Optimization of Thermophysical and Rheological Properties of Mxene Ionanofluids for Hybrid Solar Photovoltaic/Thermal Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
2.4. Uncertainty Analysis
3. Results and Discussion
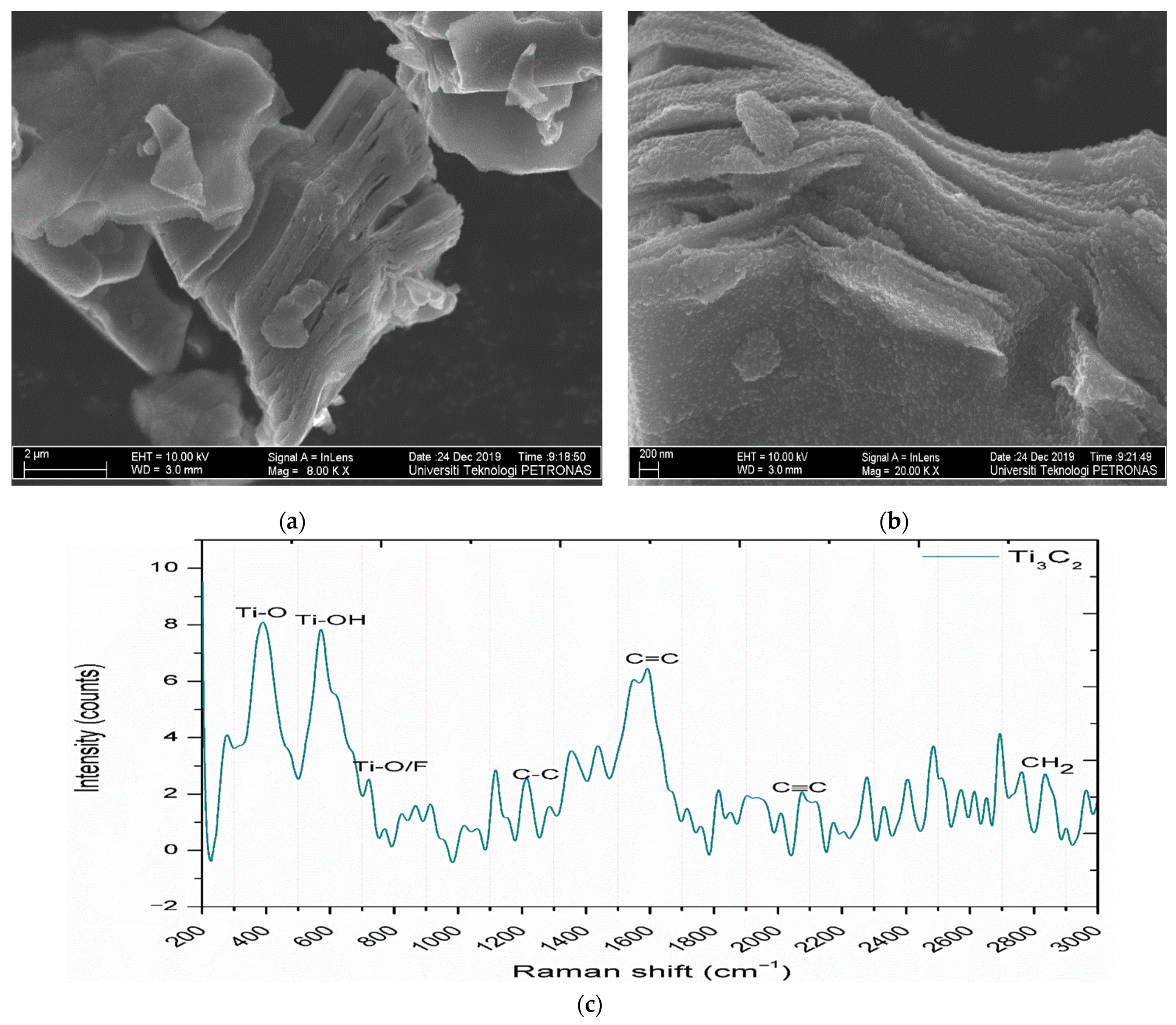
3.1. Structural and Morphological Analysis of MXene Nanoparticles
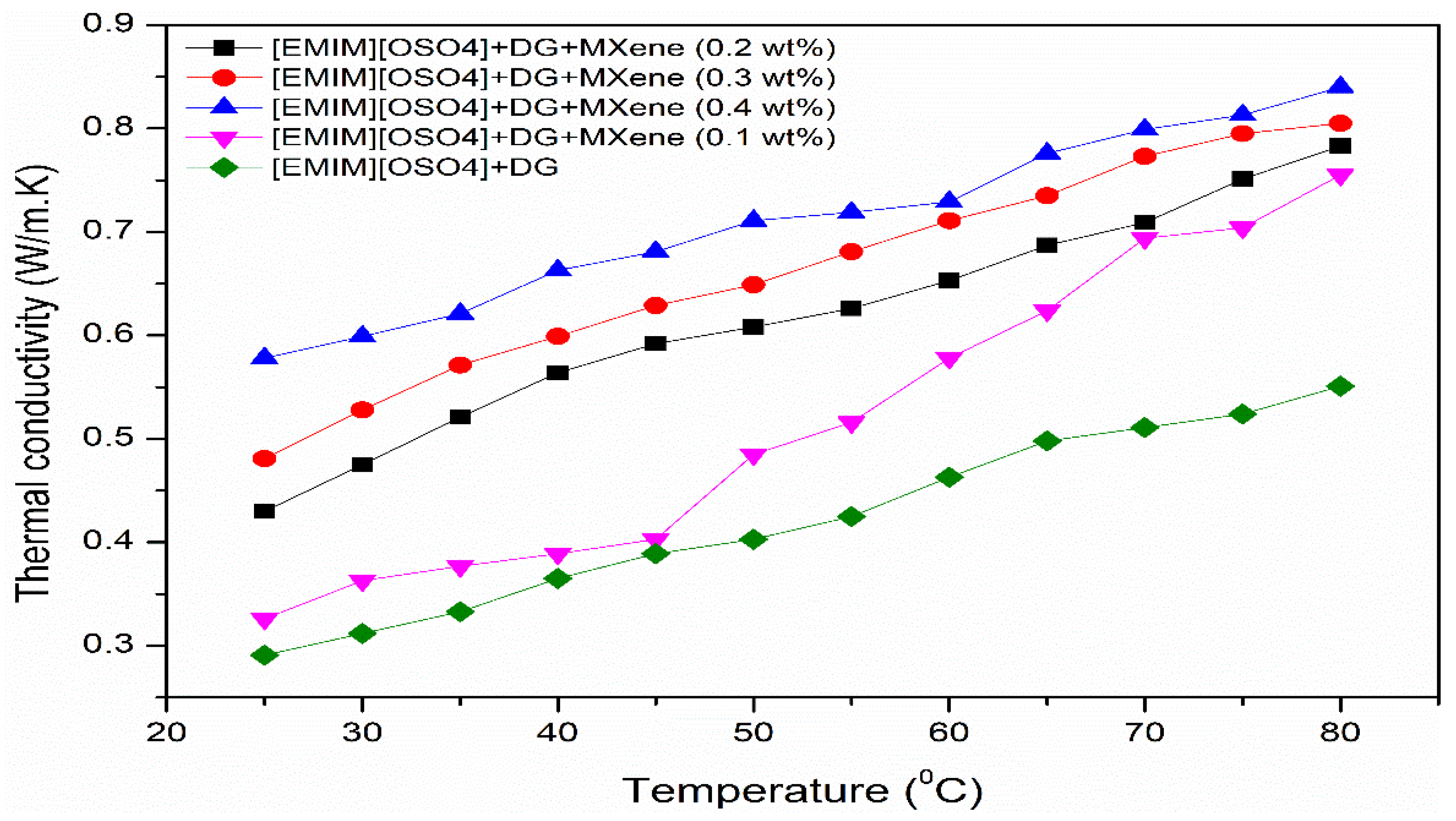
3.2. Thermal Conductivity
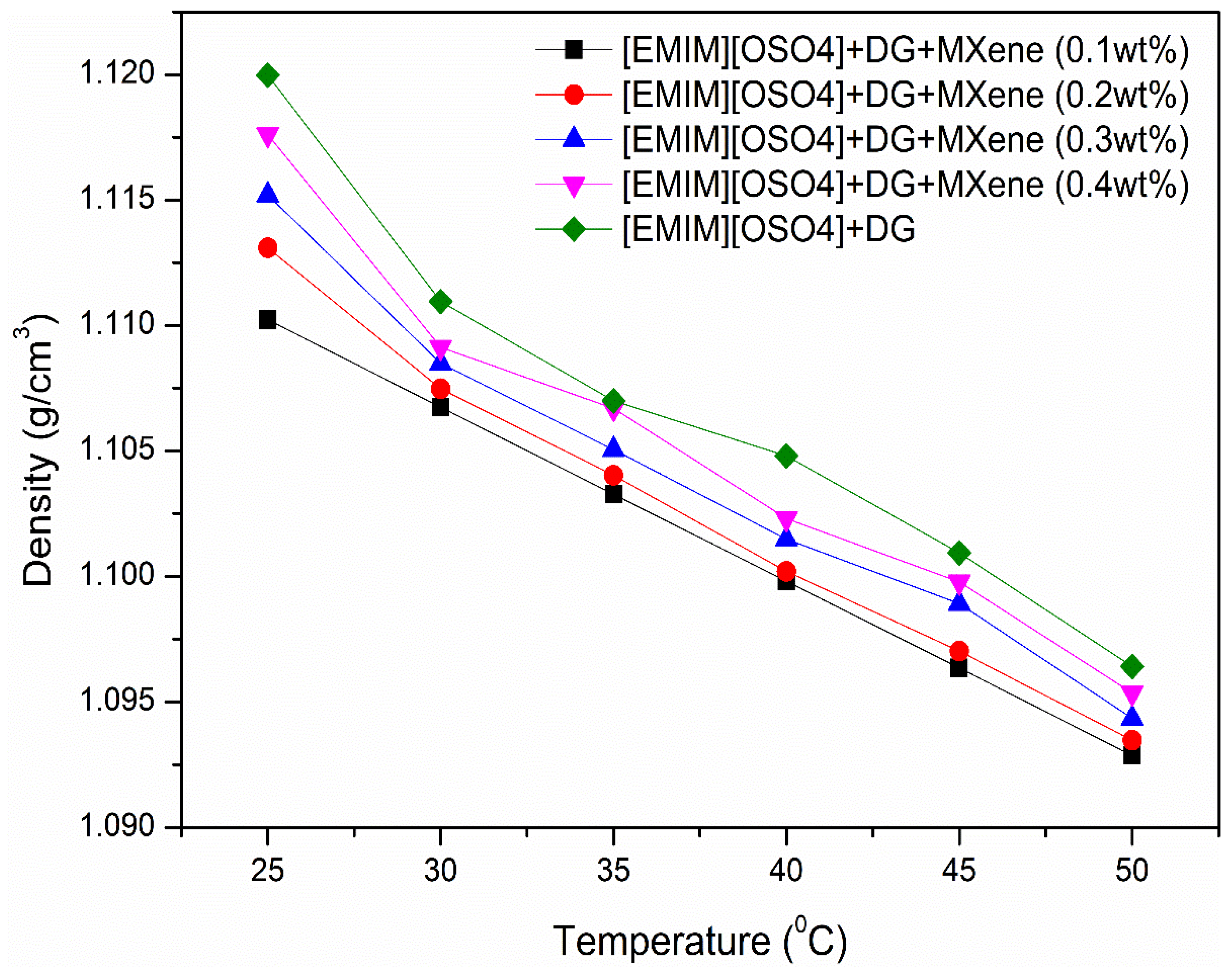
3.3. Density
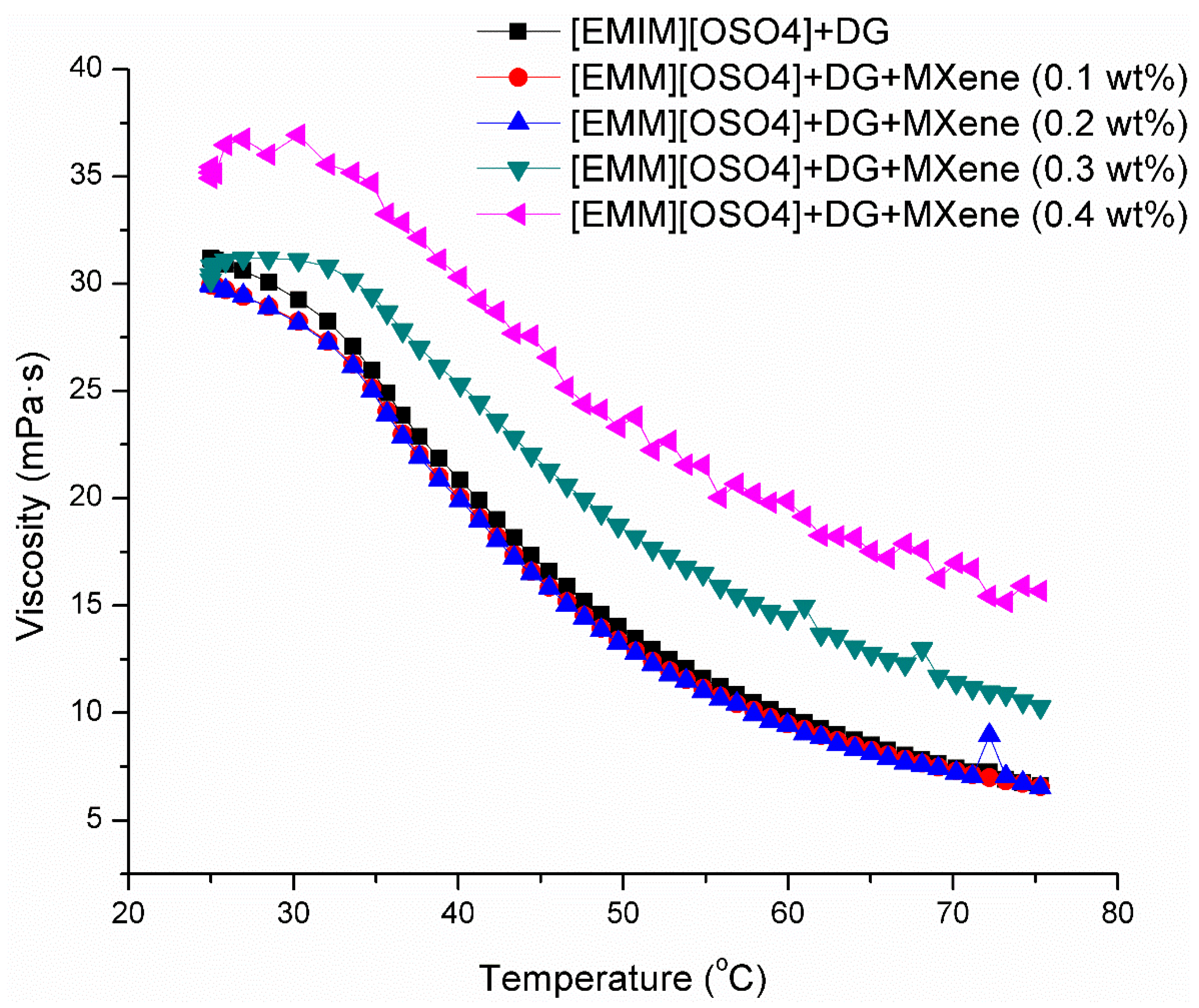
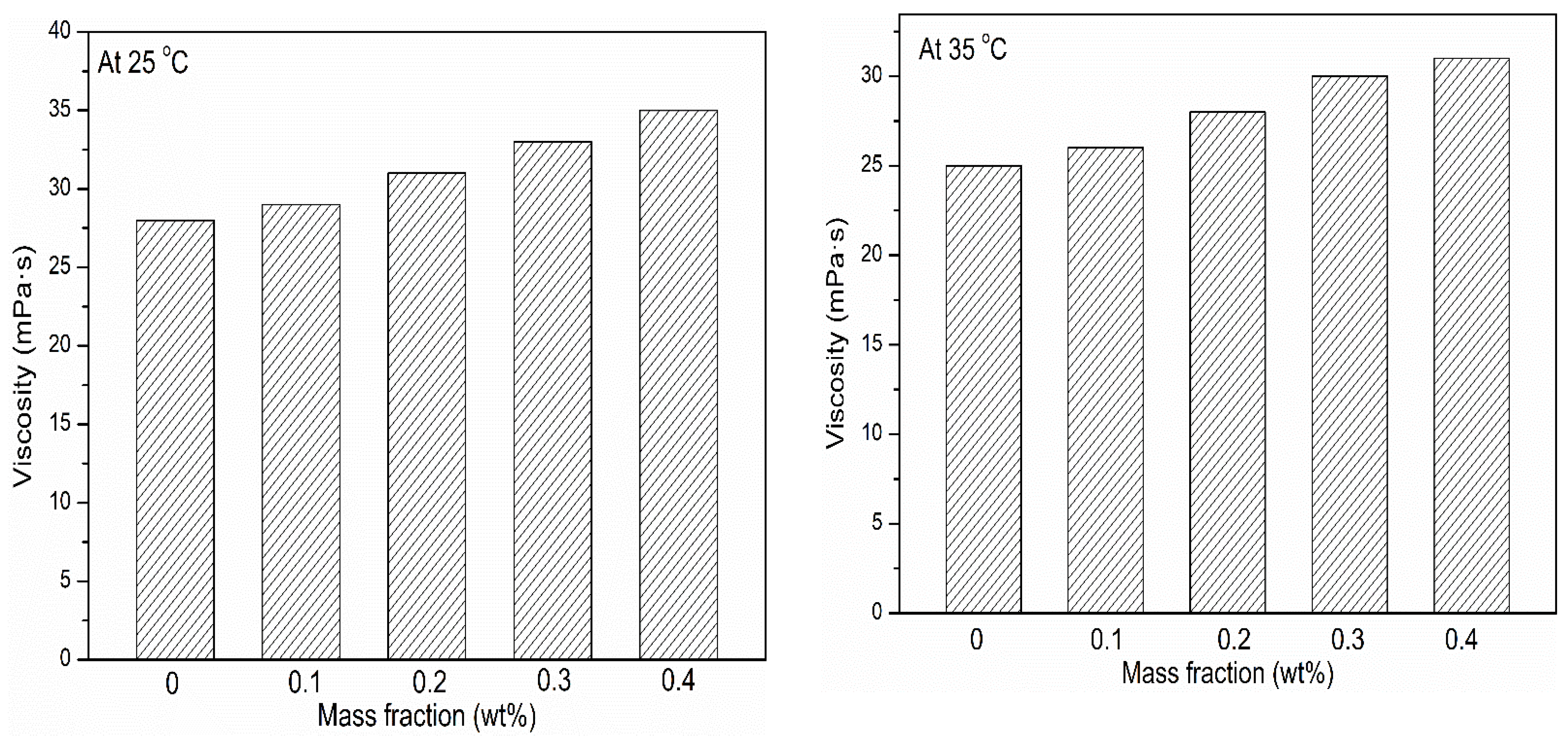
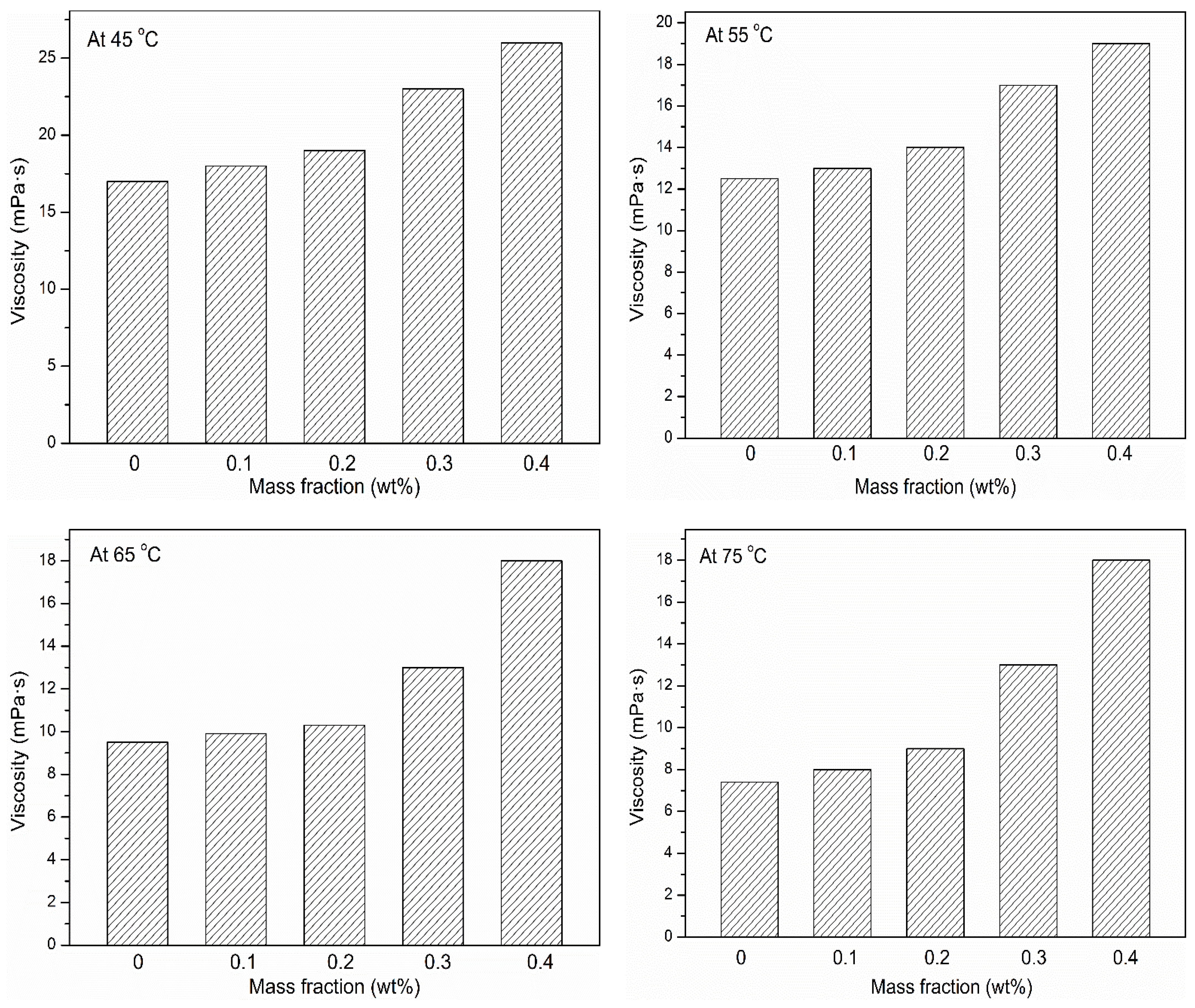
3.4. Viscosity
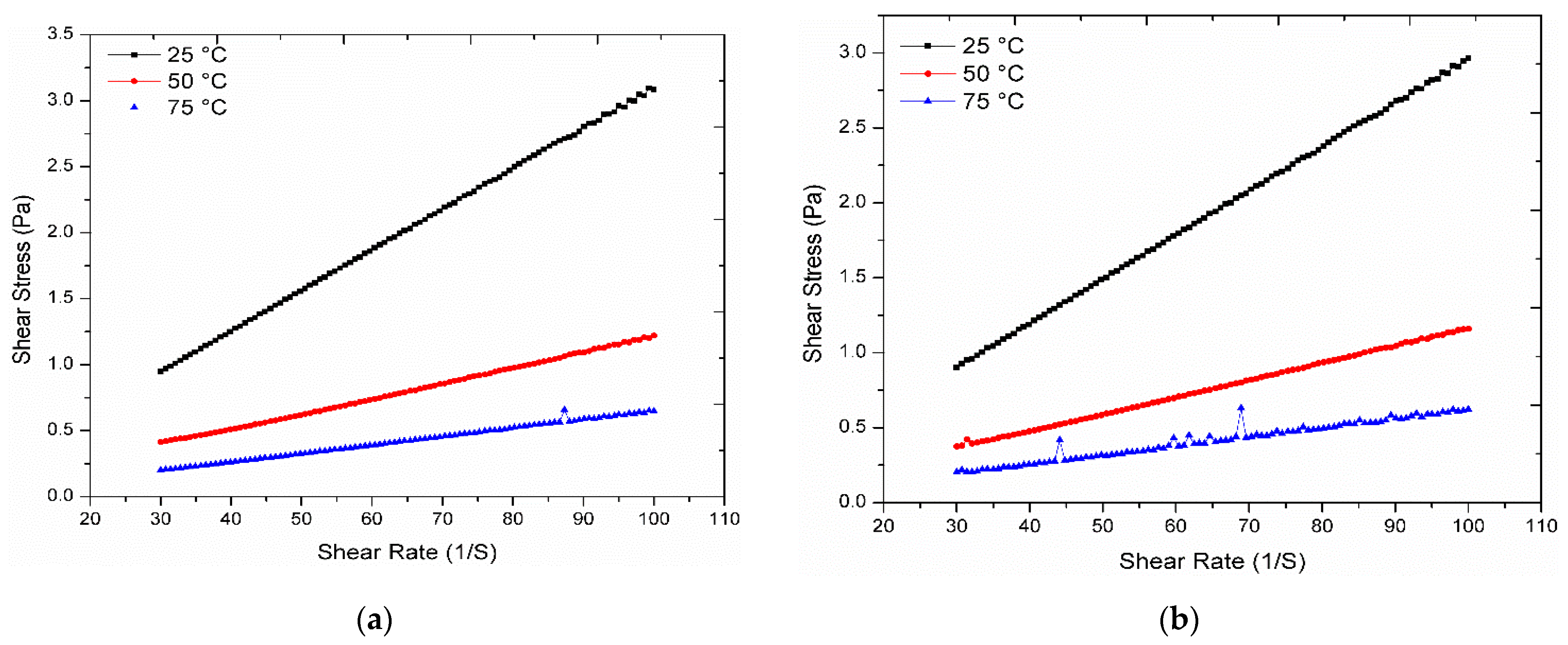
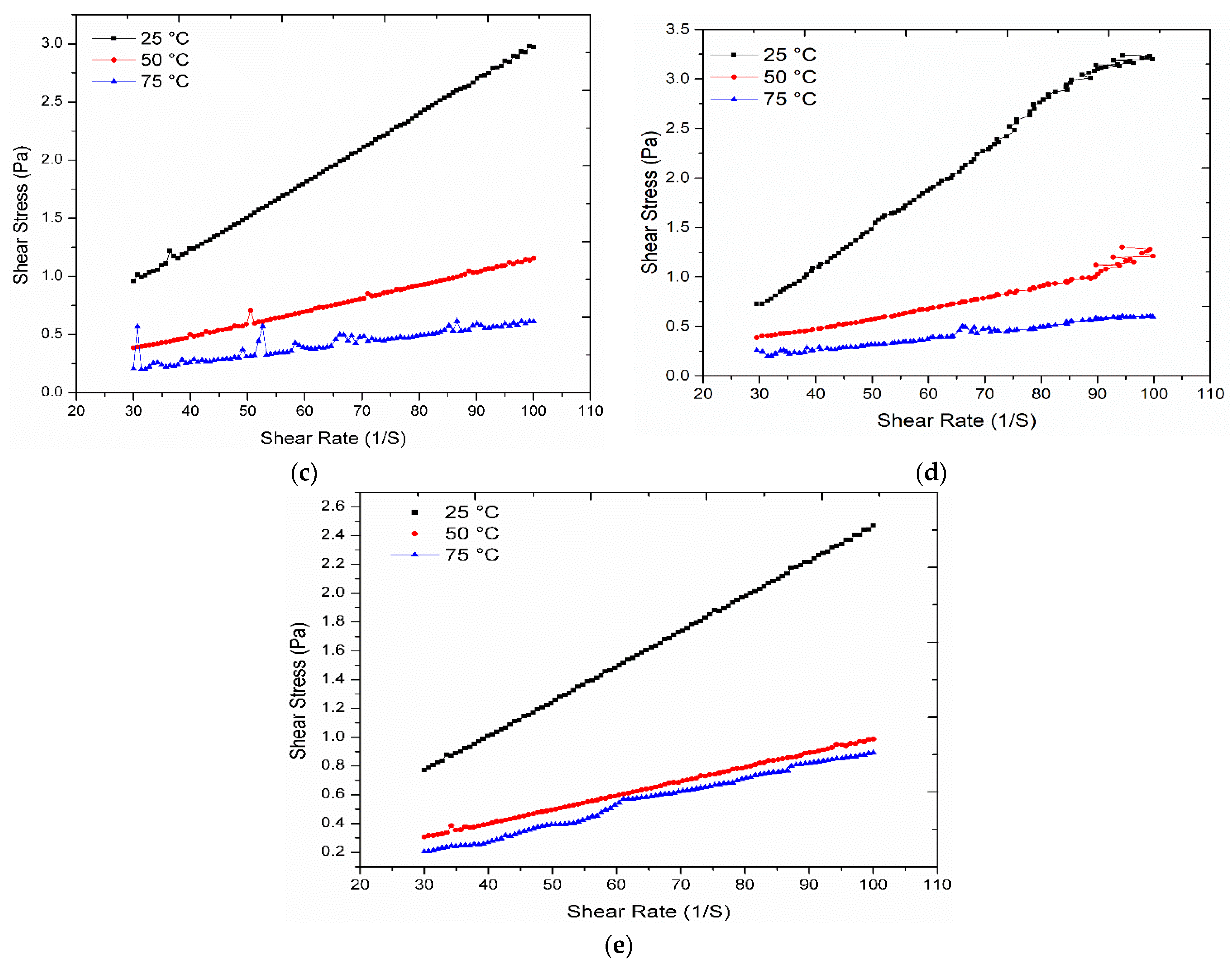
3.5. Rheological Analysis
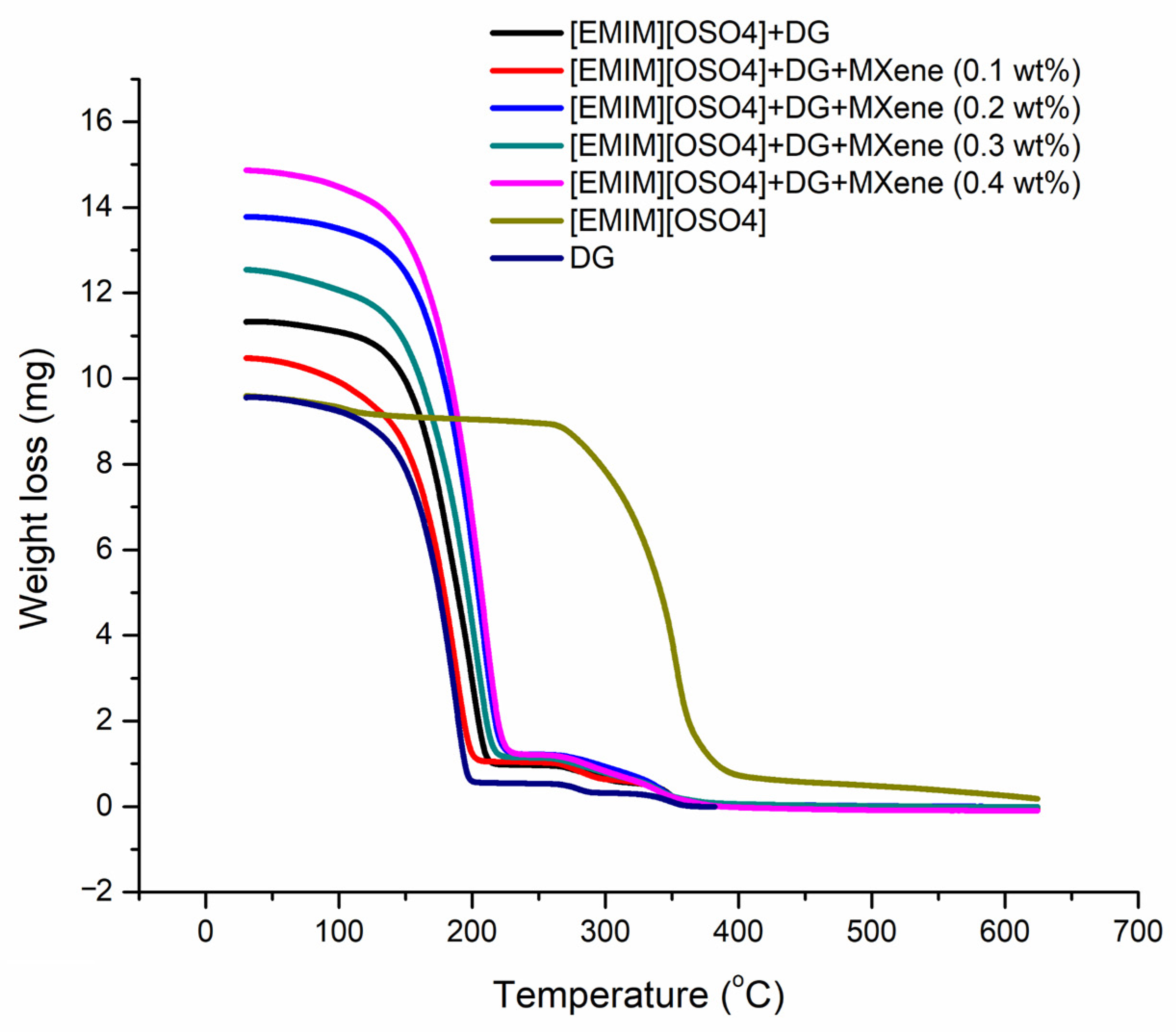
3.6. Thermal Stability
3.7. Specific Heat Capacity
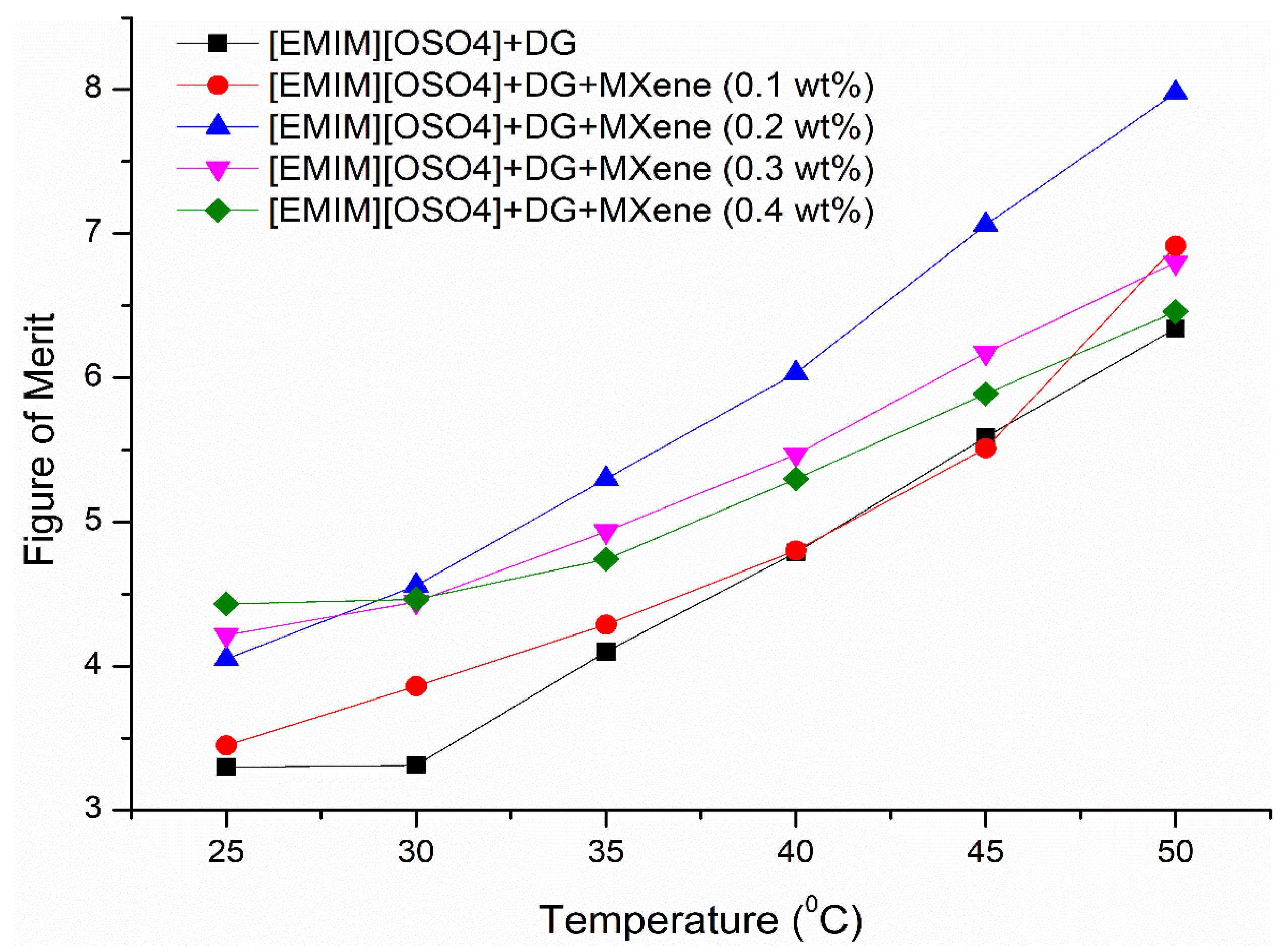
3.8. Heat Transfer Rate Efficiency of the Ionanofluids
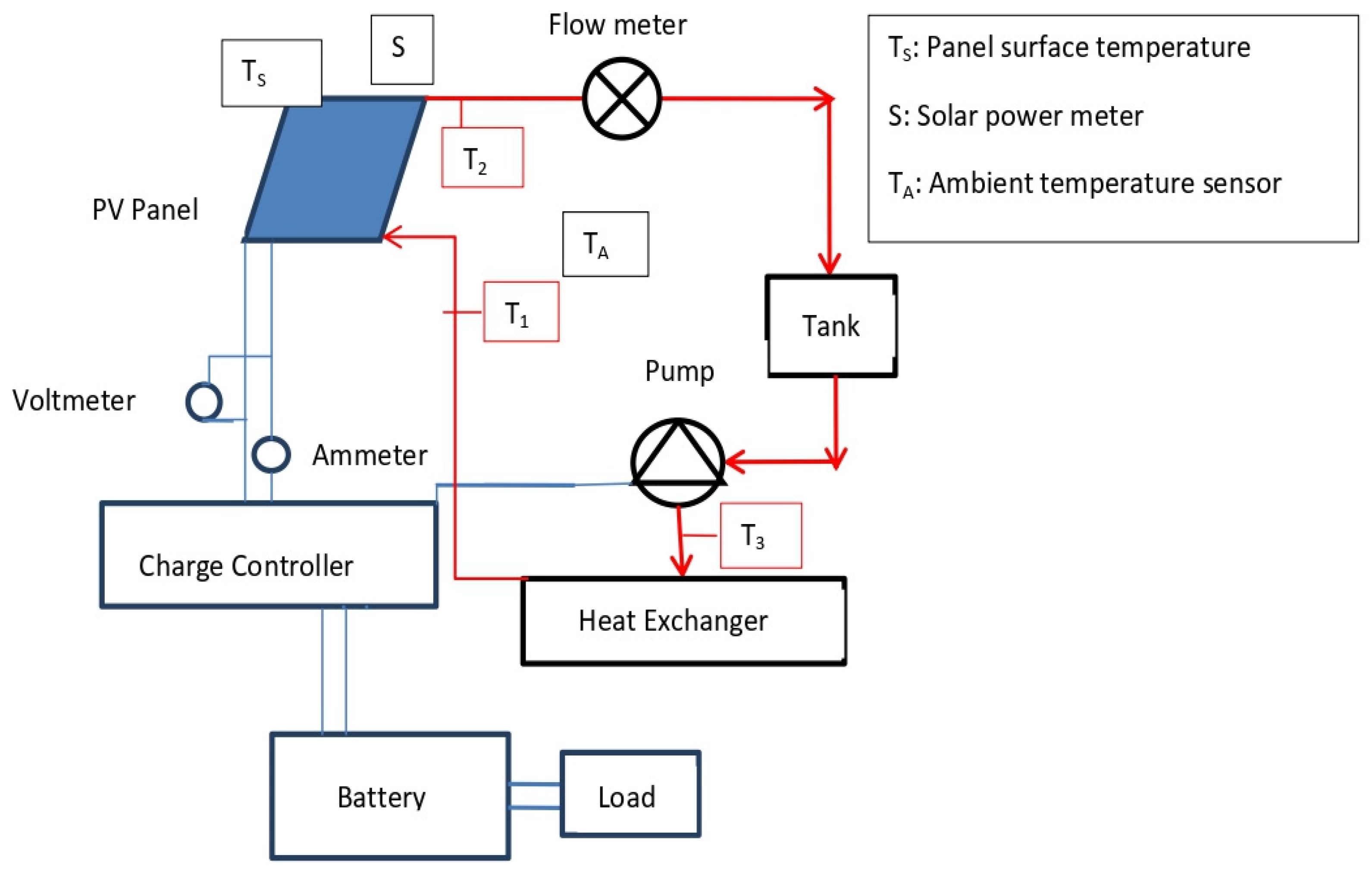
3.9. Application of the Ionic Liquid-Based MXene Nanofluid in the PVT System
3.9.1. Numerical Model and Simulation
Boundary Conditions
Meshing and Grid Independency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| [C8SO4] | 1-ethyl-3-methyl imidazolium octylsulfate |
| [EMIM][BF4] | 1-methyl-3-ethylimidazolium tetrafluoroborate |
| [BMIM][BF4] | 1-methyl-3-butylimidazolium tetraflouoroborate |
| [BMIM][Br] | 1-butyl-3-methylimidazolium bromide |
| [DMPI][FSI] | 1,2-dimethyl-3-propylimidazolium-bis (trifluorosulfonyl) imide |
| [C4MIM][NTf2] | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [P14,6,6,6][RO] | Trihexyl(tetradecyl)phosphonium carboxylate |
| PVT | Photovoltaic Thermal System |
| MXene | Ti3C2 |
| DEG | Diethylene Glycol |
| HTF | Heat Transfer Fluid |
| PEG | Polyethylene Glycol |
| PVDF | Polyvinylidene Fluoride |
| SA | Sodium Ascorbate |
| SO | Sodium Oxalate |
| SC | Sodium Citrate |
| SP | Sodium Phosphate |
| Ra | Rayleigh Number |
| Al2O3 | Alumina |
| SiO2 | Silicon dioxide |
| Ra | Rayleigh number |
| ϕ | Concentration |
| S/m | Siemens per meter |
| γ-Al2O3 | Gamma Alumina |
| Pa | Pascal |
| Nb | Niobium |
| Ta | Tantalum |
| Ti | Titanium |
Appendix A


References
- Omiddezyani, S.; Gharehkhani, S.; Yousefi-Asli, V.; Khazaee, I.; Ashjaee, M.; Nayebi, R.; Shemirani, F.; Houshfar, E. Experimental investigation on thermo-physical properties and heat transfer characteristics of green synthesized highly stable CoFe2O4/rGO nanofluid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125923. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Sharifpur, M.; Giwa, S.O.; Meyer, J.P. Experimental Research and Development on the Natural Convection of Suspensions of Nanoparticles—A Comprehensive Review. Nanomaterials 2020, 10, 1855. [Google Scholar] [CrossRef] [PubMed]
- Hamze, S.; Berrada, N.; Cabaleiro, D.; Desforges, A.; Ghanbaja, J.; Gleize, J.; Bégin, D.; Michaux, F.; Maré, T.; Vigolo, B.; et al. Few-Layer Graphene-Based Nanofluids with Enhanced Thermal Conductivity. Nanomaterials 2020, 10, 1258. [Google Scholar] [CrossRef]
- Khan, M.Z.U.; Uddin, E.; Akbar, B.; Akram, N.; Naqvi, A.A.; Sajid, M.; Ali, Z.; Younis, Y.; Márquez, F.P.G. Investigation of Heat Transfer and Pressure Drop in Microchannel Heat Sink Using Al2O3 and ZrO2 Nanofluids. Nanomaterials 2020, 10, 1796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Kong, M.; Alvarado, J.L. Thermophysical performance of graphene based aqueous nanofluids. Int. J. Heat Mass Transf. 2018, 119, 408–417. [Google Scholar] [CrossRef]
- Zhou, R.; Fu, S.; Li, H.; Yuan, D.; Tang, B.; Zhou, G. Experimental study on thermal performance of copper nanofluids in a miniature heat pipe fabricated by wire electrical discharge machining. Appl. Therm. Eng. 2019, 160, 113989. [Google Scholar] [CrossRef]
- Pourhoseini, S.; NaghiZadeh, N.; Hoseinzadeh, H. Effect of silver-water nanofluid on heat transfer performance of a plate heat exchanger: An experimental and theoretical study. Powder Technol. 2018, 332, 279–286. [Google Scholar] [CrossRef]
- Loni, R.; Asli-Areh, E.A.; Ghobadian, B.; Kasaeian, A.; Bellos, E. Thermal performance comparison between Al2O3/oil and SiO2/oil nanofluids in cylindrical cavity receiver based on experimental study. Renew. Energy 2018, 129, 652–665. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: A review. RSC Adv. 2018, 8, 19895–19905. [Google Scholar] [CrossRef] [Green Version]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Chudy, M.; Olszyna, A.; Birowska, M.; Popielski, M.; Majewski, J.A.; Scheibe, B.; Natu, V.; et al. On tuning the cytotoxicity of Ti3C2 (MXene) flakes to cancerous and benign cells by post-delamination surface modifications. 2D Mater. 2020, 7, 025018. [Google Scholar] [CrossRef]
- Nasrallah, G.K.; Al-Asmakh, M.; Rasool, K.; Mahmoud, K.A. Ecotoxicological assessment of Ti3C2Tx (MXene) using a zebrafish embryo model. Environ. Sci. Nano 2018, 5, 1002–1011. [Google Scholar] [CrossRef]
- Lina, P.; Xiea, J.; Hea, Y.; Lub, X.; Lia, W.; Fanga, J.; Yana, S.; Zhanga, L.; Sheng, X.; Chena, Y. MXene aerogel-based phase change materials toward solar energy conversion. Sol. Energy Mater. Sol. Cells 2020, 206, 110229. [Google Scholar] [CrossRef]
- Lua, X.; Huangb, H.; Zhangb, X.; Linc, P.; Huangd, J.; Sheng, X.; Zhangc, L.; Pingqua, J. Novel light-driven and electro-driven polyethylene glycol/two-dimensional MXene form-stable phase change material with enhanced thermal conductivity and electrical conductivity for thermal energy storage. Compos. Part B Eng. 2019, 177, 107372. [Google Scholar] [CrossRef]
- Wu, C.-W.; Unnikrishnan, B.; Chen, I.-W.P.; Harroun, S.G.; Chang, H.-T.; Huang, C.-C. Excellent oxidation resistive MXene aqueous ink for micro-supercapacitor application. Energy Storage Mater. 2020, 25, 563–571. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Gao, F.; Wang, Y.; Shen, X.; He, N.; Zhu, J.; Chen, Y.; Wan, X.; Lian, X.; et al. Formation of new MXene film using spinning coating method with DMSO solution and its application in advanced memristive device. Ceram. Int. 2019, 45, 19467–19472. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, Y.; Cheng, H.; Lu, Z. The potential application of 2D Ti2CT2 (T = C, O and S) monolayer MXenes as anodes for Na-ion batteries: A theoretical study. Comput. Mater. Sci. 2019, 163, 267–277. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118382. [Google Scholar] [CrossRef]
- Rajavel, K.; Luo, S.; Wan, Y.; Yu, X.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C. 2D Ti3C2Tx MXene/polyvinylidene fluoride (PVDF) nanocomposites for attenuation of electromagnetic radiation with excellent heat dissipation. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105693. [Google Scholar] [CrossRef]
- De Castro, C.A.N.; Lourenço, M.J.V.; Ribeiro, A.P.C.; Langa, E.; Vieira, S.I.C.; Goodrich, P.; Hardacre, C. Thermal Properties of Ionic Liquids and IoNanofluids of Imidazolium and Pyrrolidinium Liquids. J. Chem. Eng. Data 2009, 55, 653–661. [Google Scholar] [CrossRef]
- Murshed, S.S.; de Castro, C.N.; Lourenço, M.J.V.; França, J.; Ribeiro, A.P.C.; Vieira, S.I.C.; Queirós, C.S. Ionanofluids as Novel Fluids for Advanced Heat Transfer Applications. Int. J. Phys. Math. Sci. 2011, 5, 579–582. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; ElSinawi, A. Thermal Conductivity Enhancement Phenomena in Ionic Liquid-Based Nanofluids (Ionanofluids). Aust. J. Chem. 2019, 72, 21–33. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; ElSinawi, A. Ionic liquid-based nanofluids (ionanofluids) for thermal applications: An experimental thermophysical characterization. Pure Appl. Chem. 2019, 91, 1309–1340. [Google Scholar] [CrossRef]
- Jóźwiak, B.; Dzido, G.; Zorębski, E.; Kolanowska, A.; Jȩdrysiak, R.; Dziadosz, J.; Libera, M.; Boncel, S.; Dzida, M. Remarkable Thermal Conductivity Enhancement in Carbon-Based Ionanofluids: Effect of Nanoparticle Morphology. ACS Appl. Mater. Interfaces 2020, 12, 38113–38123. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.P.; Del Río, J.M.L.; Fernández, J.; Lugo, L. Tribological performance of silicon nitride and carbon black Ionanofluids based on 1-ethyl-3-methylimidazolium methanesulfonate. J. Mol. Liq. 2020, 319, 114335. [Google Scholar] [CrossRef]
- Jóźwiak, B.; Boncel, S. Rheology of ionanofluids—A review. J. Mol. Liq. 2020, 302, 112568. [Google Scholar] [CrossRef]
- Valkenburg, M.E.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Paul, T.C.; Mahamud, R.; Khan, J.A. Multiphase modeling approach for ionic liquids (ILs) based nanofluids: Improving the performance of heat transfer fluids (HTFs). Appl. Therm. Eng. 2019, 149, 165–172. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Zhao, J.; Gao, H. Measurement of thermal conductivity, viscosity and density of ionic liquid [EMIM][DEP]-based nanofluids. Chin. J. Chem. Eng. 2016, 24, 331–338. [Google Scholar] [CrossRef]
- Minea, A.A.; El-Maghlany, W.M. Natural convection heat transfer utilizing ionic nanofluids with temperature-dependent thermophysical properties. Chem. Eng. Sci. 2017, 174, 13–24. [Google Scholar] [CrossRef]
- Soman, D.P.; Karthika, S.; Kalaichelvi, P.; Radhakrishnan, T. Impact of viscosity of nanofluid and ionic liquid on heat transfer. J. Mol. Liq. 2019, 291, 111349. [Google Scholar] [CrossRef]
- Cao, P.; Li, Y.; Wu, Y.; Chen, H.; Zhang, J.; Cheng, L.; Niu, T. Role of base fluid on enhancement absorption properties of Fe3O4/ionic liquid nanofluids for direct absorption solar collector. Sol. Energy 2019, 194, 923–931. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M.; et al. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Asadi, A.; Alarifi, I.M.; Ali, V.; Nguyen, H.M. An experimental investigation on the effects of ultrasonication time on stability and thermal conductivity of MWCNT-water nanofluid: Finding the optimum ultrasonication time. Ultrason. Sonochem. 2019, 58, 104639. [Google Scholar] [CrossRef]
- Li, F.; Li, L.; Zhong, G.; Zhai, Y.; Li, Z. Effects of ultrasonic time, size of aggregates and temperature on the stability and viscosity of Cu-ethylene glycol (EG) nanofluids. Int. J. Heat Mass Transf. 2019, 129, 278–286. [Google Scholar] [CrossRef]
- Mahbubul, I.; Elcioglu, E.B.; Saidur, R.; Amalina, M. Optimization of ultrasonication period for better dispersion and stability of TiO2—Water nanofluid. Ultrason. Sonochem. 2017, 37, 360–367. [Google Scholar] [CrossRef]
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Żyła, G.; Estellé, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state-of-the-art review. Powder Technol. 2019, 352, 209–226. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Rahman, S.; Aslfattahi, N.; Rashedi, A. Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids. Appl. Sci. 2020, 10, 8943. [Google Scholar] [CrossRef]
- Hu, M.; Li, Z.; Hu, T.; Zhu, S.; Zhang, C.; Wang, X. High-Capacitance Mechanism for Ti3C2Tx MXene by in Situ Electrochemical Raman Spectroscopy Investigation. ACS Nano 2016, 10, 11344–11350. [Google Scholar] [CrossRef]
- França, J.M.P.; Vieira, S.I.C.; Lourenço, M.J.V.; Murshed, S.M.S.; De Castro, C.A.N. Thermal Conductivity of [C4mim][(CF3SO2)2N] and [C2mim][EtSO4] and Their IoNanofluids with Carbon Nanotubes: Experiment and Theory. J. Chem. Eng. Data 2013, 58, 467–476. [Google Scholar] [CrossRef]
- Marcinkowski, Ł.; Szepiński, E.; Milewska, M.J.; Kloskowski, A. Density, sound velocity, viscosity, and refractive index of new morpholinium ionic liquids with amino acid-based anions: Effect of temperature, alkyl chain length, and anion. J. Mol. Liq. 2019, 284, 557–568. [Google Scholar] [CrossRef]
- Mariano, A.; Pastoriza-Gallego, M.J.; Lugo, L.; Mussari, L.; Piñeiro, M.M. Co3O4 ethylene glycol-based nanofluids: Thermal conductivity, viscosity and high pressure density. Int. J. Heat Mass Transf. 2015, 85, 54–60. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Costa, A.J.L.; Esperança, J.M.S.S.; Marrucho, I.M.; Rebêlo, L. Densities and Viscosities of 1-Ethyl-3-methylimidazoliumn-Alkyl Sulfates. J. Chem. Eng. Data 2011, 56, 3433–3441. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zou, C. An experimental study on β-cyclodextrin modified carbon nanotubes nanofluids for the direct absorption solar collector (DASC): Specific heat capacity and photo-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2020, 204, 110240. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Shahabuddin, S.; Saha, B.B. Influence of solvents on the enhancement of thermophysical properties and stability of multi-walled carbon nanotubes nanofluid. Nanotechnology 2020, 31, 235402. [Google Scholar] [CrossRef] [PubMed]
- Mouromtseff, I.E. Water and Forced-Air Cooling of Vacuum Tubes Nonelectronic Problems in Electronic Tubes. Proc. IRE 1942, 30, 190–205. [Google Scholar] [CrossRef]
- Simons, R.E. Calculation corner: Comparing heat transfer rates of liquid coolants using the Mouromtseff number. Electron. Cool. 2006, 12, 10. [Google Scholar]
- Cengel, Y.A.; Klein, S.; Beckman, W. Heat Transfer: A Practical Approach; McGraw-Hill: New York, NY, USA, 1998; Volume 141. [Google Scholar]
- Esfe, M.H.; Saedodin, S.; Mahian, O.; Wongwises, S. Thermal conductivity of Al2O3/water nanofluids. J. Therm. Anal. Calorim. 2014, 117, 675–681. [Google Scholar] [CrossRef]
- Jarahnejad, M.; Haghighi, E.B.; Saleemi, M.; Nikkam, N.; Khodabandeh, R.; Palm, B.; Toprak, M.S.; Muhammed, M. Experimental investigation on viscosity of water-based Al2O3 and TiO2 nanofluids. Rheol. Acta 2015, 54, 411–422. [Google Scholar] [CrossRef]
- Teng, T.-P.; Hung, Y.-H. Estimation and experimental study of the density and specific heat for alumina nanofluid. J. Exp. Nanosci. 2012, 9, 707–718. [Google Scholar] [CrossRef]
- Sekhar, Y.R.; Sharma, K. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2015, 10, 86–102. [Google Scholar] [CrossRef]







| References | Material | Additives | Applications | Findings |
|---|---|---|---|---|
| Lin et al. [13] | Ti3C2 | PEG4000 | Solar energy conversion |
|
| Lu et al. [14] | Ti3C2 | PEG | Thermal energy storage |
|
| Wu et al. [15] | Ti3C2 | SA SO SC SP | Micro-Super capacitor |
|
| Zhang et al. [16] | Ti3C2 | Dimethyl sulfoxide Absolute ethanol Deionized water | Memristor |
|
| Xiao et al. [17] | Ti2C Ti2CC2 Ti2CO2 Ti2CS2 | Sodium-ion | Na-ion Batteries |
|
| Rong et al. [18] | Ti3C2 | CdS | Photocatalysis |
|
| Rajavel et al. [19] | Ti3C2 | PVDF | Heat dissipation |
|
| Sample | Weight (%) | Total Mass (gram) | Mass ± 0.001 g | ||
|---|---|---|---|---|---|
| [C8SO4] | Diethylene Glycol | MXene Nanoparticle | |||
| [C8SO4]+DEG | 0 | 50 | 5 | 45 | 0 |
| [C8SO4]+DEG+MXene | 0.1 | 50 | 4.995 | 44.955 | 0.05 |
| [C8SO4]+DEG+MXene | 0.2 | 50 | 4.990 | 44.91 | 0.1 |
| [C8SO4]+DEG+MXene | 0.3 | 50 | 4.985 | 44.865 | 0.15 |
| [C8SO4]+DEG+MXene | 0.4 | 50 | 4.980 | 44.82 | 0.2 |
| Device | Quantity | Accuracy | Maximum Uncertainty (In Experiments) |
|---|---|---|---|
| Thermal property analyzer (Tempos) | Thermal conductivity | ±10% | 0.04 W/m·K |
| Density meter | Density | ±0.001 g/cm3 | 0.003 g/cm3 |
| Rheometer | Viscosity | ±1% | 1.15 mPa·s |
| Rheology | ±1% | 0.06 Pa | |
| Differential Scanning Calorimetry | Specific heat capacity | ±2% | 0.08 J/g·K |
| Thermogravimetric Analyzer | Thermal stability | ±0.02% | 0.09% |
| FTIR Spectrometer | Light transmittance | ±1% | 0.22% |
| Serial No. | Size of Mesh (No. of Elements) | Temperature of Cell (°C) |
|---|---|---|
| 1 | 2.5 × 105 | 42.341 |
| 2 | 4 × 105 | 43.872 |
| 3 | 6 × 105 | 44.003 |
| 4 | 8 × 105 | 44.118 |
| 5 | 1.5 × 106 | 45.200 |
| 6 | 3.5 × 106 | 45.201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakthavatchalam, B.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Rashedi, A.; Khanam, T. Optimization of Thermophysical and Rheological Properties of Mxene Ionanofluids for Hybrid Solar Photovoltaic/Thermal Systems. Nanomaterials 2021, 11, 320. https://doi.org/10.3390/nano11020320
Bakthavatchalam B, Habib K, Saidur R, Aslfattahi N, Yahya SM, Rashedi A, Khanam T. Optimization of Thermophysical and Rheological Properties of Mxene Ionanofluids for Hybrid Solar Photovoltaic/Thermal Systems. Nanomaterials. 2021; 11(2):320. https://doi.org/10.3390/nano11020320
Chicago/Turabian StyleBakthavatchalam, Balaji, Khairul Habib, R. Saidur, Navid Aslfattahi, Syed Mohd Yahya, A. Rashedi, and Taslima Khanam. 2021. "Optimization of Thermophysical and Rheological Properties of Mxene Ionanofluids for Hybrid Solar Photovoltaic/Thermal Systems" Nanomaterials 11, no. 2: 320. https://doi.org/10.3390/nano11020320
APA StyleBakthavatchalam, B., Habib, K., Saidur, R., Aslfattahi, N., Yahya, S. M., Rashedi, A., & Khanam, T. (2021). Optimization of Thermophysical and Rheological Properties of Mxene Ionanofluids for Hybrid Solar Photovoltaic/Thermal Systems. Nanomaterials, 11(2), 320. https://doi.org/10.3390/nano11020320







