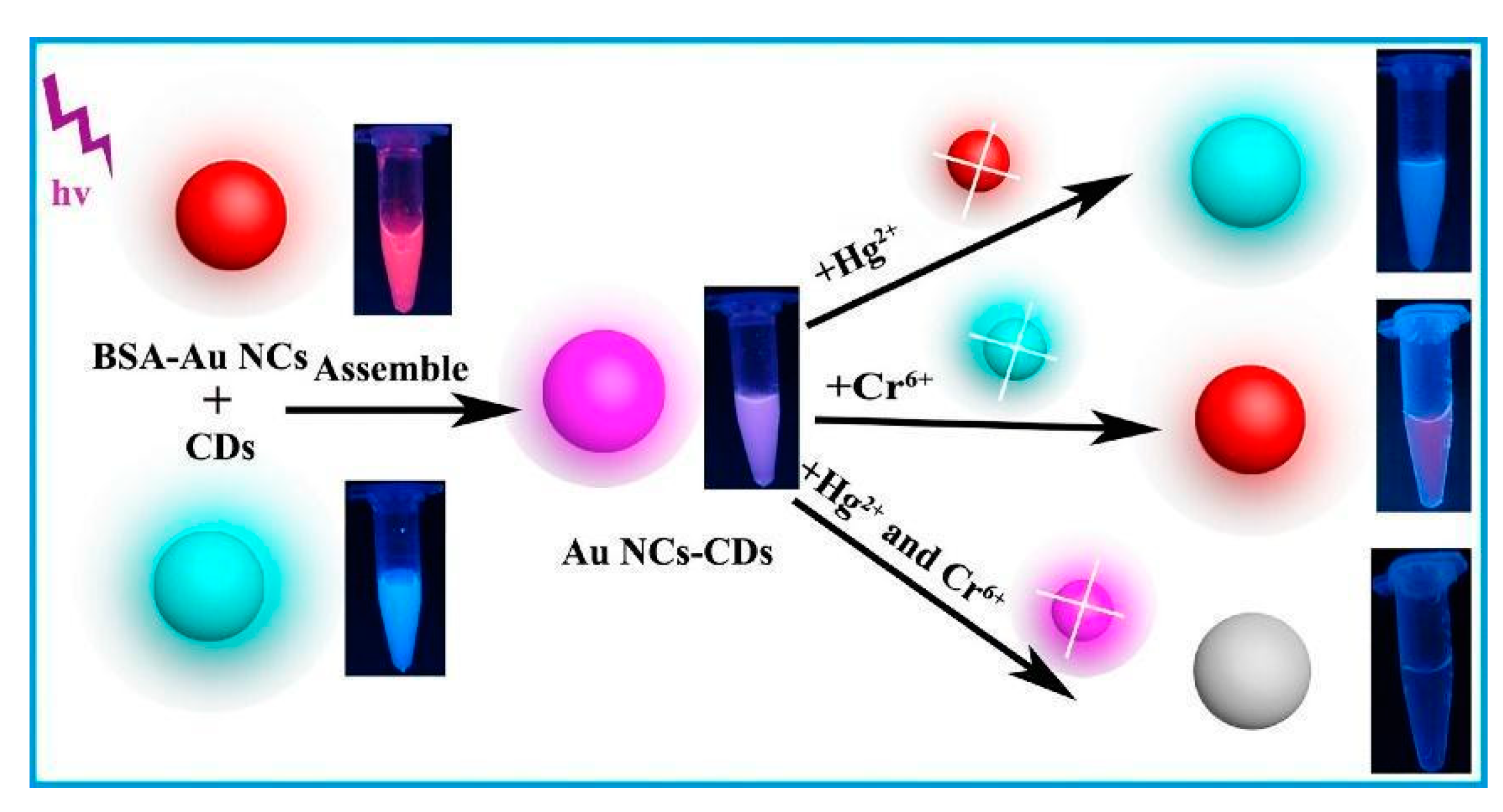
| AuNCs-BSA and CDs, IFE mechanism for Cr(VI) and Au-Hg interaction for Hg(II) | Cr(VI) Hg(II) | 5.34 nM 1.85 nM | [81] |
| AuNCs-GSH Cr(III) ions may induce aggregation or superficial imperfections of NCs | Cr(III) | 2.5 µg/L | [82] |
| AuNCs-GSH redox interaction between Cr(VI) and GSH | Cr(VI) | 0.5 µg/L | [82] |
| Orange-emitting fluorescence AuNCs with methitione sensor based on AIE | Cd(II) | 12.25 nM | [83] |
| AuNCs-MUA-CE sensor based on CHEF or AIE in cases of Cd(II) and Zn(II), while Cu(II) ions induce a not fluorescent aggregation of NCs | Cd(II) Zn(II) Cu(II) | 12 nM 16 nM 26 nM | [67] |
| AuNCs-BSA, Co(II) ions induce intersystem crossing, while Cd(II) induce FRET on the system | Cd(II) Co(II) | - - | [84] |
| AuNCs-CQDs ratiometric-based sensor, Cd(II) induces an electrostatic interaction with AgNCs ligands, quenching the PL emission only of the AgNCs | Cd(II) | 105 nM | [74] |
| OMRTH-AgNPs, Cd(II) induces aggregation of NPs | Cd(II) | 10−8 M | [85] |
| R-PE-AgNPs, Cu(II) induces aggregation of NPs | Cu(II) | 19 nM | [87] |
| AuNCs-BSA, Cu(II) ions reversible interact with BSA ligands, while Hg(II) ions irreversible interact with Au on the NCs surface | Cu(II) Hg(II) | 300 nM 8 nM | [89] |
| SiNPs, Mn(II) induces aggregation of NPs | Mn(II) | 1.1 µM | [97] |
| CuNCs-Thymine, Mn(II) induces AIE on the system | Mn(II) | 10 µM | [98] |
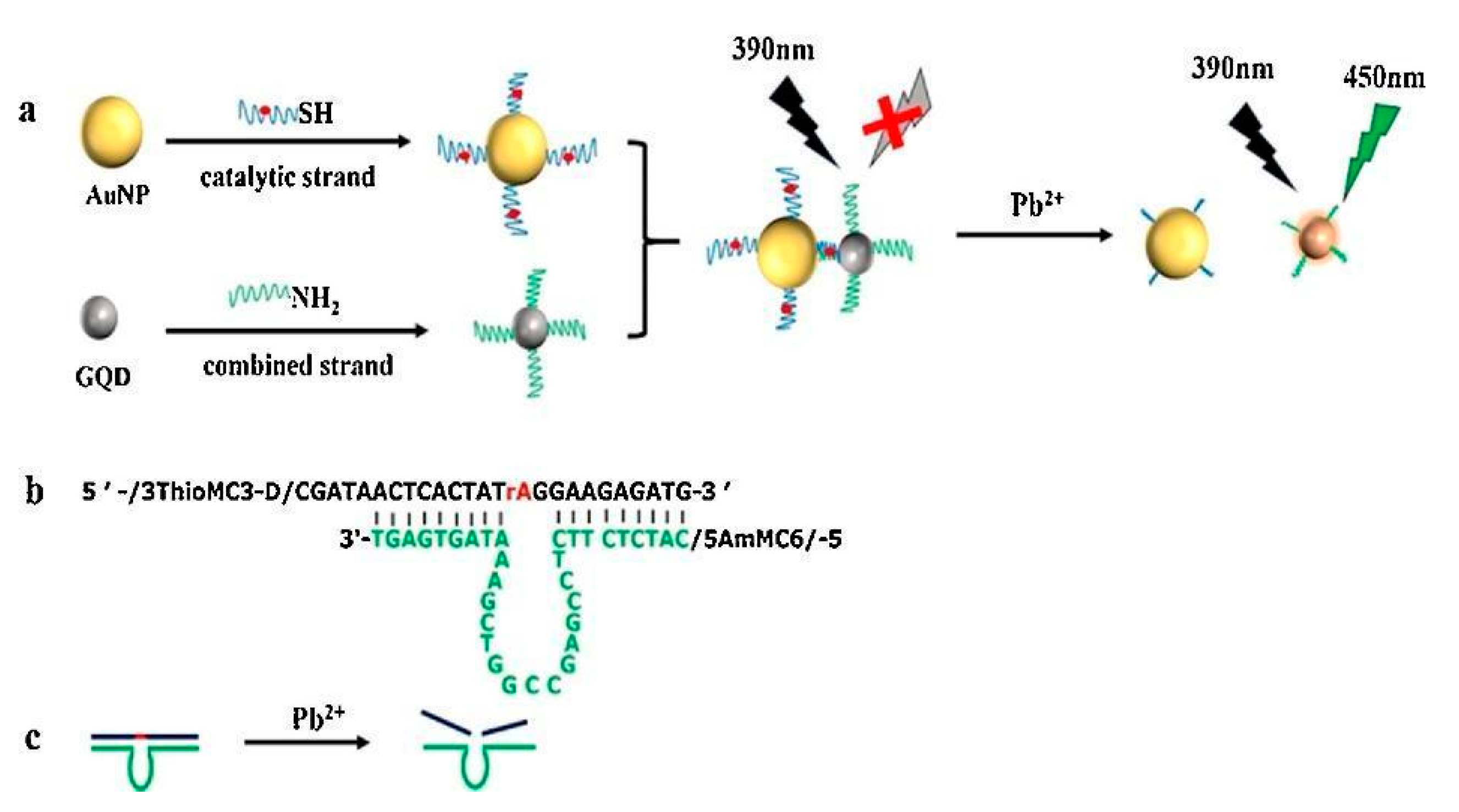
| DNA-AgNCs, Pb(II) ions interact with DNA strands, giving rise to more stable molecular conformation, PL emission is enhanced | Pb(II) | 0.3 nM | [99] |
| GQDs-AuNPs, Pb(II) ions induce the separation of GQDs and AuNPS, restoring the GQDs emission | Pb(II) | 16.7 nM | [100] |
| AuNPs-AO, Pb(II) ions inhibit the SET between AO and AuNPs | Pb(II) | 44 nM | [101] |
| Au(I)-SG complexes, Pb(II) ions promote the AIE on Au(I) complexes | Pb(II) | 0.1 µM | [102] |
| Detection Technique | Analyte | LOD | Ref. |
|---|---|---|---|
| Direct Metal-NP | |||
| FRET between BNQDs and AuNPs | Paraoxon | 33 ng/L | [61] |
| NSET of AuNPs on the fluorescence of fluorescein | Fenitrothion | 9.41 nM | [63] |
| Recovery of luminescence of B,N-doped CQDs quenched by Au-NP + thiocoline | Carbaryl | 0.06 µg/L | [110] |
| Modulation of luminescence resonance energy transfer (LRET) between UCNPs and AuNPs | Acetamiprid | 15 ng/L | [111] |
| Fenpropathrin | 240 ng/L | ||
| Chlorothalonil | 11 ng/L | ||
| RB-Ag/AuNPs turn-on effect due to interaction between pesticide and RB-Ag/AuNPs | Parathion methyl | 1.8 pg/mL | [112] |
| Competitive adsorption between RB and thiodicarb on the surface of AuNPs | Thiodicarb | 0.08 ppm | [35] |
| Aptasensors | |||
| Negatively charged UCNPs and cationic-polymer encapsulated GNPs exploit the FRET mechanism between malathion and UCNPs-GNPs | Malathion | 1.42 nM | [62] |
| Fluorescent aptasensor by modulation of IFE on CDs fluorescence | Acetamiprid | 1.08 μg/L | [73] |
| Aptamer-modified UCNPs based on FRET | Diazinon | 23 ng/L | [113] |
| Fluorescence quenching of specific aptamer by AuNPs | Carbendazim | 2.33 nM | [114] |
| Colorimetric method with Acetamiprid-binding aptamer functionalized with AuNPs | Acetamiprid | 0.36 nM | [115] |
| Antibodies, nucleic acids, amino acids | |||
| Adenosine Triphosphate-Modified AuNPs by colorimetric method | Ethoprophos | 37.0 nM | [118] |
| Colorimetric aptasensors based on DNA and AuNPs | Tobramycin | 23.3 nM | [119] |
| Fluorescent polarization immunoassay using a broad-specificity monoclonal antibody | Organophosphate pesticides | 10 ng/mL | [120] |
| Fluorescence quenching-based recombinant fluorescent-peptide tracer | Imidaclothiz | 8.00 ng/mL (sensitivity) | [122] |
| AuNP functionalized with fluorescently labelled oligonucleotide | Triazophos | 0.007 μg/L | [123] |
| Patathion | 0.009 μg/L | ||
| Chlorpyrifos | 0.087 μg/L | ||
| Immunochromatographic strip | Fenitrothion-IgG | 5 mg/mL | [124] |
| Fluorescence-based immunoassay based on the quenching effects of functionalized AuNCs | Imidacloprid | 1.3 ng/mL (IC50) | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burratti, L.; Ciotta, E.; De Matteis, F.; Prosposito, P. Metal Nanostructures for Environmental Pollutant Detection Based on Fluorescence. Nanomaterials 2021, 11, 276. https://doi.org/10.3390/nano11020276
Burratti L, Ciotta E, De Matteis F, Prosposito P. Metal Nanostructures for Environmental Pollutant Detection Based on Fluorescence. Nanomaterials. 2021; 11(2):276. https://doi.org/10.3390/nano11020276
Chicago/Turabian StyleBurratti, Luca, Erica Ciotta, Fabio De Matteis, and Paolo Prosposito. 2021. "Metal Nanostructures for Environmental Pollutant Detection Based on Fluorescence" Nanomaterials 11, no. 2: 276. https://doi.org/10.3390/nano11020276






