(Bio)Nanotechnology in Food Science—Food Packaging
Abstract
:1. Introduction
2. Food Packaging
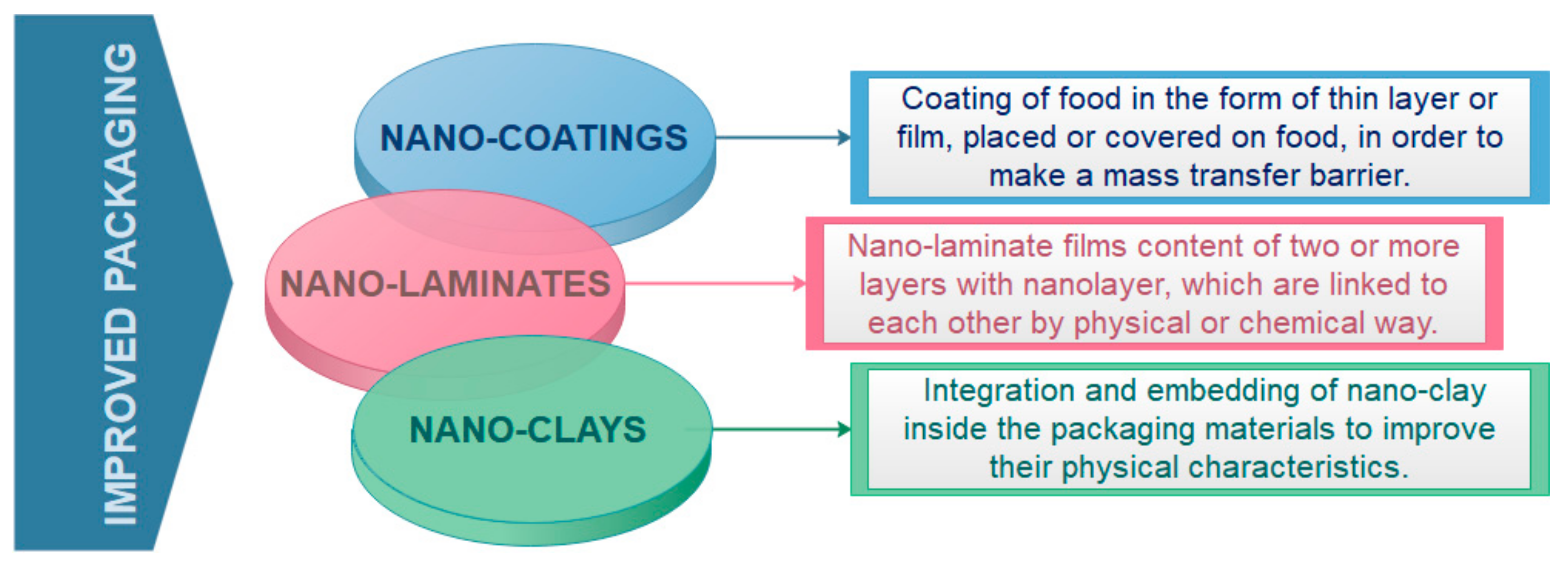
2.1. Improved Food Packaging
2.2. Active Packaging
Antimicrobial Active Packaging
2.3. Smart Packaging
2.4. Bio-Based Packaging
2.4.1. Starch-Based Nanomaterial
2.4.2. Cellulose-Based Nanomaterial
2.4.3. Chitosan-Based Nanomaterial
3. Safety and Environmental Concerns of (Bio)Nanotechnology Implementation in Food Packaging
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luttge, R. Chapter 4—Nanotechnology. In Microfabrication for Industrial Applications; Luttge, R., Ed.; Micro and Nano Technologies; William Andrew Publishing: Boston, MA, USA, 2011; pp. 91–146. ISBN 978-0-8155-1582-1. [Google Scholar]
- He, X.; Deng, H.; Hwang, H. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, J.J. Chapter 11—Bionanotechnology. In Nanotechnology, 2nd ed.; Ramsden, J.J., Ed.; Micro and Nano Technologies; William Andrew Publishing: Oxford, UK, 2016; pp. 263–278. ISBN 978-0-323-39311-9. [Google Scholar]
- Dasgupta, N.; Ranjan, S.; Mundekkad, D.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanotechnology in agro-food: From field to plate. Food Res. Int. 2015, 69, 381–400. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ghorbani, H.; Wood, D.A.; Khoshmardan, M.A. A hybrid nanocomposite of poly(styrene-methyl methacrylate- acrylic acid)/clay as a novel rheology-improvement additive for drilling fluids. J. Polym. Res. 2019, 26, 33. [Google Scholar] [CrossRef]
- Samadi, A.; Klingberg, H.; Jauffred, L.; Kjær, A.; Bendix, P.M.; Oddershede, L.B. Platinum nanoparticles: A non-toxic, effective and thermally stable alternative plasmonic material for cancer therapy and bioengineering. Nanoscale 2018, 10, 9097–9107. [Google Scholar] [CrossRef]
- Kuswandi, B.; Moradi, M. Improvement of food packaging based on functional nanomaterial. In Nanotechnology: Applications in Energy, Drug and Food; Siddiquee, S., Melvin, G.J.H., Rahman, M.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 309–344. ISBN 978-3-319-99602-8. [Google Scholar]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology in food processing. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 49–55. ISBN 978-0-12-384953-3. [Google Scholar]
- Khare, S.; Williams, K.; Gokulan, K. Nanotechnology. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 893–900. ISBN 978-0-12-384733-1. [Google Scholar]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticle-loaded chitosan-starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater. Sci. Eng. C 2010, 30, 891–897. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X.; Bi, X.; Liu, X.; Shui, Y.; Lin, H.; et al. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruits and vegetables. Molecules 2019, 24, 1695. [Google Scholar] [CrossRef] [PubMed]
- Calva-Estrada, S.J.; Jimenez-Fernandez, M.; Lugo-Cervantes, E. Protein-based films: Advances in the development of biomaterials applicable to food packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Kuswandi, B. Environmental friendly food nano-packaging. Environ. Chem. Lett. 2017, 15, 205–221. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Dasgupta, N.; Chakraborty, A.R.; Melvin Samuel, S.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanoscience and nanotechnologies in food industries: Opportunities and research trends. J. Nanopart. Res. 2014, 16, 2464. [Google Scholar] [CrossRef]
- Brody, A.L. Case studies on nanotechnologies for food packaging. Food Technol. 2007, 61, 102–107. [Google Scholar]
- Kim, S.W.; Cha, S.-H. Thermal, mechanical, and gas barrier properties of ethylene—Vinyl alcohol copolymer-based nanocomposites for food packaging films: Effects of nanoclay loading. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Arora, A.; Choudhary, V.; Sharma, D.K. Effect of clay content and clay/surfactant on the mechanical, thermal and barrier properties of polystyrene/organoclay nanocomposites. J. Polym. Res. 2011, 18, 843–857. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Wood, D.; Williams, T.G.; Avena-Bustillos, R.J.; McHugh, T.H. Nanocomposite edible films from mango puree reinforced with cellulose nanofibers. J. Food Sci. 2009, 74, N31–N35. [Google Scholar] [CrossRef]
- Gabr, M.H.; Okumura, W.; Ueda, H.; Kuriyama, W.; Uzawa, K.; Kimpara, I. Mechanical and thermal properties of carbon fiber/polypropylene composite filled with nano-clay. Compos. Part B. 2015, 69, 94–100. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Patra, D.; Srivastava, P.; Kumar, A.; Ramalingam, C. Bovine serum albumin interacts with silver nanoparticles with a “side-on” or “end on” conformation. Chem. Biol. Interact. 2016, 253, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Bhatia, S. Natural polymers vs synthetic polymer. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer: Cham, Switzerland, 2016; pp. 95–118. ISBN 978-3-319-41129-3. [Google Scholar]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of starch composition and molecular weight on physicochemical properties of biodegradable films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.-X.; de Jesus Avena-Bustillos, R.; de Fátima Ferreira Soares, N.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A Review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; ISBN 978-3-527-64676-0. [Google Scholar]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible films and coatings containing bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Majeed, K.; Jawaid, M.; Hassan, A.; Abu Bakar, A.; Abdul Khalil, H.P.S.; Salema, A.A.; Inuwa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef]
- Adame, D.; Beall, G.W. Direct measurement of the constrained polymer region in polyamide/clay nanocomposites and the implications for gas diffusion. Appl. Clay Sci. 2009, 42, 545–552. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Thomas, V.M.; Yoo, C.G.; Ozcan, S.; Deng, Y.; Nelson, K.; Ragauskas, A.J. Recent advancements of plant-based natural fiber—Reinforced composites and their applications. Compos. Part B 2020, 200, 108254. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-reinforced polymer composites: Manufacturing, properties, and applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic starch—Waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef]
- Shi, A.-M.; Wang, L.-J.; Li, D.; Adhikari, B. Characterization of starch films containing starch nanoparticles: Part 1: Physical and mechanical properties. Carbohydr. Polym. 2013, 96, 593–601. [Google Scholar] [CrossRef]
- Piyada, K.; Waranyou, S.; Thawien, W. Mechanical, thermal and structural properties of rice starch films reinforced with rice starch nanocrystals. Int. Food Res. J. 2013, 20, 439–449. [Google Scholar]
- Wang, Y.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Preparation and characterization of corn starch bio-active edible packaging films based on zein incorporated with orange-peel oil. Antioxidants 2019, 8, 391. [Google Scholar] [CrossRef]
- Fathi Achachlouei, B.; Zahedi, Y. Fabrication and characterization of CMC-based nanocomposites reinforced with sodium montmorillonite and TiO2 nanomaterials. Carbohydr. Polym. 2018, 199, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X.; He, M. Improving thermal, mechanical, and barrier properties of feather keratin/polyvinyl alcohol/tris(hydroxymethyl)aminomethane nanocomposite films by incorporating sodium montmorillonite and TiO2. Nanomaterials 2019, 9, 298. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and characterization of active films based on starch-PVA, containing silver nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martin-Belloso, O. Photo-protection and controlled release of folic acid using edible alginate/chitosan nanolaminates. J. Food Eng. 2018, 229, 72–82. [Google Scholar] [CrossRef]
- Chawla, V.; Ruoho, M.; Weber, M.; Abou Chaaya, A.; Taylor, A.A.; Charmette, C.; Miele, P.; Bechelany, M.; Michler, J.; Utke, I. Fracture mechanics and oxygen gas barrier properties of Al2O3/ZnO nanolaminates on PET deposited by atomic layer deposition. Nanomaterials 2019, 9, 88. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalhoc, S.; Quintas, M.A.C.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Mangiacapra, P.; Gorrasi, G.; Sorrentino, A.; Vittoria, V. Biodegradable nanocomposites obtained by ball milling of pectin and montmorillonites. Carbohydr. Polym. 2006, 64, 516–523. [Google Scholar] [CrossRef]
- Kusmono; Abdurrahim, I. Water sorption, antimicrobial activity, and thermal and mechanical properties of chitosan/clay/glycerol nanocomposite films. Heliyon 2019, 5, e02342. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Al-Saleh, M.A.; Al-Samhan, M.M.; Al-Enezi, S.T.; Al-Banna, A.H.; Abraham, G. Investigation of polypropylene-montmorillonite clay nanocomposite films containing a pro-degradant additive. J. Polym. Environ. 2017, 1, 275–290. [Google Scholar] [CrossRef]
- Cesur, S.; Koroglu, C.; Yalcin, H.T. Antimicrobial and biodegradable food packaging applications of polycaprolactone/organo nanoclay/chitosan polymeric composite films. J. Vinyl Addit. Technol. 2018, 24, 376–387. [Google Scholar] [CrossRef]
- Toro-Marquez, L.A.; Merino, D.; Gutierrez, T.J. Bionanocomposite films prepared from corn starch with and without nanopackaged Jamaica (hibiscus sabdariffa) flower extract. Food Bioprocess. Technol. 2018, 11, 1955–1973. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Oxygen scavenging films in food packaging. Environ. Chem. Lett. 2018, 16, 523–538. [Google Scholar] [CrossRef]
- Sadeghi, K.; Lee, Y.; Seo, J. Ethylene scavenging systems in packaging of fresh produce: A review. Food Rev. Int. 2019, 37, 155–176. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, S.; He, G.; He, Q.; Shi, B. Effect of Pe/Ag2o nano-packaging on the quality of apple slices. J. Food Qual. 2011, 34, 171–176. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture absorbers for food packaging applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation systems for antimicrobial food packaging components: An update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of nanomaterials in food packaging and its advanced future prospects. J. Inorg. Organomet. Polym. 2020, 30, 5180–5192. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Ragavan, V.; Weng, X.; Chand, R. Biosensors for sustainable food engineering: Challenges and perspectives. Biosensors 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Verma, A.K. Comprehensive review on application of edible film on meat and meat products: An eco-friendly approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Conte, A.; Alessandro, M.; Nobile, D. Chapter 31—Use of metal nanoparticles for active packaging applications. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 399–406. ISBN 978-0-12-800723-5. [Google Scholar]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications–A review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jimenez, A.; Garrigos, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active chicken meat packaging based on polylactide films and bimetallic Ag-Cu nanoparticles and essential oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.B. Antimicrobial activity of buckwheat starch films containing zinc oxide nanoparticles against listeria monocytogenes on mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Marcous, A.; Rasouli, S.; Ardestani, F. Low-density polyethylene films loaded by titanium dioxide and zinc oxide nanoparticles as a new active packaging system against Escherichia Coli O157:H7 in fresh calf minced meat. Packag. Technol. Sci. 2017, 30, 693–701. [Google Scholar] [CrossRef]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Lotfi, S.; Ahari, H.; Sahraeyan, R. The effect of silver nanocomposite packaging based on melt mixing and sol-gel methods on shelf life extension of fresh chicken stored at 4 °C. J. Food Saf. 2019, 39, e12625. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Jacob, H.; Luciano, G.; Bini, T.B.; Almusallam, A. Polylactide/poly(ε-caprolactone)/zinc oxide/clove essential oil composite antimicrobial films for scrambled egg packaging. Food Packag. Shelf Life 2019, 21, 100355. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Longo, M.; de Oliveira, J.L.; da Rosa, C.G.; de Lima Veeck, A.P.; de Aquino, R.S.; Masiero, A.V.; Bertoldi, F.C.; Manique Barreto, P.L.; Nunes, M.R. Nanocomposite poly (ethylene oxide) films functionalized with silver nanoparticles synthesized with acca sellowiana extracts. Colloid. Surf. A 2020, 602, 125125. [Google Scholar] [CrossRef]
- Gohargani, M.; Lashkari, H.; Shirazinejad, A. Study on biodegradable chitosan-whey protein-based film containing bionanocomposite TiO2 and Zataria multiflora essential oil. J. Food Qual. 2020, 2020, 8844167. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of reinforced ZnO Nanoparticle-incorporated gelatin bionanocomposite film with chitosan nanofiber for packaging of chicken fillet and cheese as food models. Food Bioprocess Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Bahrami, A.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Ghanbarzadeh, B.; Salehi, R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Paosen, S.; Vongkamjan, K.; Voravuthikunchai, S.P. Enhancement of food shelf life with polyvinyl alcohol-chitosan nanocomposite films from bioactive eucalyptus leaf extracts. Food Biosci. 2020, 36, 100609. [Google Scholar] [CrossRef]
- Rezaei, M.; Pirsa, S.; Chavoshizadeh, S. Photocatalytic/antimicrobial active film based on wheat gluten/ZnO nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2654–2665. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.-H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef] [PubMed]
- Valencia, L.; Nomena, E.M.; Mathew, A.P.; Velikov, K.P. Biobased cellulose nanofibril–oil composite films for active edible barriers. ACS Appl. Mater. Interfaces 2019, 11, 16040–16047. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. Available online: https://pubmed.ncbi.nlm.nih.gov/29155200/ (accessed on 27 October 2020). [CrossRef]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef]
- Perez Espitia, P.J.; Ferreira Soares, N.d.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Alves Medeiros, E.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Atares, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and Nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Development of chitosan functionalized magnetic nanoparticles with bioactive compounds. Nanomaterials 2020, 10, 1913. [Google Scholar] [CrossRef] [PubMed]
- Križnik, L.; Vasić, K.; Knez, Ž.; Leitgeb, M. Hyper-activation of ß-galactosidase from aspergillus Oryzae via immobilization onto amino-silane and chitosan magnetic maghemite nanoparticles. J. Clean. Prod. 2018, 179, 225–234. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Caliandro, R. Enzymes and advanced materials for active food packaging. In Proceedings of the 3rd International Conference on Food and Beverage Packaging, Rome, Italy, 16–18 July 2018. [Google Scholar]
- Jebali, A.; Hekmatimoghaddam, S.; Behzadi, A.; Rezapor, I.; Mohammadi, B.H.; Jasemizad, T.; Yasini, S.A.; Javadzadeh, M.; Amiri, A.; Soltani, M.; et al. Antimicrobial activity of nanocellulose conjugated with allicin and lysozyme. Cellulose 2013, 20, 2897–2907. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control 2012, 26, 387–392. [Google Scholar] [CrossRef]
- Wong, D.E.; Dai, M.; Talbert, J.N.; Nugen, S.R.; Goddard, J.M. Biocatalytic polymer nanofibers for stabilization and delivery of enzymes. J. Mol. Catal. B 2014, 110, 16–22. [Google Scholar] [CrossRef]
- Madhusudan, P.; Chellukuri, N.; Shivakumar, N. Smart packaging of food for the 21st century—A review with futuristic trends, their feasibility and economics. Mater. Today Proc. 2018, 5, 21018–21022. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nanomaterial-based optical indicators: Promise, opportunities, and challenges in the development of colorimetric systems for intelligent packaging. Nano Res. 2019, 12, 489–500. [Google Scholar] [CrossRef]
- Fuertes, G.; Soto, I.; Vargas, M.; Valencia, A.; Sabattin, J.; Carrasco, R. Nanosensors for a monitoring system in intelligent and active packaging. J. Sens. 2015, 2016, e7980476. [Google Scholar] [CrossRef]
- Caon, T.; Martelli, S.M.; Fakhouri, F.M. New Trends in the Food Industry: Application of Nanosensors in Food Packaging; Grumezescu, A.M., Ed.; Academic Press: London, UK, 2017; Volume 8, pp. 773–804. ISBN 978-0-12-804372-1. [Google Scholar]
- Li, Z.; Sheng, C. Nanosensors for food safety. J. Nanosci. Nanotechnol. 2014, 14, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An untapped resource for food packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.K.D.; Solanki, A.; Debnath, A.; Nayyar, A.; El-Sappagh, S.; Kwak, K. Advancing modern healthcare with nanotechnology, nanobiosensors, and internet of nano things: Taxonomies, applications, architecture, and challenges. IEEE Access 2020, 8, 65230–65266. [Google Scholar] [CrossRef]
- Eggins, B.R. Chemical sensors and biosensors. In Analytical Techniques in the Sciences, 2nd ed.; Wiley and Sons Ltd: Hoboken, NJ, USA, 2002; ISBN 978-0-471-89914-3. [Google Scholar]
- Mak, A.C.; Osterfeld, S.J.; Yu, H.; Wang, S.X.; Davis, R.W.; Jejelowo, O.A.; Pourmand, N. Sensitive giant magnetoresistive-based immunoassay for multiplex mycotoxin detection. Biosens. Bioelectron. 2010, 25, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Actis, P.; Jejelowo, O.; Pourmand, N. UltraSensitive mycotoxin detection by STING sensors. Biosens. Bioelectron. 2010, 26, 333–337. [Google Scholar] [CrossRef]
- Chiao, D.-J.; Shyu, R.-H.; Hu, C.-S.; Chiang, H.-Y.; Tang, S.-S. Colloidal gold-based immunochromatographic assay for detection of botulinum neurotoxin type B. J. Chromatogr. B 2004, 809, 37–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, F.-G.; Li, Y.-S.; Zhang, Y.-Y.; Zhang, J.-H.; Lu, S.-Y.; Ren, H.-L.; Liu, Z.-S. Colloidal gold probe-based immunochromatographic assay for the rapid detection of brevetoxins in fishery product samples. Biosens. Bioelectron. 2009, 24, 2744–2747. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Xu, D.; Shim, B.S.; Zhu, Y.; Sun, F.; Liu, L.; Peng, C.; Jin, Z.; Xu, C.; et al. Simple, rapid, sensitive, and versatile SWNT-paper sensor for environmental toxin detection competitive with ELISA. Nano Lett. 2009, 9, 4147–4152. [Google Scholar] [CrossRef]
- Zamolo, V.A.; Valenti, G.; Venturelli, E.; Chaloin, O.; Marcaccio, M.; Boscolo, S.; Castagnola, V.; Sosa, S.; Berti, F.; Fontanive, G.; et al. Highly sensitive electrochemiluminescent nanobiosensor for the detection of palytoxin. ACS Nano 2012, 6, 7989–7997. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Matharu, Z.; Sumana, G.; Solanki, P.R.; Kim, C.G.; Malhotra, B.D. Antibody immobilized cysteamine functionalized-gold nanoparticles for aflatoxin detection. Thin Solid Film. 2010, 519, 1213–1218. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.; Wu, Y.; Weng, B.; Liu, Y.; Lu, Z.; Li, C.M.; Yu, C. Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosens. Bioelectron. 2016, 78, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Villamizar, R.A.; Maroto, A.; Rius, F.X.; Inza, I.; Figueras, M.J. Fast detection of salmonella infants with carbon nanotube field effect transistors. Biosens. Bioelectron. 2008, 24, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.E.; Pillai, S.; Ram, M.K.; Kumar, A.; Singh, S.R. Electrochemical impedance-based DNA sensor using a modified single walled carbon nanotube electrode. Mater. Sci. Eng. C 2011, 31, 821–825. [Google Scholar] [CrossRef]
- Hasan, M.R.; Pulingam, T.; Appaturi, J.N.; Zifruddin, A.N.; Teh, S.J.; Lim, T.W.; Ibrahim, F.; Leo, B.F.; Thong, K.L. Carbon nanotube-based aptasensor for sensitive electrochemical detection of whole-cell salmonella. Anal. Biochem. 2018, 554, 34–43. [Google Scholar] [CrossRef]
- Yamada, K.; Kim, C.-T.; Kim, J.-H.; Chung, J.-H.; Lee, H.G.; Jun, S. Single walled carbon nanotube-based junction biosensor for detection of Escherichia Coli. PLoS ONE 2014, 9, e105767. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Bangar, M.A.; Baldrich, E.; Muñoz, F.J.; Mulchandani, A. Conducting polymer nanowire-based chemiresistive biosensor for the detection of bacterial spores. Biosens. Bioelectron. 2010, 25, 2309–2312. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, S.-H.; Chuang, Y.-C.; Lu, Y.-C.; Shen, T.Y.; Chang, C.A.; Lin, C.-S. Disposable amperometric immunosensing strips fabricated by Au nanoparticles-modified screen-printed carbon electrodes for the detection of foodborne pathogen Escherichia Coli O157:H7. Biosens. Bioelectron. 2008, 23, 1832–1837. [Google Scholar] [CrossRef]
- Davis, D.; Guo, X.; Musavi, L.; Lin, C.-S.; Chen, S.-H.; Wu, V.C.H. Gold nanoparticle-modified carbon electrode biosensor for the detection of listeria monocytogenes. Ind. Biotechnol. 2013, 9, 31–36. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of salmonella typhimurium and staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Y.; Zhang, T.; Jiang, Y. Rapid and sensitive detection of salmonella typhimurium using aptamer-conjugated carbon dots as fluorescence probe. Anal. Methods 2015, 7, 1701–1706. [Google Scholar] [CrossRef]
- Wang, D.-B.; Tian, B.; Zhang, Z.-P.; Wang, X.-Y.; Fleming, J.; Bi, L.-J.; Yang, R.-F.; Zhang, X.-E. Detection of bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “road closure”. Biosens. Bioelectron. 2015, 67, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Suaifan, G.A.R.Y.; Alhogail, S.; Zourob, M. Paper-based magnetic nanoparticle-peptide probe for rapid and quantitative colorimetric detection of Escherichia Coli O157:H7. Biosens. Bioelectron. 2017, 92, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yu, Z.; Liu, D.; Xu, C.; Lai, W. Developing a novel immunochromatographic test strip with gold magnetic bifunctional nanobeads (GMBN) for efficient detection of salmonella choleraesuis in milk. Food Control 2016, 59, 507–512. [Google Scholar] [CrossRef]
- Pandey, A.; Gurbuz, Y.; Ozguz, V.; Niazi, J.H.; Qureshi, A. Graphene-interfaced electrical biosensor for label-free and sensitive detection of foodborne pathogenic, E. Coli O157:H7. Biosens. Bioelectron. 2017, 91, 225–231. [Google Scholar] [CrossRef]
- Kara, M.; Uzun, L.; Kolayli, S.; Denizli, A. Combining molecular imprinted nanoparticles with surface plasmon resonance nanosensor for chloramphenicol detection in honey. J. Appl. Polym. Sci. 2013, 129, 2273–2279. [Google Scholar] [CrossRef]
- Wu, M.; Tang, W.; Guimarães, J.; Wang, Q.; He, P.; Fang, Y. Electrochemical detection of Sudan I using a multi-walled carbon nanotube/chitosan composite modified glassy carbon electrode. Am. J. Anal. Chem. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Vamvakaki, V.; Chaniotakis, N.A. Pesticide detection with a liposome-based nano-biosensor. Biosens. Bioelectron. 2007, 22, 2848–2853. [Google Scholar] [CrossRef]
- Devaramani, S.; Malingappa, P. Synthesis and characterization of cobalt nitroprusside nano particles: Application to sulfite sensing in food and water samples. Electrochim. Acta 2012, 85, 579–587. [Google Scholar] [CrossRef]
- Ping, H.; Zhang, M.; Li, H.; Li, S.; Chen, Q.; Sun, C.; Zhang, T. Visual detection of melamine in raw milk by label-free silver nanoparticles. Food Control 2012, 23, 191–197. [Google Scholar] [CrossRef]
- Su, H.; Fan, H.; Ai, S.; Wu, N.; Fan, H.; Bian, P.; Liu, J. Selective determination of melamine in milk samples using 3-mercapto-1-propanesulfonate-modified gold nanoparticles as colorimetric probe. Talanta 2011, 85, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Khalilzadeh, M.A.; Karimi-Maleh, H. A new strategy for determination of bisphenol A in the presence of Sudan I using a ZnO/CNTs/Ionic liquid paste electrode in food samples. Food Chem. 2014, 158, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Majidi, M.R.; Fadakar Bajeh Baj, R.; Naseri, A. Carbon nanotube-ionic liquid (CNT–IL) nanocamposite modified sol-gel derived carbon-ceramic electrode for simultaneous determination of sunset yellow and tartrazine in food samples. Food Anal. Methods 2013, 6, 1388–1397. [Google Scholar] [CrossRef]
- Jamali, T.; Karimi-Maleh, H.; Khalilzadeh, M.A. A novel nanosensor based on Pt:Co nanoalloy ionic liquid carbon paste electrode for voltammetric determination of vitamin B9 in food samples. Food Sci. Technol. 2014, 57, 679–685. [Google Scholar] [CrossRef]
- Wu, R.-J.; Lin, D.-J.; Yu, M.-R.; Chen, M.H.; Lai, H.-F. Ag@SnO2 Core—Shell material for use in fast-response ethanol sensor at room operating temperature. Sens. Actuat. B 2013, 178, 185–191. [Google Scholar] [CrossRef]
- Scandurra, G.; Arena, A.; Ciofi, C.; Saitta, G. Electrical characterization and hydrogen peroxide sensing properties of Gold/Nafion:Polypyrrole/MWCNTs electrochemical devices. Sensors 2013, 13, 3878–3888. [Google Scholar] [CrossRef]
- Liang, K.-Z.; Mu, W.-J. ZrO2/DNA-derivated polyion hybrid complex membrane for the determination of hydrogen peroxide in milk. Ionics 2008, 14, 533–539. [Google Scholar] [CrossRef]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-based smart materials for food packaging and sensors—A review. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Pathak, V.M. Navneet review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Avérous, L. Chapter 21—Polylactic acid: Synthesis, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 433–450. ISBN 978-0-08-045316-3. [Google Scholar]
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Van den Oever, M.; Molenveld, K.; van der Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics: Facts and Figures: Focus on Food Packaging in the Netherlands; Wageningen University and Research: Wageningen, The Netherlands, 2017; ISBN 978-94-6343-121-7. [Google Scholar]
- Flores, S.; Famá, L.; Rojas, A.M.; Goyanes, S.; Gerschenson, L. Physical properties of tapioca-starch edible films: Influence of filmmaking and potassium sorbate. Food Res. Int. 2007, 40, 257–265. [Google Scholar] [CrossRef]
- Sadeghizadeh-Yazdi, J.; Habibi, M.; Kamali, A.A.; Banaei, M. Application of edible and biodegradable starch-based films in food packaging: A systematic review and meta-analysis. Curr. Res. Nutr. Food Sci. 2019, 7, 624–637. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Ashori, A.; Bahrami, R. Modification of physico-mechanical properties of chitosan-tapioca starch blend films using nano graphene. Polym. Plast. Technol. Eng. 2014, 53, 312–318. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Indu, C.; Radhakrishnan, E.K. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent PH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef]
- Yoon, S.-D.; Park, M.-H.; Byun, H.-S. Mechanical and water barrier properties of starch/PVA composite films by adding nano-sized poly(methyl methacrylate-co-acrylamide) particles. Carbohydr. Polym. 2012, 1, 676–686. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Xiao, L.; Lin, D.; Yang, Y.; Wang, H.; Yang, Y.; Wu, D.; Chen, H.; Zhang, Q.; et al. Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int. J. Biol. Macromol. 2019, 123, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, S.S.; Lim, S.-T. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Chang, P.R.; Huneault, M.A. Comparative study on the films of poly(vinyl alcohol)/pea starch nanocrystals and poly(vinyl alcohol)/native pea starch. Carbohydr. Polym. 2008, 73, 8–17. [Google Scholar] [CrossRef]
- Dai, L.; Qiu, C.; Xiong, L.; Sun, Q. Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem. 2015, 174, 82–88. [Google Scholar] [CrossRef]
- Xu, C.; Chen, C.; Wu, D. The starch nanocrystal filled biodegradable poly(ε-caprolactone) composite membrane with highly improved properties. Carbohydr. Polym. 2018, 182, 115–122. [Google Scholar] [CrossRef]
- Tian, H.; Xu, G. Processing and characterization of glycerol-plasticized soy protein plastics reinforced with citric acid-modified starch nanoparticles. J. Polym. Environ. 2011, 19, 582–588. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Thielemans, W.; Magnin, A.; Boufi, S. Starch nanocrystals and starch nanoparticles from waxy maize as nanoreinforcement: A comparative study. Carbohydr. Polym. 2016, 143, 310–317. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Islam Sarker, M.Z.; Fazita, M.R.N.; Syakir, M.I.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Behrooz, R.; Rezaei, M.; Miraki, R. Reducing water sensitivity of alginate bio-nanocomposite film using cellulose nanoparticles. Int. J. Biol. Macromol. 2013, 54, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Boumail, A.; Salmieri, S.; Klimas, E.; Tawema, P.O.; Bouchard, J.; Lacroix, M. Characterization of trilayer antimicrobial diffusion films (ADFs) based on methylcellulose-polycaprolactone composites. J. Agric. Food Chem. 2013, 61, 811–821. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Qasim, U.; Osman, A.I.; Al-Muhtaseb, A.H.; Farrell, C.; Al-Abri, M.; Ali, M.; Vo, D.-V.N.; Jamil, F.; Rooney, D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Sa, N.M.S.M.; Mattos, A.L.A.; Silva, L.M.A.; Brito, E.S.; Rosa, M.F.; Azeredo, H.M.C. From cashew byproducts to biodegradable active materials: Bacterial cellulose-lignin-cellulose nanocrystal nanocomposite films. Int. J. Biol. Macromol. 2020, 161, 1337–1345. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Wang, Y.; Yu, H.-Y.; Ouyang, Z.; Asad, R.A.M.; Mu, M.; Lu, Y.; Yao, J.; Zhang, L. Supermagnetic cellulose nanocrystal hybrids reinforced PHBV nanocomposites with high sensitivity to intelligently detect water vapor. Ind. Crop. Prod. 2020, 154, 112704. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Wang, K.; Xu, H. Development and evaluation of soy protein isolate-based antibacterial nanocomposite films containing cellulose nanocrystals and zinc oxide nanoparticles. Food Hydrocoll. 2020, 106, 105898. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ahmadi, P.; Ehsani, A. Development of an active packaging system containing zinc oxide nanoparticles for the extension of chicken fillet shelf life. Food Sci. Nutr. 2020, 8, 5461–5473. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Sun, L.; Kong, F.; Lin, M.; Mustapha, A. Preparation of cellulose nanofibril/titanium dioxide nanoparticle nanocomposites as fillers for PVA-based packaging and investigation into their intestinal toxicity. Int. J. Biol. Macromol. 2020, 156, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M. Applications of chitosan as food packaging materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Radhakrishnan, Y.; Gopal, G.; Lakshmanan, C.C.; Nandakumar, K.S. Chitosan nanoparticles for generating novel systems for better applications: A review. Mol. Genet. Med. 2015, 9, 1–10. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-based (nano)materials for novel biomedical applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. Funct. Chitosan 2020, 457–489. [Google Scholar] [CrossRef]
- Zubair, M.; Arshad, M.; Pradhan, R.A.; Ullah, A. Chapter 20—Chitosan/chitin-based composites for food packaging applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 641–670. ISBN 978-0-12-817966-6. [Google Scholar]
- Kadam, D.; Momin, B.; Palamthodi, S.; Lele, S.S. Physicochemical and functional properties of chitosan-based nano-composite films incorporated with biogenic silver nanoparticles. Carbohydr. Polym. 2019, 211, 124–132. [Google Scholar] [CrossRef]
- Lin, D.; Yang, Y.; Wang, J.; Yan, W.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Szabo, P.; Daugaard, A.E.; Baschetti, M.G. Effect of crystallinity on water vapor sorption, diffusion, and permeation of PLA-based nanocomposites. ACS Omega 2020, 5, 15362–15369. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef] [PubMed]
- Potrc, S.; Krasevac Glaser, T.; Vesel, A.; Poklar Ulrih, N.; Fras Zemljic, L. Two-layer functional coatings of chitosan particles with embedded catechin and pomegranate extracts for potential active packaging. Polymers 2020, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Upadhyay, S.N.; Niranjan, K.; Mishra, P.K. Antimicrobial biodegradable chitosan-based composite nano-layers for food packaging. Int. J. Biol. Macromol. 2020, 157, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Mechanism of action of electrospun chitosan-based nanofibers against meat spoilage and pathogenic bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Wootthikanokkhan, J.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Mechanical and antibacterial properties of the chitosan coated cellulose paper for packaging applications: Effects of molecular weight types and concentrations of chitosan. Int. J. Biol. Macromol. 2020, 155, 1510–1519. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. 2020, 309, 125520. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer films based on chitosan/potato protein/linseed Oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. 2020, 332, 127375. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef]
- Su, H.; Huang, C.; Liu, Y.; Kong, S.; Wang, J.; Huang, H.; Zhang, B. Preparation and characterization of cinnamomum essential oil-chitosan nanocomposites: Physical, structural, and antioxidant activities. Processes 2020, 8, 834. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Silva Pereira, B.C.; Lopes, G.K.; Andrade, C.T. Encapsulation and antioxidant activity of assai pulp oil (euterpe oleracea) in chitosan/alginate polyelectrolyte complexes. Food Hydrocoll. 2020, 109, 106097. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Al-Nabulsi, A.; Osaili, T.; Sawalha, A.; Olaimat, A.N.; Albiss, B.A.; Mehyar, G.; Ayyash, M.; Holley, R. Antimicrobial activity of chitosan coating containing ZnO nanoparticles against E. Coli 0157:H7 on the surface of white brined cheese. Int. J. Food Microbiol. 2020, 334, 108838. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.-M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef]
- Xia, Y.; Rubino, M.; Auras, R. Release of nanoclay and surfactant from polymer—Clay nanocomposites into a food simulant. Environ. Sci. Technol. 2014, 48, 13617–13624. [Google Scholar] [CrossRef]
- Han, W.; Yu, Y.; Li, N.; Wang, L. Application and safety assessment for nano-composite materials in food packaging. Chin. Sci. Bull. 2011, 56, 1216–1225. [Google Scholar] [CrossRef]
- Oberdörster, G.; Stone, V.; Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 2007, 1, 2–25. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Brandelli, A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Sci. Hum. Wellness 2020, 9, 8–20. [Google Scholar] [CrossRef]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in medicine: A focus on vascular oxidative stress. Oxid. Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Echegoyen, Y.; Nerín, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Vass, I.Z.; Deák, Z.; Paul, K.; Kovács, S.; Vass, I. Interaction of nanoparticles with biological systems. Acta Biol. Szeged. 2015, 59, 225–245. [Google Scholar]
- Kumar, V.; Sharma, M.; Khare, T.; Wani, S.H. Chapter 17—Impact of nanoparticles on oxidative stress and responsive antioxidative defense in plants. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 393–406. ISBN 978-0-12-811487-2. [Google Scholar]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of oligomers from a food contact biopolymer based on polylactic acid (PLA) and polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in drug delivery and antimicrobially active materials. Polymers 2020, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets. Nanotechnology for Food Packaging Market—A Global Market and Regional Analysis: Focus on (Product, Application, Industry Outlook, Region and Country Analysis)—Analysis and Forecast, 2019–2025. Available online: https://www.researchandmarkets.com/reports/5181729/nanotechnology-for-food-packaging-market-a (accessed on 13 November 2020).
| 1 | High exhibited activity against bacteria and fungi was obtained. Ag release into the non-polar food simulants was lower than into polar simulants. |






| Type | Representative | E Number | ADI Value |
|---|---|---|---|
| polyols | glycerol | E422 | not specified |
| sorbitol | E420 | not specified | |
| polyethylene glycerol | E1521 | 0–10 mg/kg body weight | |
| sugars | glucose | - | - |
| sucrose | - | - | |
| lipids | monoglycerides | E471 | not specified |
| phospholipids | (lecithin E322) | not specified | |
| natural source | triglycerides from vegetable oil | (sorbitan tris tearate E492) | 0-25 mg/kg body weight |
| fatty acid esters | - | - |
| Nano-Technology Method | Composition of Nanomaterial | Function/Properties | Reference |
|---|---|---|---|
| NANO- COATING FILMS | waxy maize starch nanocrystals | Reinforcing agent in a thermoplastic waxy maize starch matrix plasticized with glycerol. | [44] |
| starch nanoparticles | Corn starch-based edible films | [45] | |
| rice starch nanocrystals | Rice starch edible films | [46] | |
| corn starch/orange-peel oil/zein nanocapsules | Edible films | [47] | |
| carboxymethyl cellulose/sodium montmorillonite clay/titanium dioxide (TiO2) | The addition of NPs decremented water vapor permeability, while moisture content, density, and glass transition temperature were incremented slightly. | [48] | |
| whey protein isolate/cellulose nanofibers/TiO2/rosemary essential oil | Improved physico-mechanical, antibacterial and antioxidant properties. | [49] | |
| potato starch/sodium montmorillonite clay/TiO2 | Water vapor permeability and UVA, UVB and UVC lights transmittance decrease upon TiO2 and sodium montmorillonite content increase. | [50] | |
| keratin/polyvinyl alcohol/tris(hydroxymethyl) aminomethane /sodium montmorillonite clay/TiO2 | Water vapor permeability, oxygen permeability, and light transmittance decrease with increase in TiO2 and montmorillonite contents. | [51] | |
| starch/ polyvinyl alcohol/Ag nanoparticles | High exhibited activity against bacteria and fungi was obtained. Ag release into the non-polar food simulants was lower than into polar simulants. | [52] | |
| NANO- LAMINATES | alginate/chitosan/folic acid | Improved stability under ultraviolet light exposure after folic acid encapsulation. | [53] |
| polyethylene terephthalate/aluminum oxide (Al2O3)/Zinc oxide (ZnO) | Good barrier properties | [54] | |
| chitosan/alginate/polyethylene terephthalate | Increase in melting energy of 39.2% in comparison to the PET film used as support, and a decrease in the decomposition temperature. | [55] | |
| NANO- CLAYS | clay montmorillonite/pectines | Diffusion of water vapor and oxygen was reduced. | [56] |
| chitosan-clay nanocomposites | Addition of clay significantly increased the strength and stiffness of neat chitosan nanocomposite. | [57] | |
| polypropylene/montmorillonite/ pro-degradant additive (TDPA®) | Permeability of oxygen decreased with increasing montmorillonite nano clay content. | [58] | |
| polycaprolactone/organo nanoclay/chitosan | Antimicrobial effect on E. coli, Pseudomonas aeruginosa, and Candida albicans. | [59] | |
| corn starch/natural montmorillonite/ anthocyanin | Active and pH-sensitive bionanocomposites with improved mechanical and thermal properties. | [60] |
| Type | Function | Agents | Reference |
|---|---|---|---|
| oxygen scavengers | prevention of fat oxidation | metallic (iron powder, activated iron, Zn …), organic (ascorbic acid, tocopherol, catechol …), inorganic (sulfite, thiosulfate, ZnO …), polymer-based (polymer metallic complex …), enzyme-based (glucose oxidase, laccase …) | [62] |
| ethylene scavengers | fruit and vegetables ripening reduction | SiO2, KMnO4, TiO2, Ag, PdCl2, Pd-impregnated zeolite, polyvinyl chloride film containing ZnO nanoparticles … | [63,64] |
| moisture absorbers | microbial growth reduction | inorganic (silica gel, natural clay (montomorillonite, zeolite), chlorides (Ca, Mg, Al, Na, K), oxides (Ca, Ba), bentonite …), organic (sorbitol, xylitol, fructose, cellulose and their derivatives), polymer-based (starch copolymers, polyvinyl alcohol, absorbent resin) | [65] |
| carbon dioxide emitters | inhibition of spoilage by microbial action | sodium bicarbonate/ascorbate and citric acid | [66,67] |
| Application of Bionanosensors | Nanomaterial (Transducer Element) | Bioreceptor | Analyte | Reference |
|---|---|---|---|---|
| toxins detection | tri-layer oxide (SiO2 10 nm/Si3O4 10 nm/SiO2 10 nm) | monoclonal antibodies for aflatoxin-B1, zearalenone and HT-2 | mycotoxins (aflatoxin-B1 and zearalenone) | [117] |
| nanopipettes from quartz capillaries | poly l-lysine, polyclonal antibody HPV16 E6 ad monoclonal antibody for HT-2 | HT-2 | [118] | |
| colloid gold nanoparticles | polyclonal antibody for botulinum neurotoxin type B and polyclonal antibody IgG | botulinum neurotoxin type B | [119] | |
| colloid gold nanoparticles | antibody PbTx Mab and polyclonal antibody IgG | brevetoxins (PbTx-1, PbTx-2, PbTx-3, PbTx-9) | [120] | |
| carbon nanotubes | antibody of microcystin-LR | microcystin-LR | [121] | |
| carbon nanotubes | bovine serum albumin, polyclonal anti-palytoxin antibodies | palytoxin | [122] | |
| gold nanoparticles | cysteamine, monoclonal antibody of aflatoxin B1 | aflatoxin B1 | [123] | |
| carbon dots | aflatoxin B1 aptamer HS-AAA AAA GTT GGG CAC GTG TTG TCT CTC TGT GTC TCG TGC CCT TCG CTA GGC CCA CA | aflatoxin B1 | [124] | |
| Poly (amidoamine) dendrimers | cysteamine, and aflatoxin B1 aptamer NH2-5′-GTT GGG CAC GTG TTG TCT CTC TGT GTC TCG TGC CCT TCG CTA GGC CCA CA-3′ | aflatoxin B1 | [125] | |
| microbes detection | single-walled carbon nanotubes | polyclonal antibody for S. enterica | S. enterica subsp. enterica serotype Infantis | [126] |
| single-walled carbon nanotubes | ssDNA probes and complementary DNA | S. enterica serovar Typhimurium | [127] | |
| multi-walled carbon nanotubes | Salmonella aptamer sequence 5′-T ATG GCG GCG TCA CCC GAC GGG GAC TTG ACA TTA TGA CAG 3′ | S. enterica | [128] | |
| single-walled carbon nanotubes | biotinylated E. coli antibodies | E. coli K-12 | [129] | |
| polypyrrol nanowires | monoclonal antibodies specific toward Bacillus globigii spores | B. globigii | [130] | |
| Au nanoparticles | E. coli O157:H7-specific antibody, E. coli O157:H7 intact cells and E. coli O157:H7-specific antibody conjugated with horseradish peroxidase (HRP) | E. coli O157:H7 | [131] | |
| Au nanoparticles | L. monocytogenes specific antibody | L. monocytogenes | [132] | |
| Fe3O4 magnetic gold nanoparticles | S. typhimurium aptamer sequence 5′-SH-TAT GGC GGC GTC ACC CGA CGG GGA CTT GAC ATT ATG ACA G-3′ and S. aureus aptamer sequence 5′-SH-GCA ATG GTA CGG TAC TTC CTC GGC ACG TTC TCA GTA GCG CTC GCT GGT CAT CCC ACA GCT ACG TCA AAA GTG CAC GCT ACT TTG CTA A-3′. | S. typhimurium and S. aureus | [133] | |
| carbon dots | amino-modified aptamers of S. typhimurium | S. typhimurium | [134] | |
| super-paramagnetic iron oxide particles | monoclonal antibody 12F6 against Bacillus anthracis | B. anthracis spores | [135] | |
| magnetic nanoparticles | E. coli O157:H7 protease | E. coli O157:H7 | [136] | |
| gold magnetic (Fe3O4) bifunctional nanobeads | anti-Salmonella choleraesuis monoclonal antibodies (11D8-D4 as the detection antibody, 5F11–B11 as the capture antibody) | S. choleraesuis | [137] | |
| graphene nanoplatelets | E. coli O157:H7-specific antibody | E. coli O157:H7 | [138] |
| Mechanical Property | Number of Cellulose Nanoparticles (% (w/w)) | Function |
|---|---|---|
| tensile property | 5 | increase by 42% |
| water vapor permeability | 5 | decrease by 28% |
| oxygen transmission | 1 | decrease by 21% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. https://doi.org/10.3390/nano11020292
Primožič M, Knez Ž, Leitgeb M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials. 2021; 11(2):292. https://doi.org/10.3390/nano11020292
Chicago/Turabian StylePrimožič, Mateja, Željko Knez, and Maja Leitgeb. 2021. "(Bio)Nanotechnology in Food Science—Food Packaging" Nanomaterials 11, no. 2: 292. https://doi.org/10.3390/nano11020292
APA StylePrimožič, M., Knez, Ž., & Leitgeb, M. (2021). (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials, 11(2), 292. https://doi.org/10.3390/nano11020292









