Real-Time Tracking of Highly Luminescent Mesoporous Silica Particles Modified with Europium β-Diketone Chelates in Living Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
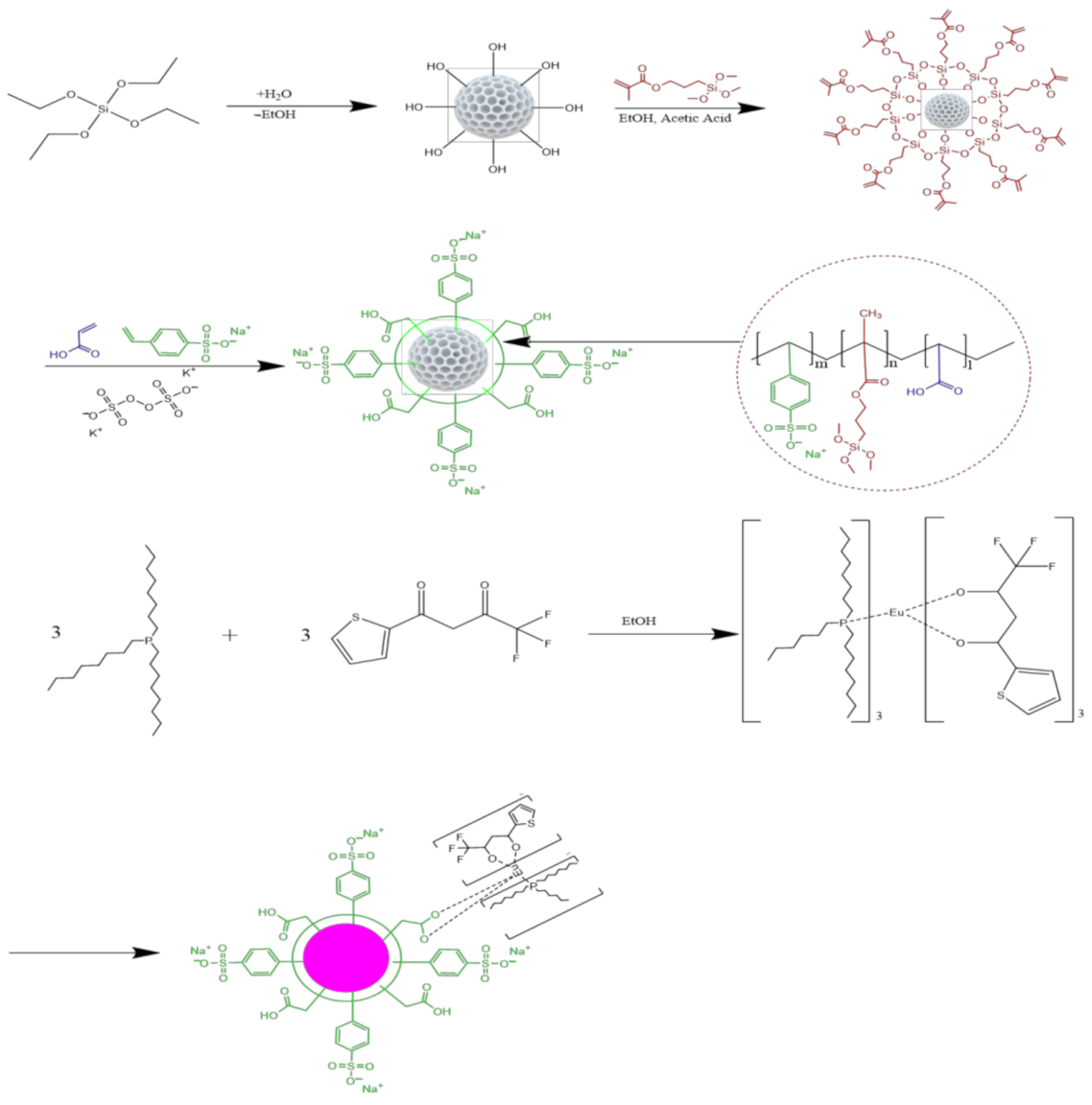
2.3. Preparation of Mesoporous SiO2 (mSiO2) Particles (MSP)
2.4. Synthesis of β-Diketone Eu(TTA)3(P(Oct)3)3 Complexes
2.5. Synthesis of Eu(TTA)3(P(Oct)3)3 Doped MSP (Eu@MSP)
2.6. Cell Lines and Cell Culture
2.7. Isolation of Bone Marrow Cells and Generation of Murine Bone Marrow-Derived Macrophage Generation
2.8. CCK-8
2.9. Flow Cytometry Analysis
2.10. Fluorescence and Holotomographic Microscopy
2.11. ELISA
3. Results
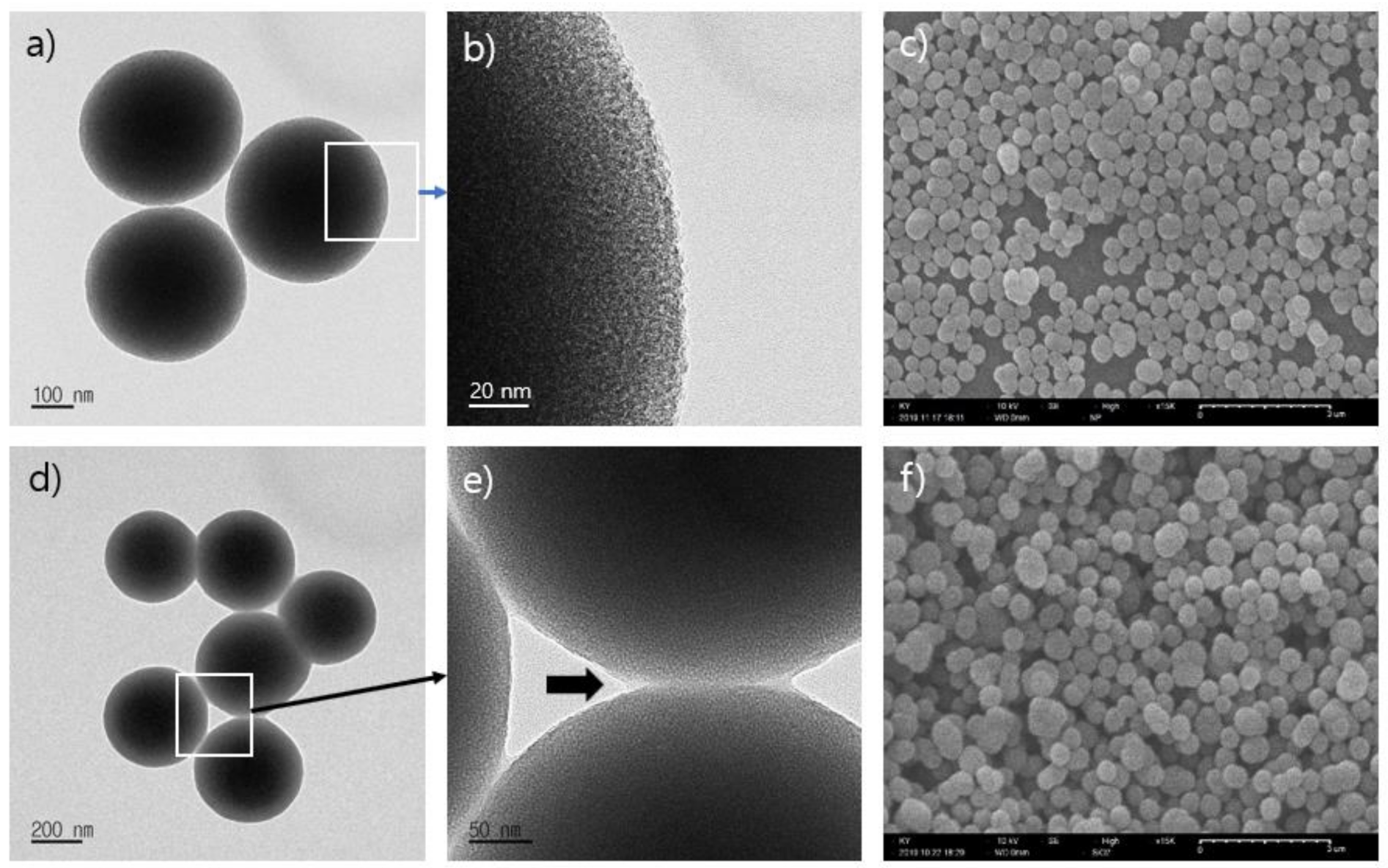
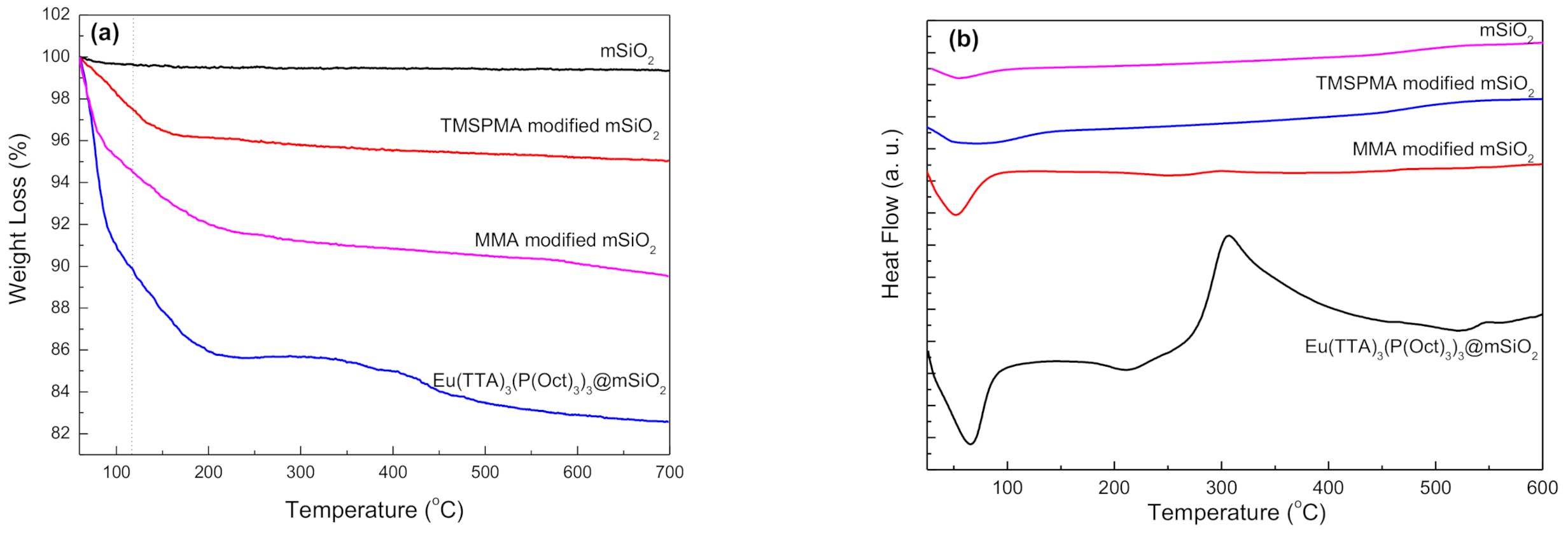
3.1. Preparation and Characterization of Eu@MSP
3.2. Photoluminescent Properties of Eu (TTA)3(P(Oct)3)3@mSiO2
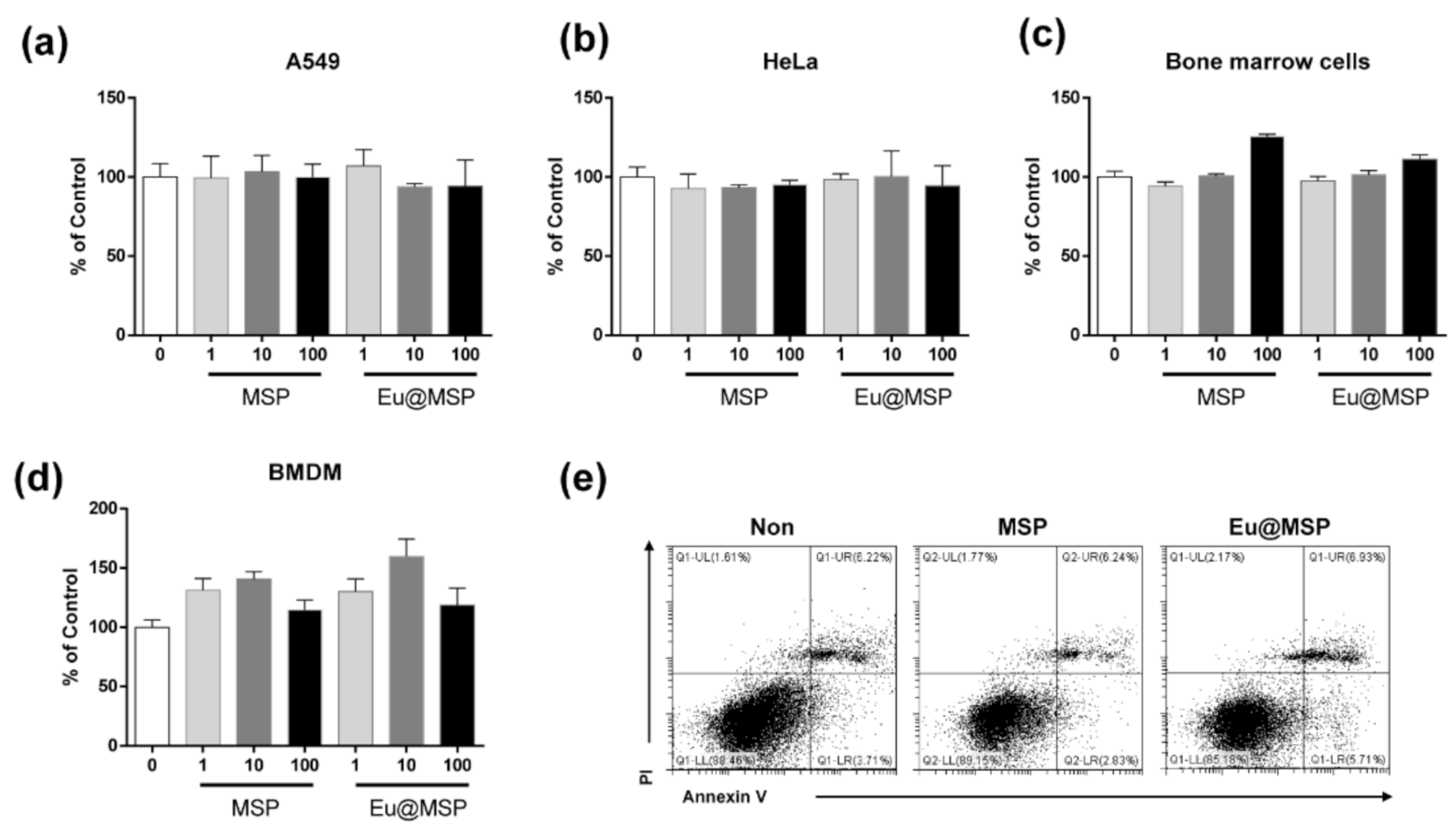
3.3. Effect of MSP and Eu@MSP on the Cytotoxicity of Various Kinds of Eukaryotic Cells
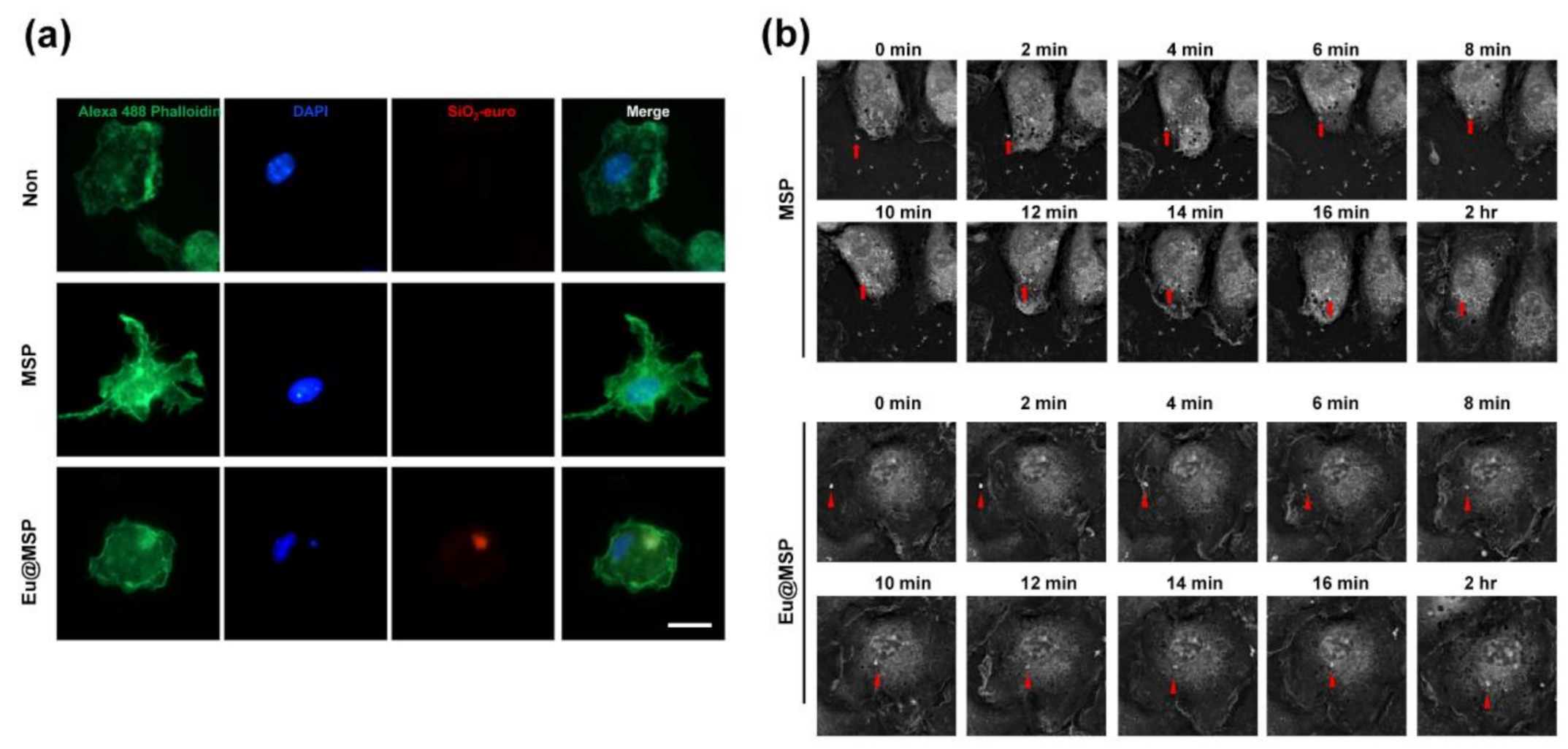
3.4. Cellular Uptake of MSP and Eu@MSP
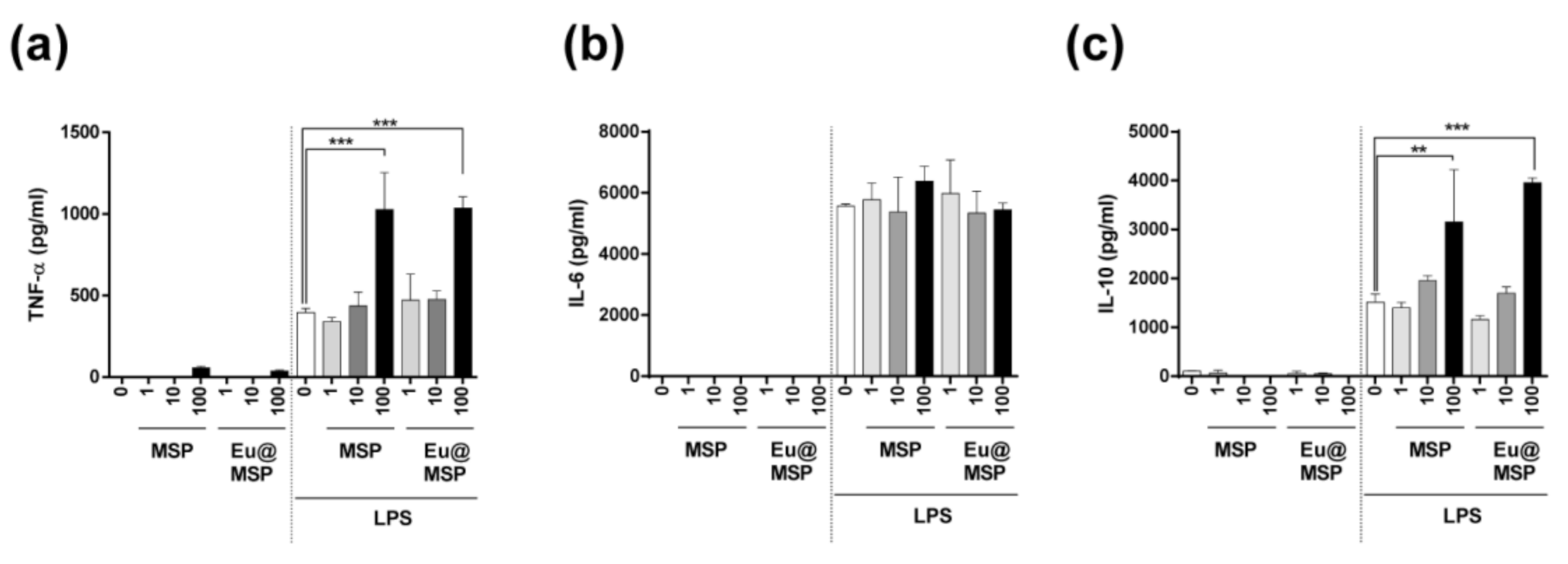
3.5. Effect of MSP and Eu@MSP on the Secretion of Cytokine by BMDMs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Z.; Li, B.; Sun, L.; Li, L.; Xu, Z.P.; Gu, Z. 2D Layered Double Hydroxide Nanoparticles: Recent Progress toward Preclinical/Clinical Nanomedicine. Small Methods 2020, 4, 1900343. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, G. Review on marine carbohydrate-based gold nanoparticles represented by alginate and chitosan for biomedical application. Carbohydr. Polym. 2020, 244, 116311. [Google Scholar] [CrossRef]
- Arsalani, S.; Oliveira, J.; Guidelli, E.J.; Araujo, J.F.D.F.; Wiekhorst, F.; Baffa, O. Synthesis of radioluminescent iron oxide nanoparticles functionalized by anthracene for biomedical applications. Coll. Surf. A Physicochem. Eng. Asp. 2020, 602, 125105. [Google Scholar] [CrossRef]
- Chen, L.; Xu, J.; Wang, Y.; Huang, R. Ultra-small MoS2 nanodots-incorporated mesoporous silica nanospheres for pH-sensitive drug delivery and CT imaging. J. Mater. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, J.; Gou, K.; Guo, Y.; Xu, X.; Li, S.; Li, H. Amino functionalized mesoporous silica with twisted rod-like shapes: Synthetic design, in vitro and in vivo evaluation for ibuprofen delivery. Microporous Mesoporous Mater. 2020, 294, 109896. [Google Scholar] [CrossRef]
- Vares, G.; Jallet, V.; Matsumoto, Y.; Rentier, C.; Takayama, K.; Sasaki, T.; Hayashi, Y.; Kumada, H.; Sugawara, H. Functionalized mesoporous silica nanoparticles for innovative boron-neutron capture therapy of resistant cancers. Nanomed. Nanotechnol. Biol. Med. 2020, 27, 102195. [Google Scholar] [CrossRef]
- Li, H.; Li, K.; Dai, Y.; Xu, X.; Cao, X.; Zeng, Q.; He, H.; Pang, L.; Liang, J.; Chen, X.; et al. In vivo near infrared fluorescence imaging and dynamic quantification of pancreatic metastatic tumors using folic acid conjugated biodegradable mesoporous silica nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1867–1877. [Google Scholar] [CrossRef]
- Giménez, C.; de la Torre, C.; Gorbe, M.; Aznar, E.; Sancenón, F.; Murguía, J.R.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P. Gated Mesoporous Silica Nanoparticles for the Controlled Delivery of Drugs in Cancer Cells. Langmuir 2015, 31, 3753–3762. [Google Scholar] [CrossRef]
- Szewczyk, A.; Prokopowicz, M. Mesoporous silica pellets—A promising oral drug delivery system? J. Drug Deliv. Sci. Technol. 2020, 56, 101491. [Google Scholar] [CrossRef]
- Flynn, J.; Mallen, S.; Durack, E.; O’Connor, P.M.; Hudson, S.P. Mesoporous matrices for the delivery of the broad spectrum bacteriocin, nisin A. J. Colloid Interface Sci. 2019, 537, 396–406. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Yang, D.; Liang, H.; Wang, Y. Luminescence enhancement of Europium(III) complexes by an ionic liquid. J. Lumin. 2019, 215, 116610. [Google Scholar] [CrossRef]
- Ortiz-Gómez, I.; Ramírez-Rodríguez, G.B.; Capitán-Vallvey, L.F.; Salinas-Castillo, A.; Delgado-López, J.M. Highly stable luminescent europium-doped calcium phosphate nanoparticles for creatinine quantification. Colloids Surf. B Biointerfaces 2020, 111337. [Google Scholar] [CrossRef]
- Yefimova, S.L.; Tkacheva, T.N.; Maksimchuk, P.O.; Bespalova, I.I.; Hubenko, K.O.; Klochkov, V.K.; Sorokin, A.V.; Malyukin, Y.V. GdVO4:Eu3+ nanoparticles—Methylene Blue complexes for PDT: Electronic excitation energy transfer study. J. Lumin. 2017, 192, 975–981. [Google Scholar] [CrossRef]
- Groult, H.; Ruiz-Cabello, J.; Pellico, J.; Lechuga-Vieco, A.V.; Bhavesh, R.; Zamai, M.; Almarza, E.; Martín-Padura, I.; Cantelar, E.; Martínez-Alcázar, M.P.; et al. Parallel Multifunctionalization of Nanoparticles: A One-Step Modular Approach for in Vivo Imaging. Bioconjug. Chem. 2015, 26, 153–160. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, W.; Huang, P.; Tu, D.; Zhou, S.; Huang, M.; Chen, X. Lanthanide-doped luminescent nano-bioprobes for the detection of tumor markers. Nanoscale 2015, 7, 4274–4290. [Google Scholar] [CrossRef]
- Bottrill, M.; Kwok, L.; Long, N.J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 2006, 35, 557–571. [Google Scholar] [CrossRef]
- Zheng, Q.; Dai, H.; Merritt, M.E.; Malloy, C.; Pan, C.Y.; Li, W.-H. A New Class of Macrocyclic Lanthanide Complexes for Cell Labeling and Magnetic Resonance Imaging Applications. J. Am. Chem. Soc. 2005, 127, 16178–16188. [Google Scholar] [CrossRef]
- Heerschap, A.; Sommers, M.G.; in ‘t Zandt, H.J.A.; Renema, W.K.J.; Veltien, A.A.; Klomp, D.W.J. Nuclear Magnetic Resonance in Laboratory Animals. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2004; Volume 385, pp. 41–63. [Google Scholar]
- Bao, G. Lanthanide complexes for drug delivery and therapeutics. J. Lumin. 2020. [Google Scholar] [CrossRef]
- Wang, S.; Wei, Z.; Li, L.; Ning, X.; Liu, Y. Luminescence imaging-guided triple-collaboratively enhanced photodynamic therapy by bioresponsive lanthanide-based nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102265. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Li, X.; Zhang, J.; Sun, J.; Xia, H.; Hua, R.; Chen, B. Fabrication, photothermal conversion and temperature sensing of novel nanoplatform-hybrid nanocomposite of NaYF4:Er3+,Yb3+@NaYF4 and Au nanorods for photothermal therapy. Mater. Res. Bull. 2019, 114, 148–155. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Zhang, H.; Fu, L.; Guo, H.; Yu, J.; Carlos, L.D.; Yang, K. Preparation and luminescence properties of covalent linking of luminescent ternary europium complexes on periodic mesoporous organosilica. Microporous Mesoporous Mater. 2008, 116, 28–35. [Google Scholar] [CrossRef]
- Iconaru, S.-L.; Motelica-Heino, M.; Predoi, D. Study on Europium-Doped Hydroxyapatite Nanoparticles by Fourier Transform Infrared Spectroscopy and Their Antimicrobial Properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Massuyeau, F.; Constantin, L.V.; Costescu, A.; Predoi, D. Synthesis, Structure, and Luminescent Properties of Europium-Doped Hydroxyapatite Nanocrystalline Powders. J. Nanomater. 2012, 2012, 942801. [Google Scholar] [CrossRef]
- Popa, C.; Ciobanu, C.; Iconaru, S.; Stan, M.; Dinischiotu, A.; Negrila, C.; Motelica-Heino, M.; Guegan, R.; Predoi, D. Systematic investigation and in vitro biocompatibility studies on mesoporous europium doped hydroxyapatite. Open Chem. 2014, 12, 1032–1046. [Google Scholar] [CrossRef]
- Driesen, K.; Van Deun, R.; Görller-Walrand, C.; Binnemans, K. Near-Infrared Luminescence of Lanthanide Calcein and Lanthanide Dipicolinate Complexes Doped into a Silica−PEG Hybrid Material. Chem. Mater. 2004, 16, 1531–1535. [Google Scholar] [CrossRef]
- Gutzov, S.; Danchova, N.; Kirilova, R.; Petrov, V.; Yordanova, S. Preparation and luminescence of silica aerogel composites containing an europium (III) phenanthroline nitrate complex. J. Lumin. 2017, 183, 108–112. [Google Scholar] [CrossRef]
- Petkova, N.; Gutzov, S.; Lesev, N.; Kaloyanova, S.; Stoyanov, S.; Deligeorgiev, T. Preparation and optical properties of silica gels doped with a new Eu(III) complex. Opt. Mater. 2011, 33, 1715–1720. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.S.; Hobson, D.; Hydbring, P. Nanoparticles for immune system targeting. Drug Discov. Today 2017, 22, 1295–1301. [Google Scholar] [CrossRef]
- Reichel, D.; Tripathi, M.; Perez, J.M. Biological Effects of Nanoparticles on Macrophage Polarization in the Tumor Microenvironment. Nanotheranostics 2019, 3, 66–88. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.K. Highly luminescent and long-term anti-photobleaching Eu(TTA)3(TOP)3 conjugated poly(St-co-DVB-co-NaSS-co-MAA) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 162–171. [Google Scholar] [CrossRef]
- Kim, J.-S.; Cha, S.-H.; Kim, W.S.; Han, S.J.; Cha, S.B.; Kim, H.M.; Kwon, K.W.; Kim, S.J.; Choi, H.-H.; Lee, J.; et al. A Novel Therapeutic Approach Using Mesenchymal Stem Cells to Protect Against Mycobacterium abscessus. Stem Cells 2016, 34, 1957–1970. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Lee, S.K.; Doh, H.; Kim, M.Y.; Kim, D.K. Real-Time Tracking of Highly Luminescent Mesoporous Silica Particles Modified with Europium β-Diketone Chelates in Living Cells. Nanomaterials 2021, 11, 343. https://doi.org/10.3390/nano11020343
Kim J-S, Lee SK, Doh H, Kim MY, Kim DK. Real-Time Tracking of Highly Luminescent Mesoporous Silica Particles Modified with Europium β-Diketone Chelates in Living Cells. Nanomaterials. 2021; 11(2):343. https://doi.org/10.3390/nano11020343
Chicago/Turabian StyleKim, Jong-Seok, Sung Ki Lee, Hansol Doh, Myeong Yun Kim, and Do Kyung Kim. 2021. "Real-Time Tracking of Highly Luminescent Mesoporous Silica Particles Modified with Europium β-Diketone Chelates in Living Cells" Nanomaterials 11, no. 2: 343. https://doi.org/10.3390/nano11020343
APA StyleKim, J.-S., Lee, S. K., Doh, H., Kim, M. Y., & Kim, D. K. (2021). Real-Time Tracking of Highly Luminescent Mesoporous Silica Particles Modified with Europium β-Diketone Chelates in Living Cells. Nanomaterials, 11(2), 343. https://doi.org/10.3390/nano11020343





