Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Tuning the Size of SiNPs: Silane Concentration and Residence Time
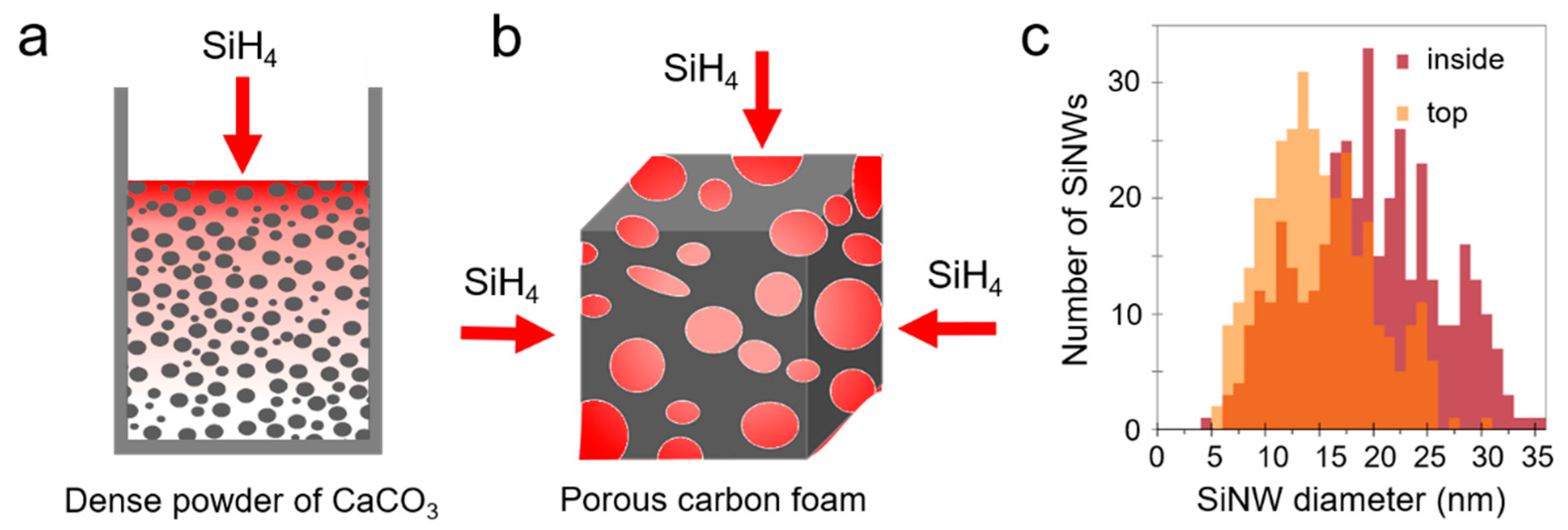
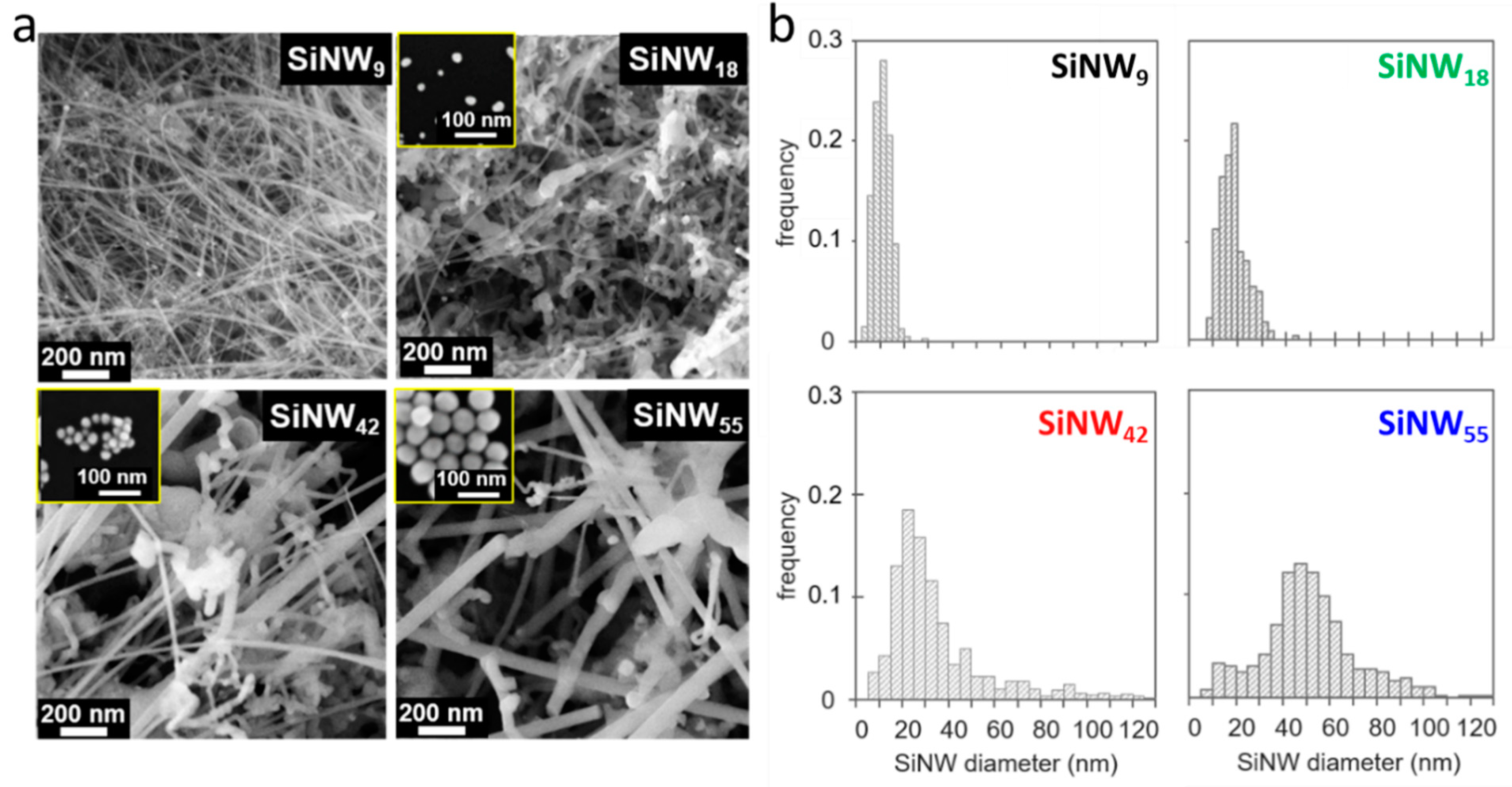
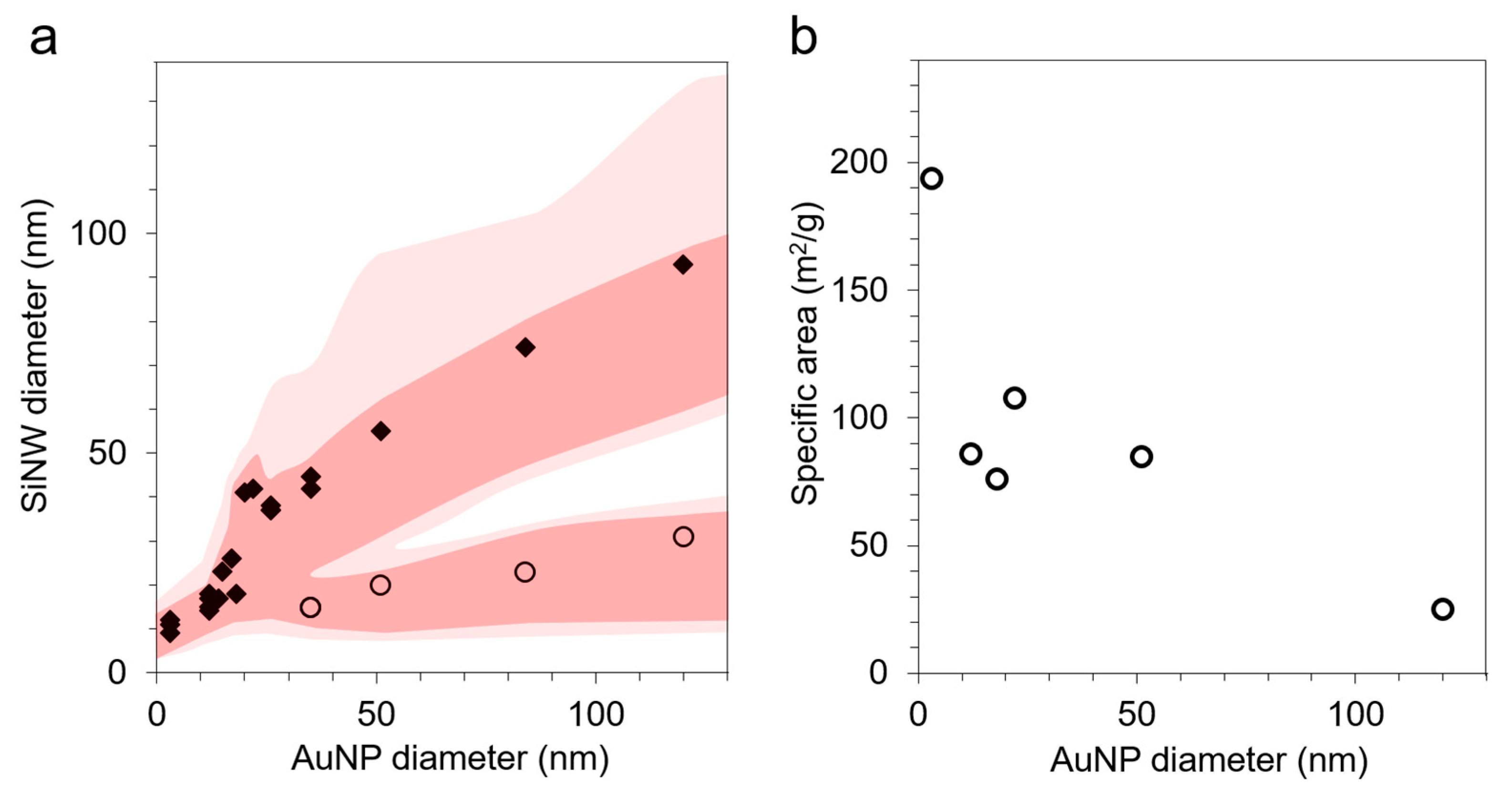
3.2. Tuning the SiNW Diameter: Catalyst Size and Silane Partial Pressure
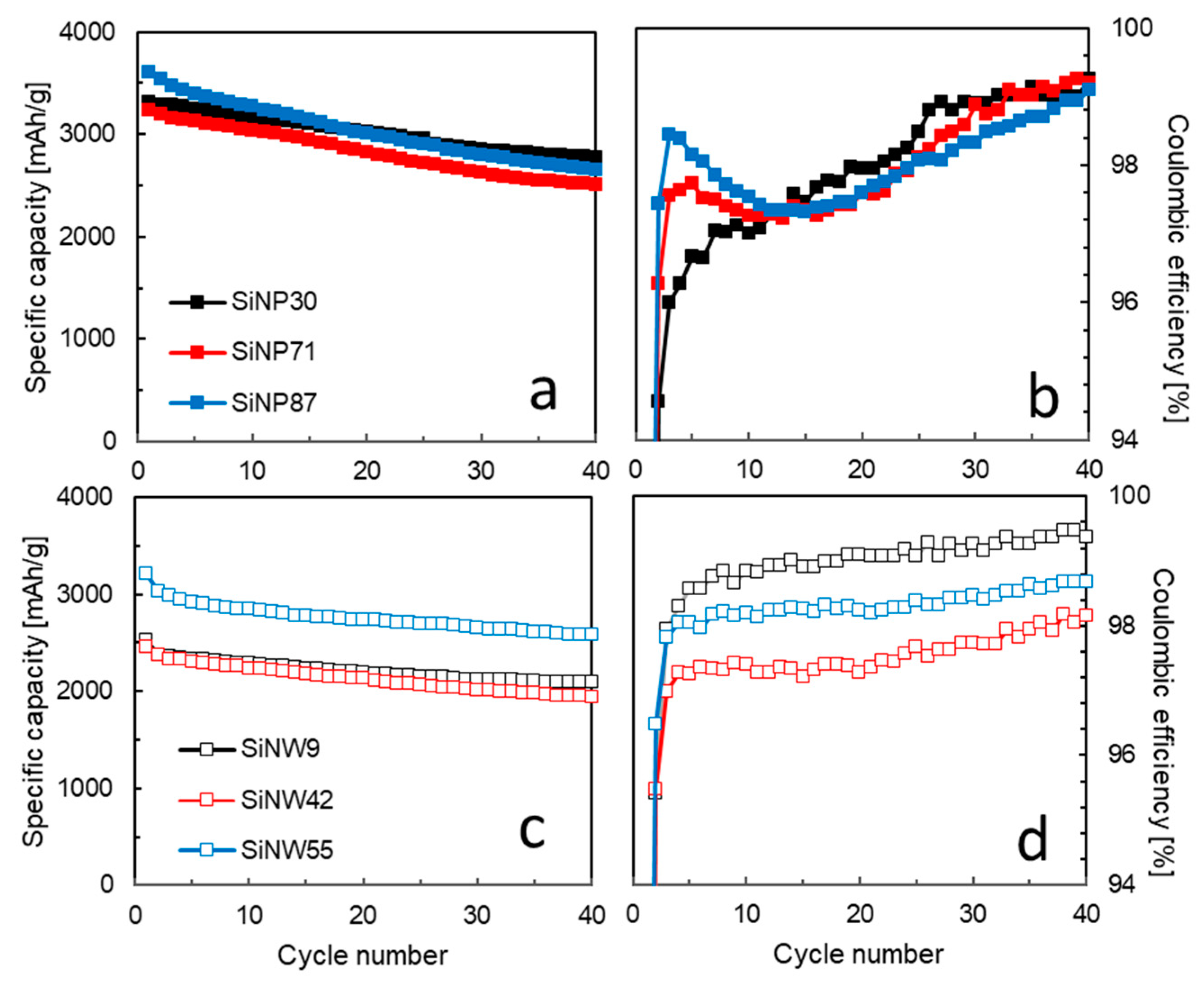
3.3. Electrochemical Performance in Li-Ion Batteries
4. Discussion
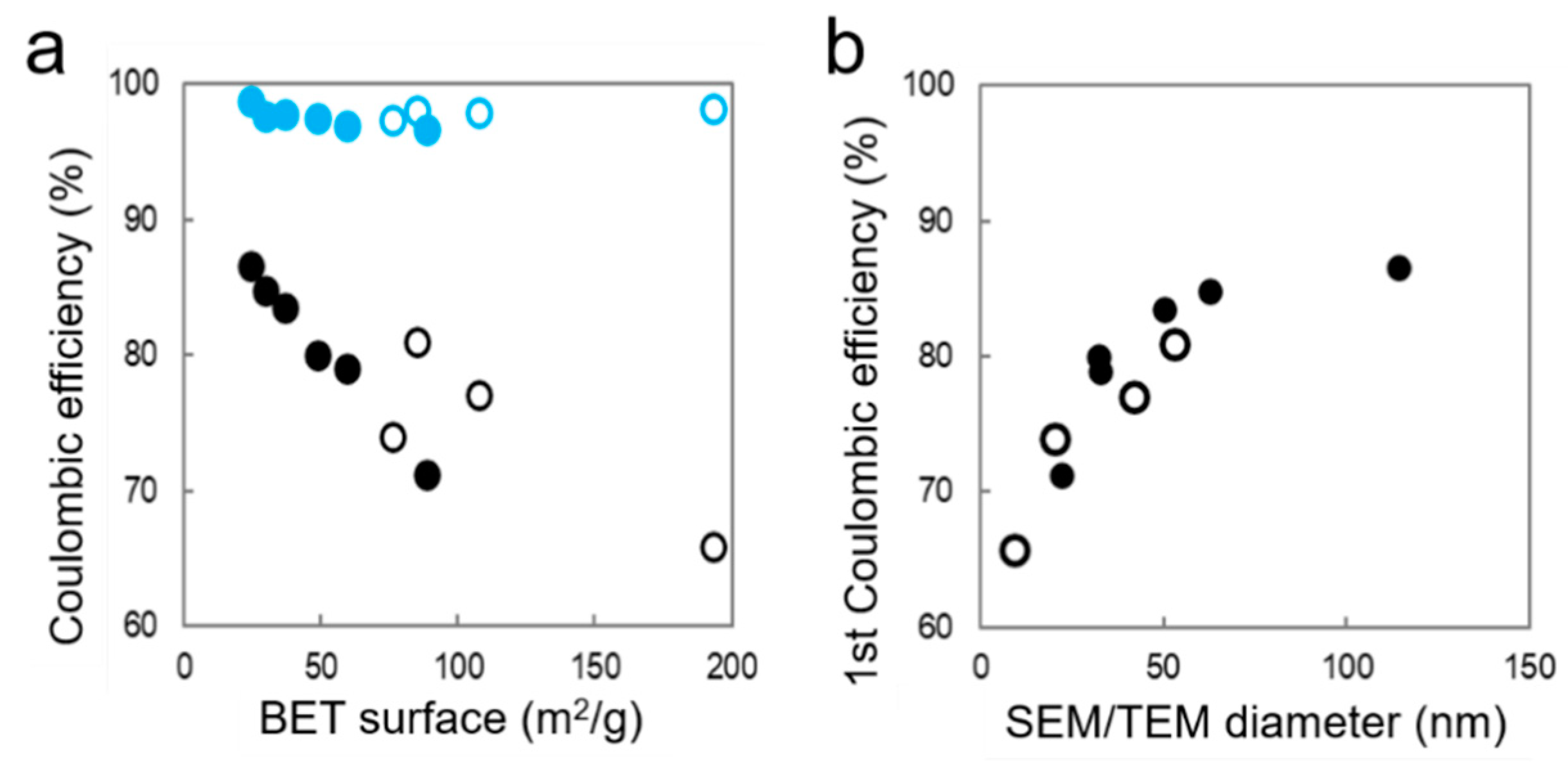
- First, the irreversible capacity loss in the first cycle increases linearly with the specific area, in a similar trend for SiNPs and SiNWs (Figure 6). This clear correlation indicates that a similar homogeneous SEI layer passivates the silicon surface, independently of Si size and shape.
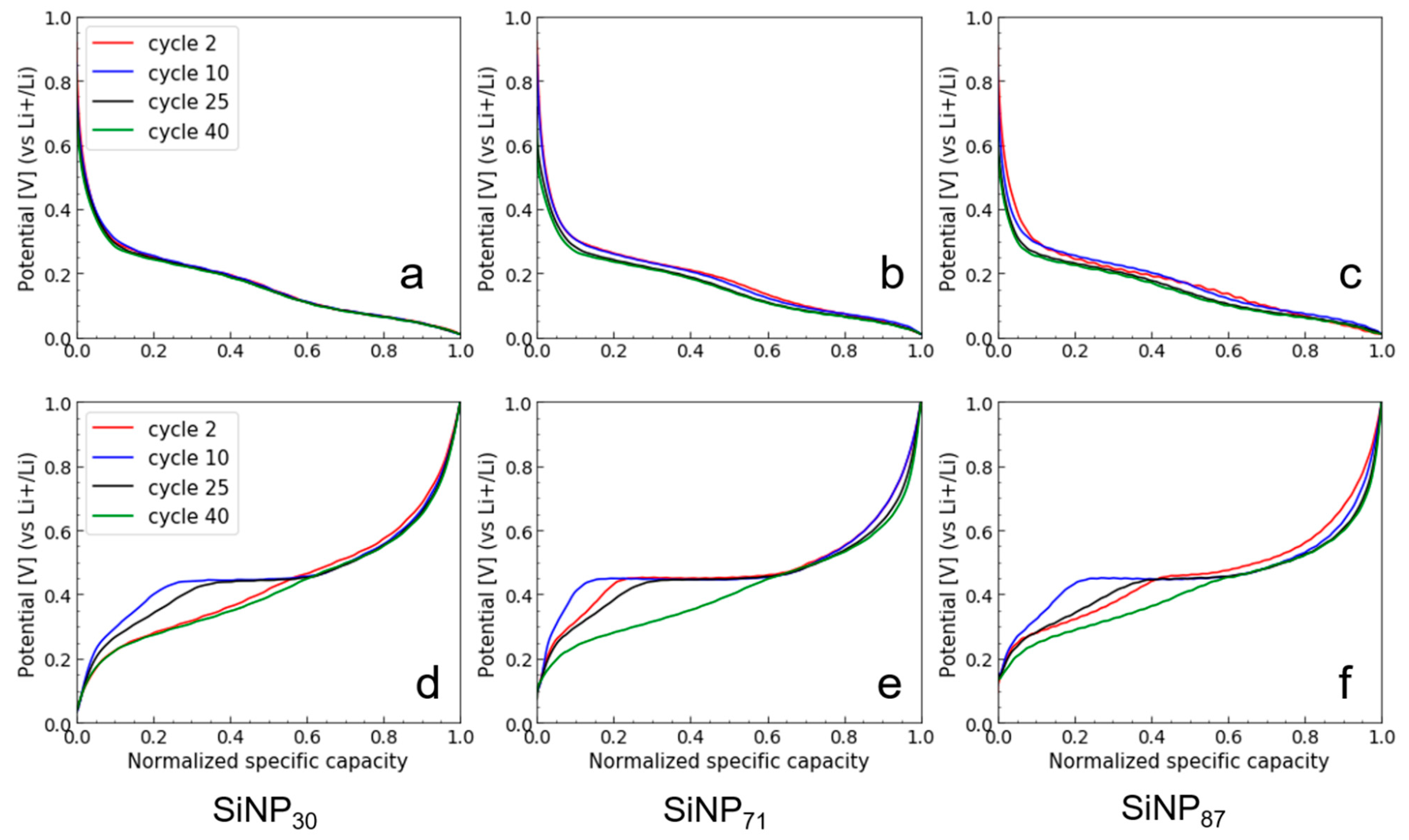
- For the SiNPs, the gas-flow process of pyrolysis produces a fine powder, which is easily incorporated in a homogeneous anode and ensures a high specific capacity independent of the SiNP size. The size strongly affects the electrochemical performance, as polarization builds up in cycling over the first 40 cycles. A crystalline Li15Si4 phase develops in the 2nd–30th cycles, having an impact on the CE behavior and enhancing capacity fade. Polarization, crystalline Li15Si4 formation, and lower CE might be attributed to electrochemical sintering [49]. The phenomenon is less apparent at lower size, thus small SiNPs should be favored for long term cycling. Alternatively, sintering is efficiently prevented by a carbon coating, conveniently obtained in the same reactor just after pyrolysis, as we recently reported [60].
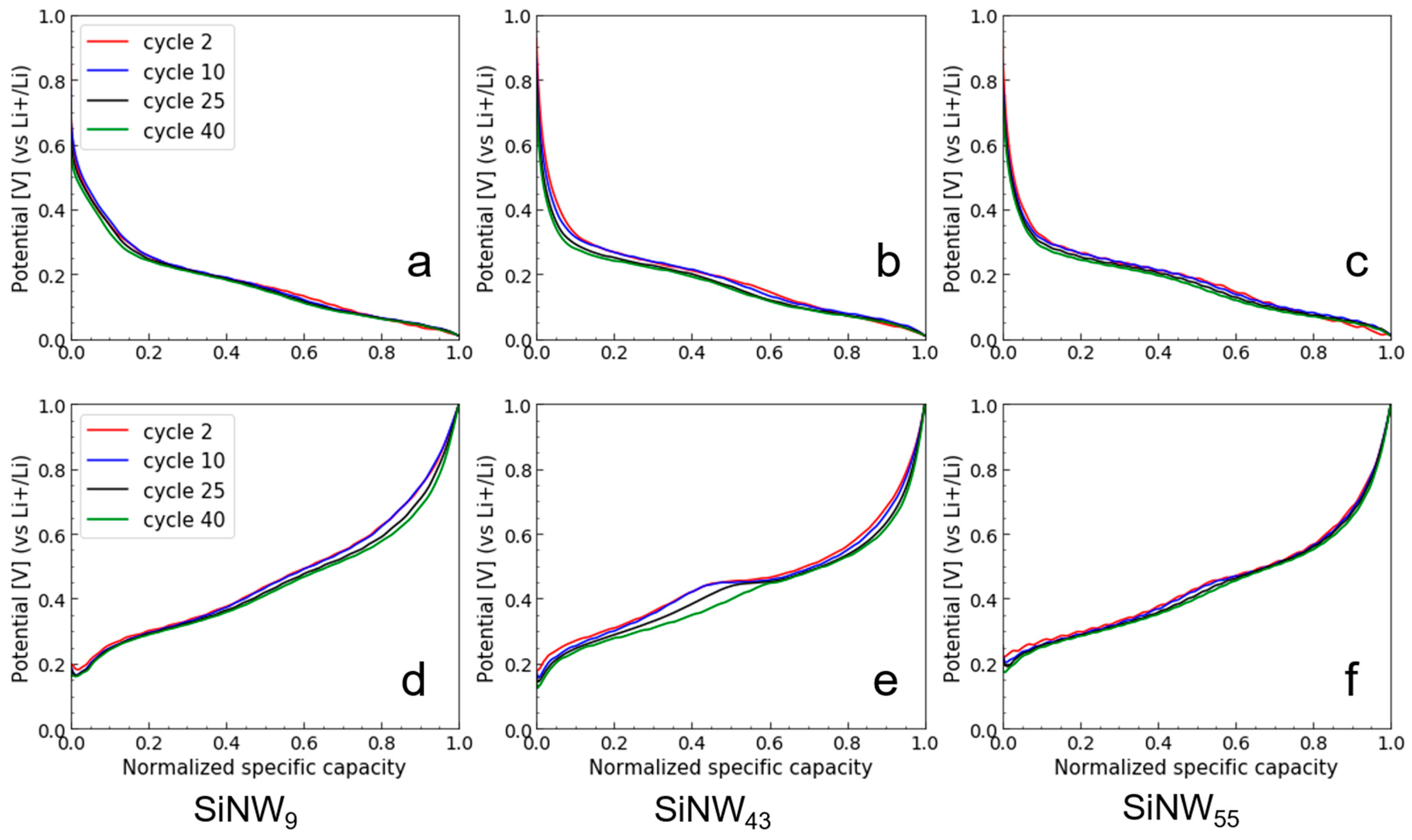
- When comparing SiNWs to SiNPs, it appears that the initial specific capacity of SiNWs is lower due to their micron-sized agglomerate form, while the CE of SiNWs is more stable and can be very high for the smallest size. It seems that the 1D shape of SiNWs brings a significant advantage in maintaining a 3D porous structure in the material, because stiff wires do not pack as tightly as particles. This 3D structure has a strong impact both on lithium diffusion and on avoiding Si electrochemical sintering.
- The effect of size for SiNWs is more complex than for SiNPs. It seems to reflect a trade-off between specific area (decreasing with diameter) and SiNW stiffness and pore size of agglomerates (increasing with diameter). We observe a minimum performance for the mid-sized SiNW42: a low specific capacity and a low Coulombic efficiency, which correlates with the formation of a higher quantity of crystalline Li15Si4 during lithiation. The larger sized SiNW55 with a smaller specific area (thus a lower irreversible capacity) and probably a larger size of pores in the agglomerates have a higher specific capacity. On the other hand, the smallest SiNW9 offer the highest Coulombic efficiency in the long run, probably due to fast Li diffusion. Additional electrode engineering is, thus, required to take full advantage of the promising performances of SiNWs. An alternative strategy consists of growing SiNWs at the surface of graphite flakes to produce directly a composite anode material [3]. This way, we could reduce micron-sized aggregation and attain full Si capacity cycling. Passivation of the SiNW surface to avoid large irreversible capacity at first cycle is another strategy to explore.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a Potential Anode Material for Li-Ion Batteries: Where Size, Geometry and Structure Matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef]
- Li, P.; Hwang, J.-Y.; Sun, Y.-K. Nano/Microstructured Silicon–Graphite Composite Anode for High-Energy-Density Li-Ion Battery. ACS Nano 2019, 13, 2624–2633. [Google Scholar] [CrossRef]
- Karuppiah, S.; Keller, C.; Kumar, P.; Jouneau, P.-H.; Aldakov, D.; Ducros, J.-B.; Lapertot, G.; Chenevier, P.; Haon, C. A Scalable Silicon Nanowires-Grown-On-Graphite Composite for High-Energy Lithium Batteries. ACS Nano 2020, 14, 12006–12015. [Google Scholar] [CrossRef]
- Berhaut, C.L.; Dominguez, D.Z.; Tomasi, D.; Vincens, C.; Haon, C.; Reynier, Y.; Porcher, W.; Boudet, N.; Blanc, N.; Chahine, G.A.; et al. Prelithiation of Silicon/Graphite Composite Anodes: Benefits and Mechanisms for Long-Lasting Li-Ion Batteries. Energy Storage Mater. 2020, 29, 190–197. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th Anniversary Article: Understanding the Lithiation of Silicon and Other Alloying Anodes for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef]
- Kim, H.; Seo, M.; Park, M.-H.; Cho, J. A Critical Size of Silicon Nano-Anodes for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. 2010, 49, 2146–2149. [Google Scholar] [CrossRef]
- Schott, T.; Robert, R.; Benito, S.P.; Ulmann, P.A.; Lanz, P.; Zürcher, S.; Spahr, M.E.; Novák, P.; Trabesinger, S. Cycling Behavior of Silicon-Containing Graphite Electrodes, Part B: Effect of the Silicon Source. J. Phys. Chem. C 2017, 121, 25718–25728. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Song, W.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; Wang, F.; Zhang, W. A Low Temperature MgH2-AlCl3-SiO2 System to Synthesize Nano-Silicon for High-Performance Li-Ion Batteries. Chem. Eng. J. 2021, 406, 126805. [Google Scholar] [CrossRef]
- Eshraghi, N.; Berardo, L.; Schrijnemakers, A.; Delaval, V.; Shaibani, M.; Majumder, M.; Cloots, R.; Vertruyen, B.; Boschini, F.; Mahmoud, A. Recovery of Nano-Structured Silicon from End-of-Life Photovoltaic Wafers with Value-Added Applications in Lithium-Ion Battery. ACS Sustain. Chem. Eng. 2020, 8, 5868–5879. [Google Scholar] [CrossRef]
- Sun, F.; Hu, Z.; Wu, L.; Chen, J.; Luo, J.; Wu, X.; Cheng, G.; Zheng, R. Reduced Graphene Oxide Wrapped Ultra-Thin Silicon Nanowires for Lithium Ion Battery Anodes. J. Phys. Conf. Ser. 2020, 1520, 012011. [Google Scholar] [CrossRef]
- Favors, Z.; Bay, H.H.; Mutlu, Z.; Ahmed, K.; Ionescu, R.; Ye, R.; Ozkan, M.; Ozkan, C.S. Towards Scalable Binderless Electrodes: Carbon Coated Silicon Nanofiber Paper via Mg Reduction of Electrospun SiO2 Nanofibers. Sci. Rep. 2015, 5, 8246. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Langklotz, U.; Pohl, D.; Tkacheva, O.; Pohl, D.; Nielsch, K.; Mikolajick, T.; Weber, W.M. Surface Related Differences between Uncoated versus Carbon-Coated Silicon Nanowire Electrodes on Performance in Lithium Ion Batteries. J. Energy Storage 2020, 27, 101052. [Google Scholar] [CrossRef]
- Cui, L.-F.; Yang, Y.; Hsu, C.-M.; Cui, Y. Carbon-Silicon Core-Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries. Nano Lett. 2009, 9, 3370–3374. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, S.; Qi, X.; Yu, Z.; Wu, Z.; Yang, J.; Lu, S. Preparation of High-Purity Straight Silicon Nanowires by Molten Salt Electrolysis. J. Energy Chem. 2020, 40, 171–179. [Google Scholar] [CrossRef]
- Song, T.; Xia, J.; Lee, J.-H.; Lee, D.H.; Kwon, M.-S.; Choi, J.-M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.I.; et al. Arrays of Sealed Silicon Nanotubes As Anodes for Lithium Ion Batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable Cycling of Double-Walled Silicon Nanotube Battery Anodes through Solid–Electrolyte Interphase Control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, M.; Hu, S.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.-G.; Liu, J. Mesoporous Silicon Sponge as an Anti-Pulverization Structure for High-Performance Lithium-Ion Battery Anodes. Nat. Commun. 2014, 5, 4105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; Chen, Y.-A.; Huang, C.-L.; Su, J.-T.; Hsieh, C.-T.; Lu, S.-Y. Small Highly Mesoporous Silicon Nanoparticles for High Performance Lithium Ion Based Energy Storage. Chem. Eng. J. 2020, 400, 125958. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; et al. Hierarchical Porous Silicon Structures with Extraordinary Mechanical Strength as High-Performance Lithium-Ion Battery Anodes. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Ryu, I.; Choi, J.W.; Cui, Y.; Nix, W.D. Size-Dependent Fracture of Si Nanowire Battery Anodes. J. Mech. Phys. Solids 2011, 59, 1717–1730. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Kushima, A.; Zhang, S.; Zhu, T.; Li, J.; Huang, J.Y. In Situ TEM Experiments of Electrochemical Lithiation and Delithiation of Individual Nanostructures. Adv. Energy Mater. 2012, 2, 722–741. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Ma, Z.; Li, T.; Huang, Y.L.; Liu, J.; Zhou, Y.; Xue, D. Critical Silicon-Anode Size for Averting Lithiation-Induced Mechanical Failure of Lithium-Ion Batteries. RSC Adv. 2013, 3, 7398–7402. [Google Scholar] [CrossRef]
- Sun, F.; Tan, Z.; Hu, Z.; Chen, J.; Luo, J.; Wu, X.; Cheng, G.; Zheng, R. Ultrathin Silicon Nanowires Produced by a Bi-Metal-Assisted Chemical Etching Method for Highly Stable Lithium-Ion Battery Anodes. NANO 2020, 15, 2050076. [Google Scholar] [CrossRef]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef]
- Dhalluin, F.; Baron, T.; Ferret, P.; Salem, B.; Gentile, P.; Harmand, J.C. Silicon Nanowires: Diameter Dependence of Growth Rate and Delay in Growth. Appl. Phys. Lett. 2010, 96, 133109. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gösele, U. Silicon Nanowires: A Review on Aspects of Their Growth and Their Electrical Properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef]
- Lacour, F.; Guillois, O.; Portier, X.; Perez, H.; Herlin, N.; Reynaud, C. Laser Pyrolysis Synthesis and Characterization of Luminescent Silicon Nanocrystals. Phys. E Low-Dimens. Syst. Nanostruct. 2007, 38, 11–15. [Google Scholar] [CrossRef]
- Sourice, J.; Quinsac, A.; Leconte, Y.; Sublemontier, O.; Porcher, W.; Haon, C.; Bordes, A.; De Vito, E.; Boulineau, A.; Larbi, S.J.S.; et al. One-Step Synthesis of Si@C Nanoparticles by Laser Pyrolysis: High-Capacity Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 6637–6644. [Google Scholar] [CrossRef]
- Burchak, O.; Keller, C.; Lapertot, G.; Salaün, M.; Danet, J.; Chen, Y.; Bendiab, N.; Pépin-Donat, B.; Lombard, C.; Faure-Vincent, J.; et al. Scalable Chemical Synthesis of Doped Silicon Nanowires for Energy Applications. Nanoscale 2019, 11, 22504–22514. [Google Scholar] [CrossRef]
- Bang, B.M.; Kim, H.; Song, H.-K.; Cho, J.; Park, S. Scalable Approach to Multi-Dimensional Bulk Si Anodes via Metal-Assisted Chemical Etching. Energy Environ. Sci. 2011, 4, 5013–5019. [Google Scholar] [CrossRef]
- Cannon, W.R.; Danforth, S.C.; Haggerty, J.S.; Marra, R.A. Sinterable Ceramic Powders from Laser-Driven Reactions: II, Powder Characteristics and Process Variables. J. Am. Ceram. Soc. 1982, 65, 330–335. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid-Liquid System. J. Chem. Soc. Chem. Comm. 1994, 801–802. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Ziegler, C.; Eychmüller, A. Seeded Growth Synthesis of Uniform Gold Nanoparticles with Diameters of 15−300 Nm. J. Phys. Chem. C 2011, 115, 4502–4506. [Google Scholar] [CrossRef]
- Karg, M.; Schelero, N.; Oppel, C.; Gradzielski, M.; Hellweg, T.; von Klitzing, R. Versatile Phase Transfer of Gold Nanoparticles from Aqueous Media to Different Organic Media. Chemistry 2011, 17, 4648–4654. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current Understanding of the Growth of Carbon Nanotubes in Catalytic Chemical Vapour Deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Morjan, R.E.; Nerushev, O.A.; Sveningsson, M.; Rohmund, F.; Falk, L.K.L.; Campbell, E.E.B. Growth of Carbon Nanotubes from C60. Appl. Phys. A 2004, 78, 253–261. [Google Scholar] [CrossRef]
- Langklotz, U.; Lein, T.; Schulze, C.; Weiser, M.; Krause, A.; Michaelis, A. Scalable Fabrication of Gold Nanoparticles with Adjustable Size Distribution as Catalytic Nuclei for the CVD Growth of Silicon Nanowires. Appl. Surf. Sci. 2020, 502, 144203. [Google Scholar] [CrossRef]
- Krause, A.; Dorfler, S.; Piwko, M.; Wisser, F.M.; Jaumann, T.; Ahrens, E.; Giebeler, L.; Althues, H.; Schadlich, S.; Grothe, J.; et al. High Area Capacity Lithium-Sulfur Full-Cell Battery with Prelitiathed Silicon Nanowire-Carbon Anodes for Long Cycling Stability. Sci. Rep. 2016, 6, 27982. [Google Scholar] [CrossRef]
- Gentile, P.; David, T.; Dhalluin, F.; Buttard, D.; Pauc, N.; Hertog, M.D.; Ferret, P.; Baron, T. The Growth of Small Diameter Silicon Nanowires to Nanotrees. Nanotechnology 2008, 19, 125608. [Google Scholar] [CrossRef]
- Dhalluin, F.; Desré, P.J.; den Hertog, M.I.; Rouvière, J.-L.; Ferret, P.; Gentile, P.; Baron, T. Critical Condition for Growth of Silicon Nanowires. J. Appl. Phys. 2007, 102, 094906. [Google Scholar] [CrossRef]
- Speier, J.L.; Ruth, E. Zimmerman Disproportionation of Phenylsilanes with Aluminum Chloride as the Catalyst. J. Am. Chem. Soc. 1955, 77, 6395–6396. [Google Scholar] [CrossRef]
- Gilman, H.; Miles, D.H. Disproportionation Reaction of Diphenylsilane in the Absence of Any Added Catalyst. J. Org. Chem. 1958, 23, 326–328. [Google Scholar] [CrossRef]
- Lee, D.C.; Hanrath, T.; Korgel, B.A. The Role of Precursor-Decomposition Kinetics in Silicon-Nanowire Synthesis in Organic Solvents. Angew. Chem. Int. Ed. Engl. 2005, 44, 3573–3577. [Google Scholar] [CrossRef]
- Salhi, B.; Grandidier, B.; Boukherroub, R. Controlled Growth of Silicon Nanowires on Silicon Surfaces. J. Electroceram. 2006, 16, 15–21. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Gösele, U. Growth, Thermodynamics, and Electrical Properties of Silicon Nanowires. Chem. Rev. 2010, 110, 361–388. [Google Scholar] [CrossRef]
- Winter, M.; Novák, P.; Monnier, A. Graphites for Lithium-Ion Cells: The Correlation of the First-Cycle Charge Loss with the Brunauer-Emmett-Teller Surface Area. J. Electrochem. Soc. 1998, 145, 428–436. [Google Scholar] [CrossRef]
- Hovington, P.; Dontigny, M.; Guerfi, A.; Trottier, J.; Lagacé, M.; Mauger, A.; Julien, C.M.; Zaghib, K. In Situ Scanning Electron Microscope Study and Microstructural Evolution of Nano Silicon Anode for High Energy Li-Ion Batteries. J. Power Sources 2014, 248, 457–464. [Google Scholar] [CrossRef]
- Shen, C.; Ge, M.; Luo, L.; Fang, X.; Liu, Y.; Zhang, A.; Rong, J.; Wang, C.; Zhou, C. In Situ and Ex Situ TEM Study of Lithiation Behaviours of Porous Silicon Nanostructures. Sci. Rep. 2016, 6, 31334. [Google Scholar] [CrossRef]
- Luo, L.; Wu, J.; Luo, J.; Huang, J.; Dravid, V.P. Dynamics of Electrochemical Lithiation/Delithiation of Graphene-Encapsulated Silicon Nanoparticles Studied by In-Situ TEM. Sci. Rep. 2014, 4, 3863. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2006, 154, A103. [Google Scholar] [CrossRef]
- Gan, C.; Zhang, C.; Wen, W.; Liu, Y.; Chen, J.; Xie, Q.; Luo, X. Enhancing Delithiation Reversibility of Li15Si4 Alloy of Silicon Nanoparticles-Carbon/Graphite Anode Materials for Stable-Cycling Lithium Ion Batteries by Restricting the Silicon Particle Size. ACS Appl. Mater. Interfaces 2019, 11, 35809–35819. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Mazouzi, D.; Reyter, D.; Lestriez, B.; Moreau, P.; Guyomard, D.; Roué, L. A Low-Cost and High Performance Ball-Milled Si-Based Negative Electrode for High-Energy Li-Ion Batteries. Energy Environ. Sci. 2013, 6, 2145–2155. [Google Scholar] [CrossRef]
- Iaboni, D.S.M.; Obrovac, M.N. Li15Si4 Formation in Silicon Thin Film Negative Electrodes. J. Electrochem. Soc. 2015, 163, A255. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, L.; Plümel, I.; Xu, G.-L.; Ren, Y.; Zuo, X.; Liu, Y.; Schulz, C.; Wiggers, H.; Amine, K.; et al. Parasitic Reactions in Nanosized Silicon Anodes for Lithium-Ion Batteries. Nano Lett. 2017, 17, 1512–1519. [Google Scholar] [CrossRef]
- Schmerling, M.; Fenske, D.; Peters, F.; Schwenzel, J.; Busse, M. Lithiation Behavior of Silicon Nanowire Anodes for Lithium-Ion Batteries: Impact of Functionalization and Porosity. ChemPhysChem 2018, 19, 123–129. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Li, X.; Hu, S.; Zhang, X.; Xu, W.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; Liu, J.; et al. In Situ TEM Study of Lithiation Behavior of Silicon Nanoparticles Attached to and Embedded in a Carbon Matrix. ACS Nano 2012, 6, 8439–8447. [Google Scholar] [CrossRef]
- Bernard, P.; Alper, J.P.; Haon, C.; Herlin-Boime, N.; Chandesris, M. Electrochemical Analysis of Silicon Nanoparticle Lithiation—Effect of Crystallinity and Carbon Coating Quantity. J. Power Sources 2019, 435, 226769. [Google Scholar] [CrossRef]
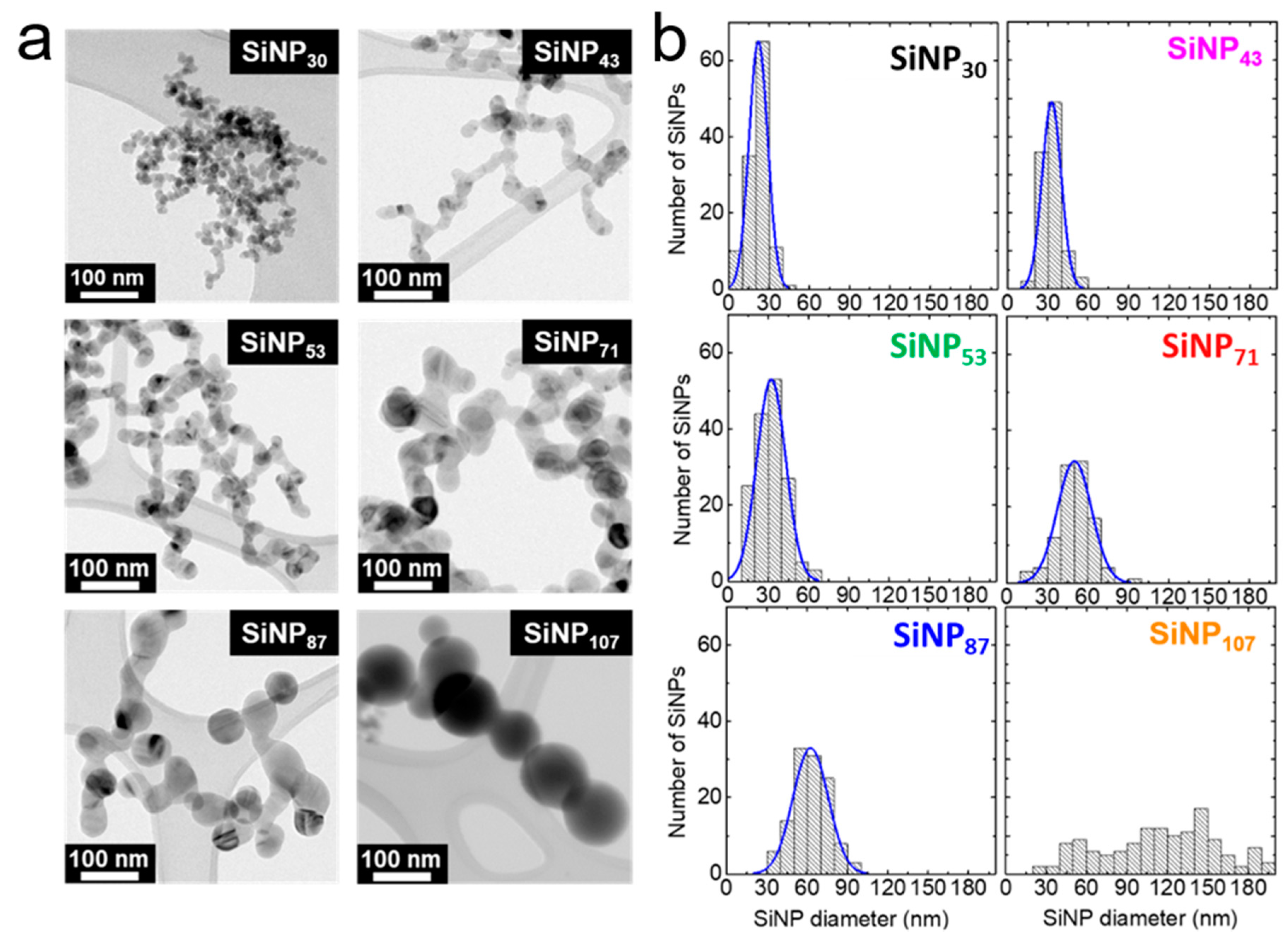

| Sample | SiH4 (sccm) | BET Specific Area (m2/g) | Diameter (nm) | Crystallite Size (nm) from XRD | |
|---|---|---|---|---|---|
| From BET | From TEM | ||||
| SiNP30 | 50 | 88 | 29 | 22 | 6 |
| SiNP43 | 100 | 60 | 43 | 33 | 25 |
| SiNP53 | 100 | 49 | 53 | 32 | 18 |
| SiNP71 | 150 | 36 | 71 | 50 | 35 |
| SiNP87 | 200 | 30 | 87 | 62 | 44 |
| SiNP107 | 234 | 24 | 107 | 114 | 63 |
| Sample | AuNP Size (nm) | BET Specific Area (m2/g) | Diameter from SEM (nm) | |
|---|---|---|---|---|
| Small Population | Large Population | |||
| SiNW9 | 1–2 | 194 | - | 9 |
| SiNW18 | 12 | 86 | - | 18 |
| SiNW20 | 18 | 76 | - | 20 |
| SiNW42 | 22 | 108 | 20 | 42 |
| SiNW55 | 51 | 85 | 23 | 55 |
| SiNW93 | 120 | 25 | 31 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, C.; Desrues, A.; Karuppiah, S.; Martin, E.; Alper, J.P.; Boismain, F.; Villevieille, C.; Herlin-Boime, N.; Haon, C.; Chenevier, P. Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery. Nanomaterials 2021, 11, 307. https://doi.org/10.3390/nano11020307
Keller C, Desrues A, Karuppiah S, Martin E, Alper JP, Boismain F, Villevieille C, Herlin-Boime N, Haon C, Chenevier P. Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery. Nanomaterials. 2021; 11(2):307. https://doi.org/10.3390/nano11020307
Chicago/Turabian StyleKeller, Caroline, Antoine Desrues, Saravanan Karuppiah, Eléa Martin, John P. Alper, Florent Boismain, Claire Villevieille, Nathalie Herlin-Boime, Cédric Haon, and Pascale Chenevier. 2021. "Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery" Nanomaterials 11, no. 2: 307. https://doi.org/10.3390/nano11020307
APA StyleKeller, C., Desrues, A., Karuppiah, S., Martin, E., Alper, J. P., Boismain, F., Villevieille, C., Herlin-Boime, N., Haon, C., & Chenevier, P. (2021). Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery. Nanomaterials, 11(2), 307. https://doi.org/10.3390/nano11020307







