Oxygen-Deficient Stannic Oxide/Graphene for Ultrahigh-Performance Supercapacitors and Gas Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
2.4. Gas-Sensing Measurements
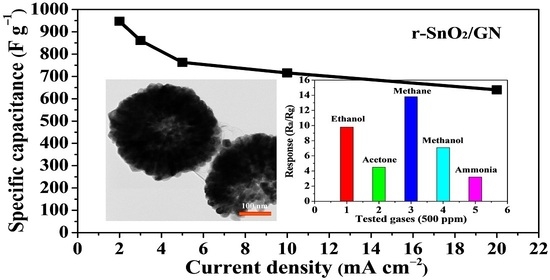
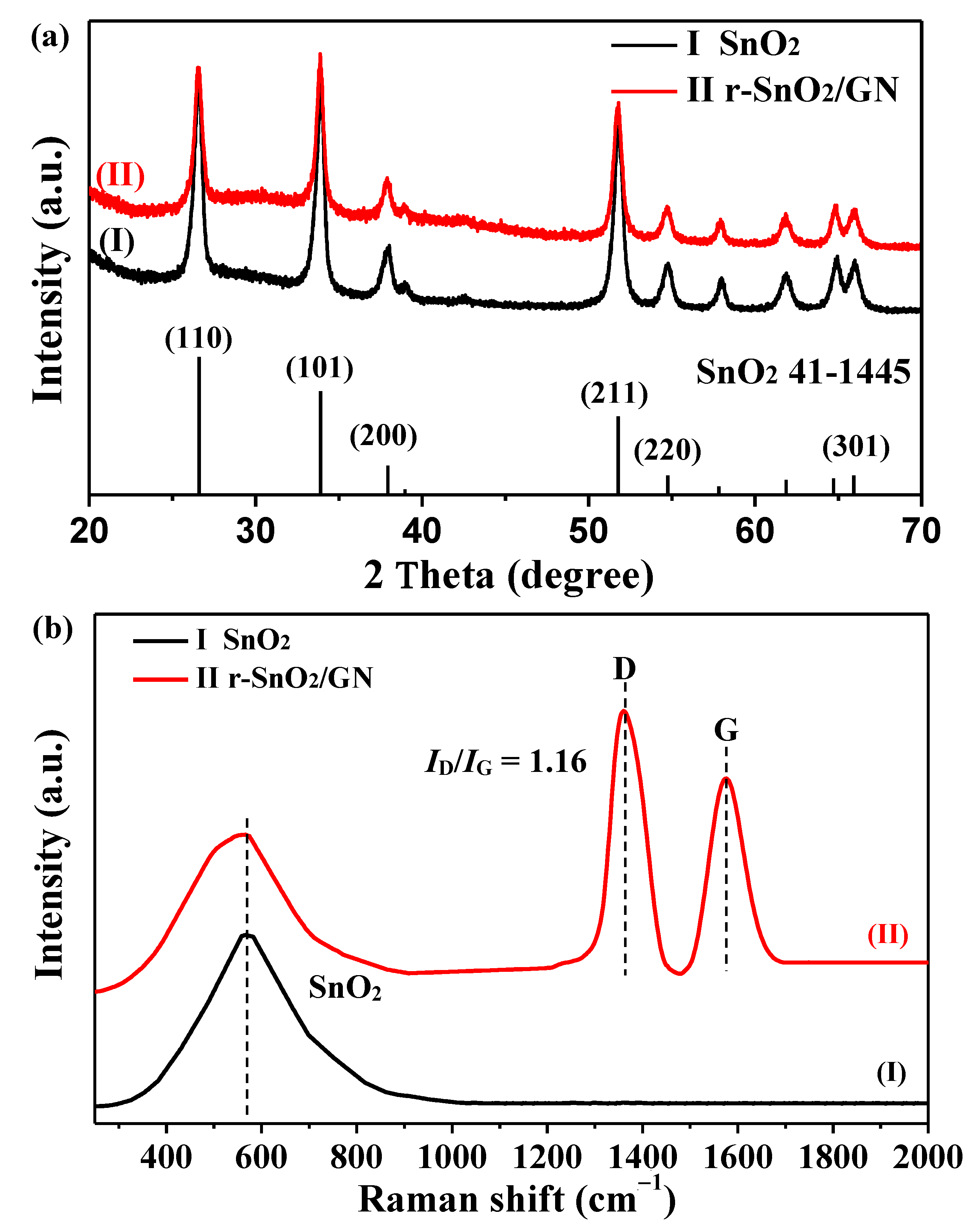
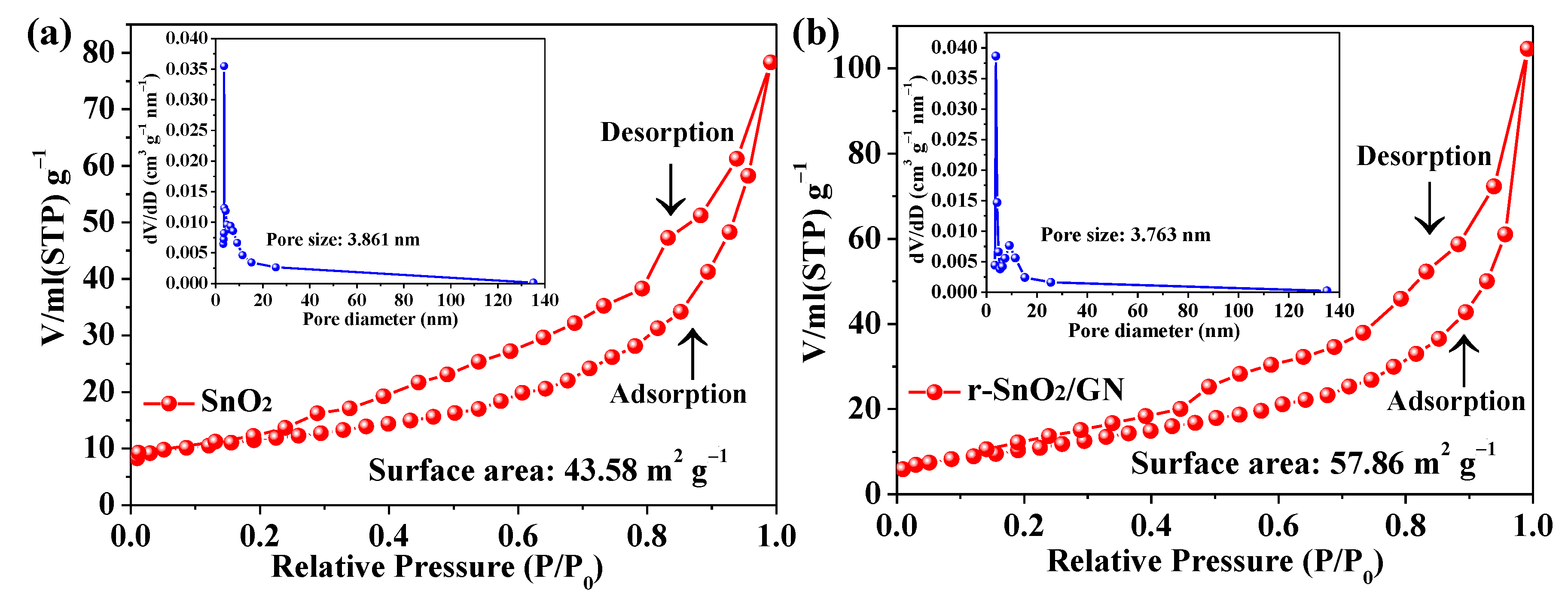
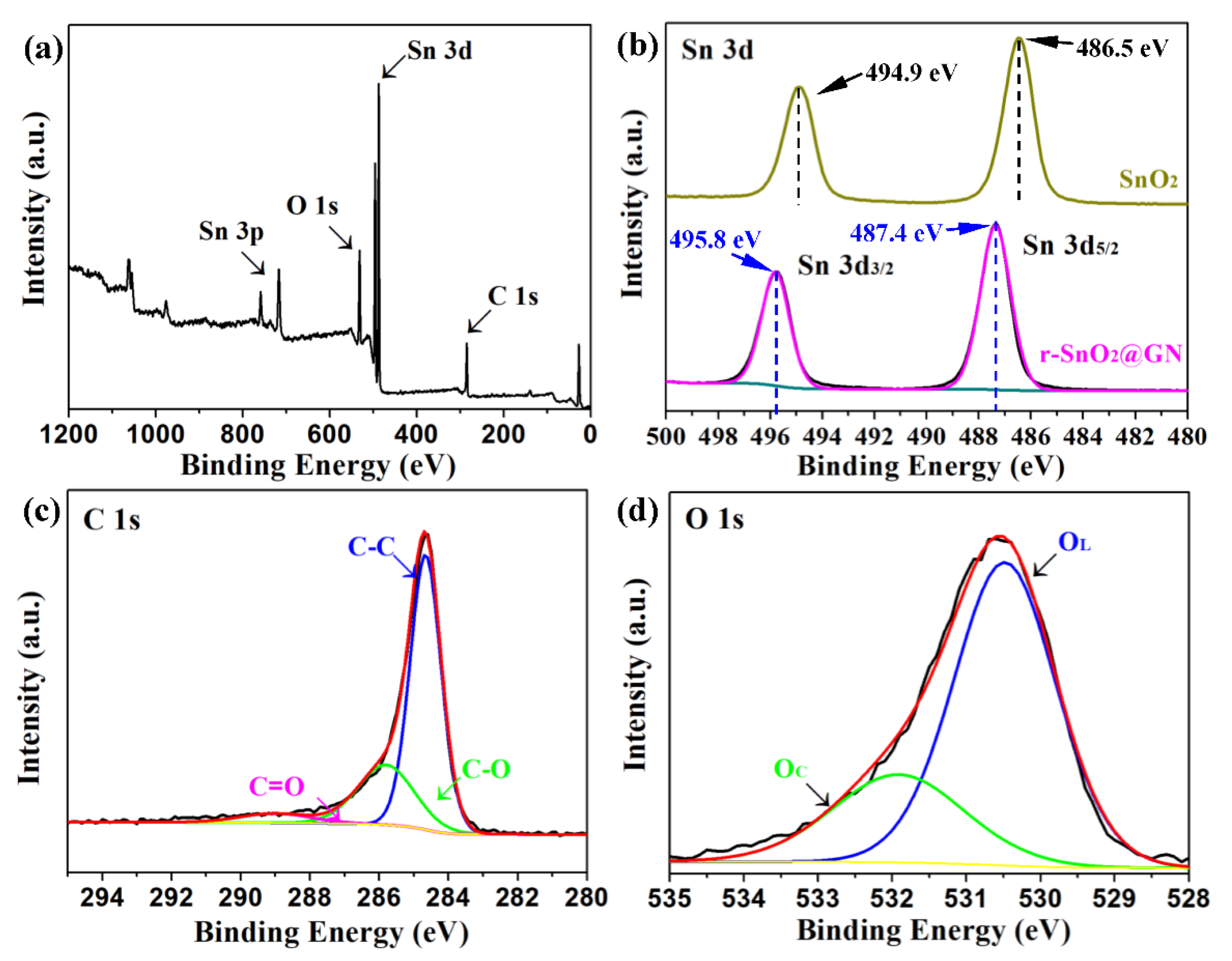
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wrapp, D.; Wang, N.S.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike In the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chen, J.; Ji, X.; Pollard, T.P.; Lü, X.J.; Sun, C.J.; Hou, S.; Liu, Q.; Liu, C.M.; Qing, T.T.; et al. Aqueous Li-Ion Battery Enabled by Halogen Conversion-Intercalation Chemistry in Graphite. Nature 2019, 569, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B. Bottled Lightning: Superbatteries, Electric Cars, and the New Lithium Economy. Nature 2011, 473, 448–449. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.L.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, 969. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Tollefson, J. Big Prize for Methane Probe. Nature 2018, 556, 283. [Google Scholar] [CrossRef]
- Le, T.H.; Wang, Y.; Liu, L.; Yang, J.N.; Yung, Y.L.; Li, G.H.; Seinfeld, J.H. Unexpected Air Pollution with Marked Emission Reductions During the COVID-19 Outbreak in China. Science 2020, 369, 702–706. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.S.; Hyeon, T.; et al. A Graphene-Based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Hong, Y.N.; Lam, J.W.Y. Aggregation-Induced Emission: Phenomenon, Mechanism and Applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable Carbon Nanotube Strain Sensor for Human-Motion Detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Mannsfeld, S.C.B.; Tee, B.C.K.; Stoltenberg, R.M.; Chen, C.V.H.H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z.N. Highly Sensitive Flexible Pressure Sensors with Microstructured Rubber Dielectric Layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Z.; Zhang, X.G.; Su, L.H.; Gao, B.; Shen, L.F. Facile Synthesis and Self-assembly of Hierarchical Porous NiO Nano/Micro Spherical Superstructures for High Performance Supercapacitors. J. Mater. Chem. 2009, 19, 5772–5777. [Google Scholar] [CrossRef]
- Kim, J.H.; Zhu, K.; Yan, Y.F.; Perkins, C.L.; Frank, A.J. Microstructure and Pseudocapacitive Properties of Electrodes Constructed of Oriented NiO-TiO2 Nanotube Arrays. Nano Lett. 2010, 10, 4099–4104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, J.; Wang, Y.B.; Wei, T.; Zhang, M.L.; Jing, X.Y.; Fan, Z.J. Three-Dimensional Flower-Like and Hierarchical Porous Carbon Materials as High-Rate Performance Electrodes for Supercapacitors. Carbon 2014, 67, 119–127. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and Ethanol Sensing Characteristics of ZnO Nanowire Gas Sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D Graphene-Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for High-Performance Supercapacitor Applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Luan, Y.X.; Nie, G.D.; Zhao, X.W.; Qiao, N.; Liu, X.C.; Wang, H.; Zhang, X.N.; Chen, Y.Q.; Long, Y.Z. The Integration of SnO2 Dots and Porous Carbon Nanofibers for Flexible Supercapacitors. Electrochim. Acta 2019, 308, 121–130. [Google Scholar] [CrossRef]
- Cao, M.L.; Cheng, W.L.; Ni, X.H.; Hu, Y.; Han, G.P. Lignin-Based Multi-Channels Carbon Nanofibers @ SnO2 Nanocomposites for High-Performance Supercapacitors. Electrochim. Acta 2020, 345, 136172. [Google Scholar] [CrossRef]
- Choudhari, A.; Bhanvase, B.A.; Saharan, V.K.; Salame, P.H.; Hunge, Y. Sonochemical Preparation and Characterization of rGO/SnO2 Nanocomposite: Electrochemical and Gas Sensing Performance. Ceram. Int. 2020, 46, 11290–11296. [Google Scholar] [CrossRef]
- Rani, M.U.; Naresh, V.; Damodar, D.; Muduli, S.; Martha, S.K.; Deshpande, A.S. In-Situ Formation of Mesoporous SnO2@C Nanocomposite Electrode for Supercapacitors. Electrochim. Acta 2021, 365, 137284. [Google Scholar] [CrossRef]
- Ali, A.; Ammar, M.; Yahya, Z.; Waqas, M.; Jamal, M.A.; Salhabi, E.H.M. A Honeycomb-Like ZnO/SnO2 Nanocomposite on Nickel Foam for High-Performance Asymmetric Supercapacitors. New J. Chem. 2019, 43, 10583–10589. [Google Scholar] [CrossRef]
- Zhou, G.M.; Wang, D.W.; Li, F.; Zhang, L.L.; Li, N.; Wu, Z.S.; Wen, L.; Lu, G.Q.; Cheng, H.M. Graphene-Wrapped Fe3O4 Anode Material with Improved Reversible Capacity and Cyclic Stability for Lithium Ion Batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Xu, J.; Ruan, C.H.; Li, P.X.; Xie, Y.B. Excessive Nitrogen Doping of Tin Dioxide Banorod Array Grown on Activated Carbon Fibers Substrate for Wire-Shaped Microsupercapacitor. Chem. Eng. J. 2019, 378, 122064. [Google Scholar] [CrossRef]
- Abdollahi, H.; Samkan, M.; Mohajerzadeh, M.A.; Sanaee, Z.; Mohajerzadeh, S. High-Performance Tin-Oxide Supercapacitors Using Hydrazine Functionalising Assisted by Hydrogen Plasma Treatment. Micro Nano Lett. 2019, 14, 1268–1273. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.D.; Liu, Z.H.; Yang, Y.; Sui, J.H.; Wang, H.E.; Cai, W.; Cao, G.Z. Synergistic Coupling of Lamellar MoSe2 and SnO2 Nanoparticles via Chemical Bonding at Interface for Stable and High-Power Sodium-Ion Capacitors. Chem. Eng. J. 2018, 354, 1164–1173. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhou, T.; Jiang, K.; Da, P.M.; Peng, Z.; Tang, J.; Kong, B.A.; Cai, W.B.; Yang, Z.Q.; Zheng, G.F. Reduced Mesoporous Co3O4 Nanowires as Efficient Water Oxidation Electrocatalysts and Supercapacitor Electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion Charge Storage through Oxygen Intercalation in LaMnO3 Perovskite Pseudocapacitor Electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef]
- Kang, Y.R.; Li, Z.; Xu, K.; He, X.J.; Wei, S.X.; Cao, Y.M. Hollow SnO2 Nanospheres with Single-Shelled Structure and the Application for Supercapacitors. J. Alloy. Comp. 2019, 779, 728–734. [Google Scholar] [CrossRef]
- Bunpang, K.; Wisitsoraat, A.; Tuantranont, A.; Singkammo, S.; Phanichphant, S.; Liewhiran, C. Highly Selective and Sensitive CH4 Gas Sensors Based on Flame-Spray-Made Cr-doped SnO2 Particulate Films. Sens. Actuators B Chem. 2019, 291, 177–191. [Google Scholar] [CrossRef]
- Bonu, V.; Das, A.; Prasad, A.K.; Krishna, N.G.; Dhara, S.; Tyagi, A.K. Influence of In-Plane and Bridging Oxygen Vacancies of SnO2 Nanostructures on CH4 Sensing at Low Operating Temperatures. Appl. Phys. Lett. 2014, 105, 245302. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a Novel Methane Gas Sensor Based on SnO2 Nanorods-Nanoporous Graphene Hybrid. Sens. Actuators B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Yadav, A.A. Spray Deposition of Tin Oxide Thin Films for Supercapacitor Applications: Effect of Solution Molarity. J. Mater. Sci. Mater. Electron. 2016, 27, 6985–6991. [Google Scholar] [CrossRef]
- Ramesh, S.; Yadav, H.M.; Lee, Y.J.; Hong, G.W.; Kathalingam, A.; Sivasamy, A.; Kim, H.S.; Kim, H.S.; Kim, J.H. Porous Materials of Nitrogen Doped Graphene Oxide@SnO2 Electrode for Capable Supercapacitor Application. Sci. Rep. 2019, 9, 12622. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Choi, S.D. Undoped and 0.1 wt.% Ca-Doped Pt-Catalyzed SnO2 Sensors for CH4 Detection. Sens. Actuators B Chem. 2005, 108, 119–124. [Google Scholar] [CrossRef]
- Wagner, T.; Bauer, M.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. X-ray Absorption Near-Edge Spectroscopy Investigation of the Oxidation State of Pd Species in Nanoporous SnO2 Gas Sensors for Methane Detection. Thin Solid Film. 2011, 520, 909–912. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, L.Y.; Wu, B.; Hu, N. Vertical Carbon Skeleton Introduced Three-Dimensional MnO2 Nanostructured Composite Electrodes for High-Performance Asymmetric Supercapacitors. J. Power Sources 2020, 476, 228527. [Google Scholar] [CrossRef]
- Lin, L.Y.; Liu, T.M.; Zhang, Y.; Liang, X.B.; Wang, Z.C. Enhancing Ethanol Detection by Heterostructural Silver Nanoparticles Decorated Polycrystalline Zinc Oxide Nanosheets. Ceram. Int. 2016, 42, 3138–3144. [Google Scholar] [CrossRef]
- Ahuja, P.; Sahu, V.; Ujjain, S.K.; Sharma, R.K.; Singh, G. Performance Evaluation of Asymmetric Supercapacitor Based on Cobalt Manganite Graphene Nanoribbons. Electrochim. Acta 2014, 146, 429–436. [Google Scholar] [CrossRef]
- Xing, M.Y.; Zhang, J.L.; Chen, F.; Tian, B.Z. An Economic Method to Prepare Vacuum Activated Photocatalysts with High Photo-Activities and Photosensitivities. Chem. Commun. 2011, 47, 4947–4949. [Google Scholar] [CrossRef] [PubMed]
- Epifani, M.; Prades, J.D.; Comini, E.; Pellicer, E.; Avella, M.; Siciliano, P.; Faglia, G.; Cirera, A.; Scotti, R.; Morazzoni, F.; et al. The Role of Surface Oxygen Vacancies in the NO2 Sensing Properties of SnO2 Nanocrystals. J. Phys. Chem. C 2008, 112, 19540–19546. [Google Scholar] [CrossRef]
- Yang, Y.J.; Wang, Y.H.; Yin, S. Oxygen Vacancies Confined in SnO2 Nanoparticles for Desirable Electronic Structure and Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2017, 420, 399–406. [Google Scholar] [CrossRef]
- Wang, R.H.; Jayakumar, A.; Xu, C.H.; Lee, J.M. Ni(OH)2 Nanoflowers/Graphene Hydrogels: A New Assembly for Supercapacitor. Acs Sustain. Chem. Eng. 2016, 4, 3736–3742. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Ghosh, M.; Ramos-Ramón, J.A.; Saha, S.; Pal, U.; Bhattacharya, S.K.; Das, S. Study on Charge Storage Mechanism in Working Electrodes Fabricated by Sol-Gel Derived Spinel NiMn2O4 Nanoparticles for Supercapacitor Application. Appl. Surf. Sci. 2019, 463, 513–525. [Google Scholar] [CrossRef]
- Xu, Y.X.; Wang, L.; Zhou, Y.F.; Guo, J.Y.; Zhang, S.Y.; Lu, Y. Synthesis of Heterostructure SnO2/Graphitic Carbon Nitride Composite for High-Performance Electrochemical Supercapacitor. J. Eelectroanal. Chem. 2019, 852, 113507. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.J.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Lu, W.B.; Ding, D.G.; Xue, Q.Z.; Du, Y.G.; Xiong, Y.; Zhang, J.Q.; Pan, X.L.; Xing, W. Great Enhancement of CH4 Sensitivity of SnO2 Based Banofibers by Heterogeneous Sensitization and Catalytic Effect. Sens. Actuators B Chem. 2018, 254, 393–401. [Google Scholar] [CrossRef]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Askarieh, M.; Carlo, A.D.; Agresti, A. Facile Synthesis of a SnO2@rGO Nanohybrid and Optimization of Its Methane-sensing Parameters. Talanta 2018, 181, 422–430. [Google Scholar] [CrossRef]
- Yao, L.J.; Li, Y.X.; Ran, Y.; Yang, Y.; Zhao, R.J.; Su, L.F.; Kong, Y.L.; Ma, D.; Chen, Y.H.; Wang, Y.D. Construction of Novel Pd–SnO2 Composite Nanoporous Structure as a High-Response Sensor for Methane Gas. J. Alloy. Comp. 2020, 826, 154063. [Google Scholar] [CrossRef]





| Electrode Materials | Electrolyte | Current Density | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|
| SnO2 | 0.5 M Na2SO4 | 1 A g−1 | 138 | [34] |
| SnO2-NGO | 6.0 M KOH | 4 A g−1 | 378 | [35] |
| SnO2/g-C3N4 | 1.0 M Na2SO4 | 1 A g−1 | 488 | [47] |
| Hollow SnO2 | 1.0 M KOH | 1 A g−1 | 332.7 | [30] |
| r-SnO2-GN | 3.0 M KOH | 2 mA cm−2 (0.57 A g−1) | 947.4 | This work |
| Sensing Materials | Temperature (°C) | CH4 Concentration | Reponses | Ref. |
|---|---|---|---|---|
| Pt-SnO2 | 400 | 1000 ppm | 1.55 b | [36] |
| Pd-SnO2 | 400 | 6600 ppm | 20 b | [37] |
| Pt-SnO2 | 350 | 1000 ppm | 4.5 b | [49] |
| SnO2-rGO | 150 | 1000 ppm | 47.6% a | [50] |
| Pd–SnO2 | 340 | 3000 ppm | 17.6 b | [51] |
| r-SnO2-GN | 140 | 1000 ppm 5000 ppm | 16.2 b 20.4 b | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Chen, S.; Deng, T.; Zeng, W. Oxygen-Deficient Stannic Oxide/Graphene for Ultrahigh-Performance Supercapacitors and Gas Sensors. Nanomaterials 2021, 11, 372. https://doi.org/10.3390/nano11020372
Lin L, Chen S, Deng T, Zeng W. Oxygen-Deficient Stannic Oxide/Graphene for Ultrahigh-Performance Supercapacitors and Gas Sensors. Nanomaterials. 2021; 11(2):372. https://doi.org/10.3390/nano11020372
Chicago/Turabian StyleLin, Liyang, Susu Chen, Tao Deng, and Wen Zeng. 2021. "Oxygen-Deficient Stannic Oxide/Graphene for Ultrahigh-Performance Supercapacitors and Gas Sensors" Nanomaterials 11, no. 2: 372. https://doi.org/10.3390/nano11020372
APA StyleLin, L., Chen, S., Deng, T., & Zeng, W. (2021). Oxygen-Deficient Stannic Oxide/Graphene for Ultrahigh-Performance Supercapacitors and Gas Sensors. Nanomaterials, 11(2), 372. https://doi.org/10.3390/nano11020372