Comparative Study of the Photocatalytic Hydrogen Evolution over Cd1−xMnxS and CdS-β-Mn3O4-MnOOH Photocatalysts under Visible Light
Abstract
:1. Introduction
2. Experimental Section
2.1. Photocatalyst Synthesis
2.2. Catalyst Characterization
2.3. Photocatalytic Tests
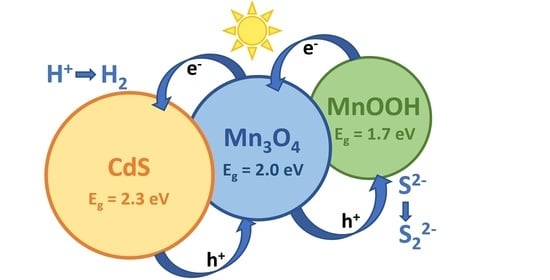
3. Results and Discussion
3.1. Photocatalyst Characterization
3.2. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Zäch, M. Nanostructured materials for photocatalytic hydrogen production. Curr. Opin. Colloid Interface Sci. 2009, 4, 260–269. [Google Scholar] [CrossRef]
- Luciani, G.; Imparato, C.; Vitiello, G. Photosensitive Hybrid Nanostructured Materials: The Big Challenges for Sunlight Capture. Catalysts 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Kozlova, E.A.; Parmon, V.N. Heterogeneous semiconductor photocatalysts for hydrogen production from aqueous solutions of electron donors. Russ. Chem. Rev. 2017, 9, 870–906. [Google Scholar] [CrossRef]
- Zamaraev, K.I.; Parmon, V.N. Potential methods and perspectives of solar energy conversion via photocatalytic processes. Catal. Rev. 1980, 22, 261–324. [Google Scholar] [CrossRef]
- Parmon, V.N.; Tributsch, H.; Bridgwater, A.V.; Hall, D.O. Chemistry for the Energy Future; Blackwell Science: Oxford, UK, 1999; p. 256. [Google Scholar]
- Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; Andreozzi, R.; et al. Near UV-Irradiation of CuOx -Impregnated TiO2 Providing Active Species for H2 Production Through Methanol Photoreforming. ChemCatChem 2019, 11, 4314–4326. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Sun, W.; Lei, Y.; Wang, Q.; Li, A.; Chen, W.; Zhou, G.; Zhang, Z.; Wang, Y.; et al. Engineering the Atomic Interface with Single Platinum Atoms for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2020, 59, 1295. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Parmon, V.N. Semiconductor Photocatalysts Based on Nanostructured Cd1 − xZnxS Solid Solutions in the Reaction of Hydrogen Evolution From Aqueous Solutions of Inorganic Electron Donors Under Visible Light. In Advanced Nanomaterials for Catalysis and Energy; Sadykov, V.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 357–391. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Kozlova, E.A.; Gerasimov, E.Y.; Rempel, A.A.; Gusev, A.I. Enhanced photocatalytic hydrogen evolution from aqueous solutions on Ag2S/Ag heteronanostructure. Int. J. Hydrog. Energy 2017, 42, 25258–25266. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Pallotti, D.K.; Silvestri, B.; Nadagouda, M.; Lettieri, S.; Luciani, G.; Andreozzi, R.; Maddalena, P.; Marotta, R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J. Hydrog. Energy 2017, 42, 28349–28362. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef] [Green Version]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Reber, J.F.; Rusek, M. Photochemical hydrogen production with platinized suspensions of cadmium sulfide and cadmium zinc sulfide modified by silver sulfide. J. Phys. Chem. US 1986, 90, 824–834. [Google Scholar] [CrossRef]
- Dong, G.; Wang, H.; Yan, Z.; Zhang, J.; Ji, X.; Lin, M.; Dahlgren, R.A.; Shang, X.; Zhang, M.; Chen, Z. Cadmium sulfide nanoparticles-assisted intimate coupling of microbial and photoelectrochemical processes: Mechanisms and environmental applications. Sci. Total Environ. 2020, 740, 140080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yoan, X.; Si, M.; Jiang, L.; Yu, H. Core-shell structured cadmium sulfide nanocomposites for solar energy utilization. Adv. Colloid Interfac. 2020, 282, 102209. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wen, M.; Zhou, C.; Li, Z.; Cheng, M.; Chen, S.; Xue, W.; Lei, L.; Yang, Y.; Xiong, W.; et al. ZnxCd1 − xS based materials for photocatalytic hydrogen evolution, pollutants degradation and carbon dioxide reduction. Appl. Catal. B Environ. 2020, 267, 1186512. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; He, X.; Zhang, B.; Song, B.; Li, S.; You, W. L-cystine-assisted hydrothermal synthesis of Mn1 − xCdxS solid solutions with hexagonal wurtzite structure for efficient photocatalytic hydrogen evolution under visible light irradiation. J. Mater. Chem. A 2014, 2, 4619–4626. [Google Scholar] [CrossRef]
- Ikeue, K.; Shiiba, S.; Machida, M. Novel visible-light-driven photocatalyst based on Mn−Cd−S for efficient H2 evolution. Chem. Mater. 2010, 22, 743–745. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, G.; Ma, Q.; Jin, Z. Amorphous NiCoB nanoalloy modified Mn0.05Cd0.95S for photocatalytic hydrogen evolution. Mol. Catal. 2020, 492, 111001. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.; Wang, P.; Liu, Y.; Huang, B.; Rozhkova, E.A.; Zhang, Q.; Wang, Z.; Dai, Y.; Lu, J. Efficient photocatalytic H2 production via rational design of synergistic spatially-separated dual cocatalysts modified Mn0.5Cd0.5S photocatalyst under visible light irradiation. Chem. Eng. J. 2018, 337, 480–487. [Google Scholar] [CrossRef]
- Dan, M.; Xiang, J.; Yang, J.; Wu, F.; Han, C.; Zhong, Y.; Zheng, K.; Yu, S.; Zhou, Y. Beyond hydrogen production: Solar−driven H2S−donating value−added chemical production over MnxCd1 − xS/CdyMn1 − yS catalyst. Appl. Catal. B Environ. 2021, 284, 119706. [Google Scholar] [CrossRef]
- Dai, D.; Wang, L.; Xiao, N.; Li, S.; Xu, H.; Liu, S.; Xu, B.; Lv, D.; Gao, Y.; Song, W.; et al. In-situ synthesis of Ni2P co-catalyst decorated Zn0.5Cd0.5S nanorods for high-quantum-yield photocatalytic hydrogen production under visible light irradiation. Appl. Catal. B Environ. 2018, 233, 194–201. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; He, Y.; Meng, S.; Xu, Y.; Chen, S.; Fu, X. Rational synthesis of MnxCd1 − xS for enhanced photocatalytic H2 evolution: Effects of S precursors and the feed ratio of Mn/Cd on its structure and performance. J. Colloid Interf. Sci. 2019, 535, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, Y.; Ma, L.; Jing, D.; Guo, L. Manganese doped cadmium sulfide nanocrystal for hydrogen production from water under visible light. Int. J. Hydrog. Energ. 2012, 37, 730–736. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Cherepanova, S.V.; Markovskaya, D.V.; Saraev, A.A.; Gerasimov, E.Y.; Parmon, V.N. Novel photocatalysts Pt/Cd1−xZnxS/ZnO/Zn(OH)2: Activation during hydrogen evolution from aqueous solutions of ethanol under visible light. Appl. Catal. B Environ. 2016, 183, 197–205. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Kozlova, E.A.; Cherepanova, S.V.; Saraev, A.A.; Gerasimov, E.Y.; Parmon, V.N. Synthesis of Pt/Zn(OH)2/Cd0.3Zn0.7S for the photocatalytic hydrogen evolution from aqueous solutions of organic and inorganic electron donors under visible light. Top. Catal. 2016, 59, 1297–1304. [Google Scholar] [CrossRef]
- Xue, L.; Peters, J.C.P.R.; Dhanabalan, S.C.; Madhaiyan, J.; Manavalan, R.K.; Ponraj, J.S. Realisation of CdS/Mn3O4 nanocomposites for potential photocatalytic applications. Micro Nano Lett. 2020, 15, 742–745. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Gribov, E.N.; Kurenkova, A.Y.; Cherepanova, S.V.; Gerasimov, E.Y.; Kozlov, D.V. Synthesis of multiphase Au/Cd0.6Zn0.4S/ZnS photocatalysts for improved photocatalytic performance. Int. J. Hydrog. Energ. 2019, 44, 23589–23599. [Google Scholar] [CrossRef]
- Stavitskaya, A.V.; Kozlova, E.A.; Kurenkova, A.Y.; Glotov, A.P.; Selischev, D.S.; Ivanov, E.V.; Kozlov, D.V.; Vinokurov, V.A.; Fakhrullin, R.F.; Lvov, Y.M. Ru/CdS quantum dots templated on clay nanotubes as visible-light-active photocatalysts: Optimization of S/Cd ratio and Ru content. Chem. Eur. J. 2020, 26, 13085. [Google Scholar] [CrossRef]
- Liu, X.; Liang, X.; Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y. Highly efficient and noble metal-free NiS modified MnxCd1 − xS solidsolutions with enhanced photocatalytic activity for hydrogen evolution under visible light irradiation. Appl. Catal. B 2017, 203, 282–288. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin—Elmer Corp.: Eden Prairie, MN, USA, 1992; p. 261. [Google Scholar]
- Kozlova, E.A.; Lyulyukin, M.N.; Markovskaya, D.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Cherepanova, S.V.; Kozlov, D.V. Photocatalytic CO2 Reduction Over Ni-Modified Cd1 − xZnxS-Based Photocatalysts: Effect of Phase Composition of Photocatalyst and Reaction Media on Reduction Rate and Product Distribution. Top. Catal. 2020, 63, 121–129. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Tiwari, P.; Malik, G.; Chandra, R. Phase-dependent structural and electrochemical properties of single crystalline MnS thin films deposited by DC reactive sputtering. J. Appl. Phys. 2018, 124, 195106. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Zeng, P.; Lio, J.; Wang, J.; Peng, T. One-pot hydrothermal synthesis of MoS2-modified Mn0.5Cd0.5S solid solution for boosting H2 production activity under visible light. Catal. Sci. Technol. 2019, 9, 762–771. [Google Scholar] [CrossRef]
- Chen, R.; Ao, Y.; Wang, C.; Wang, P. 2D ultrathin CoP modified MnxCd1 − xS with controllable band structure and robust photocatalytic performance for hydrogen generation. Dalton Trans. 2019, 48, 14783–14791. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900. [Google Scholar] [CrossRef]
- Wu, P.; Dai, S.; Chen, G.; Zhao, S.; Xu, Z.; Fu, M.; Chen, P.; Chen, Q.; Jin, X.; Qiu, Y.; et al. Interfacial effects in hierarchically porous α-MnO2/Mn3O4 heterostructures promote photocatalytic oxidation activity. Appl. Catal. B 2020, 268, 118418. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zhang, J.; Zuo, W.; Ding, Y. Efficient removal of chromium from water by Mn3O4@ ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Appl. Catal. B 2017, 214, 126–136. [Google Scholar] [CrossRef]
- Li, N.; He, M.; Lu, X.; Liang, L.; Li, R.; Yan, B.; Chen, G. Enhanced norfloxacin degradation by visible-light-driven Mn3O4/γ-MnOOH photocatalysis under weak magnetic field. Sci. Total Environ. 2020, 143268, in press. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, X.; Wang, P.; Huang, B.; Zhang, Q. Enhanced photocatalytic H2 production of Mn0.5Cd0.5S solid solution through loading transition metal sulfides XS (X = Mo, Cu, Pd) cocatalysts. Appl. Surf. Sci. 2018, 430, 515–522. [Google Scholar] [CrossRef]








| Sample | Cd1−xMnxS | Phase Composition | Eg, eV | W, μmol H2 min−1 | AQE, % | ||
|---|---|---|---|---|---|---|---|
| CS 1, nm | a 2, Å | x | |||||
| Mnx Series | |||||||
| Mn0.0 (CdS) | 7.1 | 5.84 | 0.00 | CdS | 2.25 | 0.02 | 0.12 |
| Mn0.05 | 13 | 5.83 | 0.03 | Cd0.97Mn0.03S | 2.30 | 0.04 | 0.22 |
| Mn0.1 | 8.3 | 5.82 | 0.07 | Cd0.93Mn0.07S | 2.31 | 0.04 | 0.22 |
| Mn0.2 | 8.4 | 5.80 | 0.20 | Cd0.80Mn0.20S | 2.35 | 0.09 | 0.52 |
| Mn0.4 | 5.2 | 5.77 | 0.35 | Cd0.65Mn0.35S | 2.41 | 0.41 | 2.34 |
| Mn0.6 | 4.9 | 5.77 | 0.35 | Cd0.65Mn0.35S-MnS (cub) | 2.38 | 0.37 | 2.10 |
| Mn0.8 | 4.3 | 5.77 | 0.35 | Cd0.65Mn0.35S-Mn0.92Cd0.08S | - | 0.21 | 1.20 |
| 10 | 5.66 | 0.92 | |||||
| Mn1.0 | 37 | - | 1.0 | MnS (hex) | - | 0.01 | 0.06 |
| Mnx(NaOH) Series | |||||||
| Mn0.0 (CdS) | 6.1 | 5.84 | 0.00 | CdS | 2.29 | 0.01 | 0.06 |
| Mn0.05 | 5.9 | 5.83 | 0.05 | Cd0.95Mn0.05S | 2.28 | 0.03 | 0.16 |
| Mn0.1 | 6.5 | 5.83 | 0.04 | Cd0.96Mn0.04S | 2.30 | 0.04 | 0.22 |
| Mn0.2 | 5.7 | 5.83 | 0.03 | Cd0.97Mn0.03S | 2.30 | 0.05 | 0.28 |
| Mn0.4 | 7.1 | 5.83 | 0.02 | Cd0.98Mn0.02S- β-Mn3O4-MnOOH | - | 0.35 0.51 3 | 2.00 2.91 3 |
| Mn0.6 | 6.4 | 5.84 | 0.02 | Cd0.98Mn0.02S- β-Mn3O4-MnOOH | - | 0.44 | 2.50 |
| Mn0.8 | 7.5 | 5.83 | 0.04 | Cd0.96Mn0.04S- β-Mn3O4 | - | 0.34 | 2.00 |
| Mn1.0 | - | - | - | β-Mn3O4 | - | 0 | 0 |
| Sample | [Mn]/ [Mn+Cd] | [S]/ [Mn+Cd] | [O]/ [Mn+Cd] | [MnS]/ [MnOx] | MnS, % | Sulfur Distribution, % | ||
|---|---|---|---|---|---|---|---|---|
| S2− | Oxy-Sulfide | SO42− | ||||||
| Mn0.4(NaOH) | 0.22 | 0.47 | 1.21 | 0.50 | 33.3 | 71.5 | 19.6 | 8.9 |
| Mn0.4(NaOH) 1 | 0.17 | 0.77 | 0.75 | 0.65 | 39.4 | 80.3 | 12.2 | 7.5 |
| Mn0.4 | 0.15 | 0.95 | 0.39 | 0.51 | 34.0 | 73.3 | 22.2 | 4.5 |
| № | Photocatalyst | Synthesis | Light Source | Cut-off Filter | Electron Donor | W, μmol h−1 g−1 | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Cd0.65Mn0.35S | Hydrothermal synthesis; Na2S | 450 nm LED | Na2S/ Na2SO3 | 444 | Current study | |
| 2 | CdS-β-Mn3O4-MnOOH | 600 | |||||
| 3 | Cd0.5Mn0.5S | Hydrothermal synthesis; L-Cysteine | 300 W Xe lamp | λ > 420 nm | Na2S/ Na2SO3 | 625 | [20] |
| 4 | 3%MoS2/Cd0.5Mn0.5S | 3950 | |||||
| 5 | Cu2−xS/Cd0.5Mn0.5S | 8090 | |||||
| 6 | Cu2−xS/Cd0.5Mn0.5S/3%MoS2 | 13800 | |||||
| 7 | Mn0.05Cd0.95S | Hydrothermal synthesis; thioacetamide | 300 W Xe lamp | λ > 420 nm | Na2S/ Na2SO3 | 1400 | [19] |
| 8 | NiCoB/Mn0.05Cd0.95S | 10500 | |||||
| 9 | Cd0.5Mn0.5S | Hydrothermal synthesis; L-Cysteine | 300 W Xe lamp | λ > 420 nm | Na2S/ Na2SO3 | 451 | [30] |
| 10 | 1%Pt/Cd0.5Mn0.5S | 2700 | |||||
| 11 | 0.3%NiS/Cd0.5Mn0.5S | 8390 | |||||
| 12 | Cd0.5Mn0.5S (L-Cysteine) | Hydrothermal synthesis | 300 W Xe lamp | λ > 420 nm | lactic acid | 444 | [23] |
| 13 | Cd0.5Mn0.5S (thioacetamide) | 1792 | |||||
| 14 | Cd0.5Mn0.5S (thiourea) | 178 | |||||
| 15 | Cd0.5Mn0.5S | Hydrothermal synthesis; L-Cysteine | 300 W Xe lamp | λ > 420 nm | Na2S/ Na2SO3 | 646 | [43] |
| 16 | 0.25% MoS2/Cd0.5Mn0.5S | 3940 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapenko, K.O.; Kurenkova, A.Y.; Bukhtiyarov, A.V.; Gerasimov, E.Y.; Cherepanova, S.V.; Kozlova, E.A. Comparative Study of the Photocatalytic Hydrogen Evolution over Cd1−xMnxS and CdS-β-Mn3O4-MnOOH Photocatalysts under Visible Light. Nanomaterials 2021, 11, 355. https://doi.org/10.3390/nano11020355
Potapenko KO, Kurenkova AY, Bukhtiyarov AV, Gerasimov EY, Cherepanova SV, Kozlova EA. Comparative Study of the Photocatalytic Hydrogen Evolution over Cd1−xMnxS and CdS-β-Mn3O4-MnOOH Photocatalysts under Visible Light. Nanomaterials. 2021; 11(2):355. https://doi.org/10.3390/nano11020355
Chicago/Turabian StylePotapenko, Ksenia O., Anna Yu. Kurenkova, Andrey V. Bukhtiyarov, Evgeny Yu. Gerasimov, Svetlana V. Cherepanova, and Ekaterina A. Kozlova. 2021. "Comparative Study of the Photocatalytic Hydrogen Evolution over Cd1−xMnxS and CdS-β-Mn3O4-MnOOH Photocatalysts under Visible Light" Nanomaterials 11, no. 2: 355. https://doi.org/10.3390/nano11020355