Smart Nanomaterials for Biomedical Applications—A Review
Abstract
:1. Introduction
2. Types of Stimuli
2.1. Physical Responsive Nanomaterials
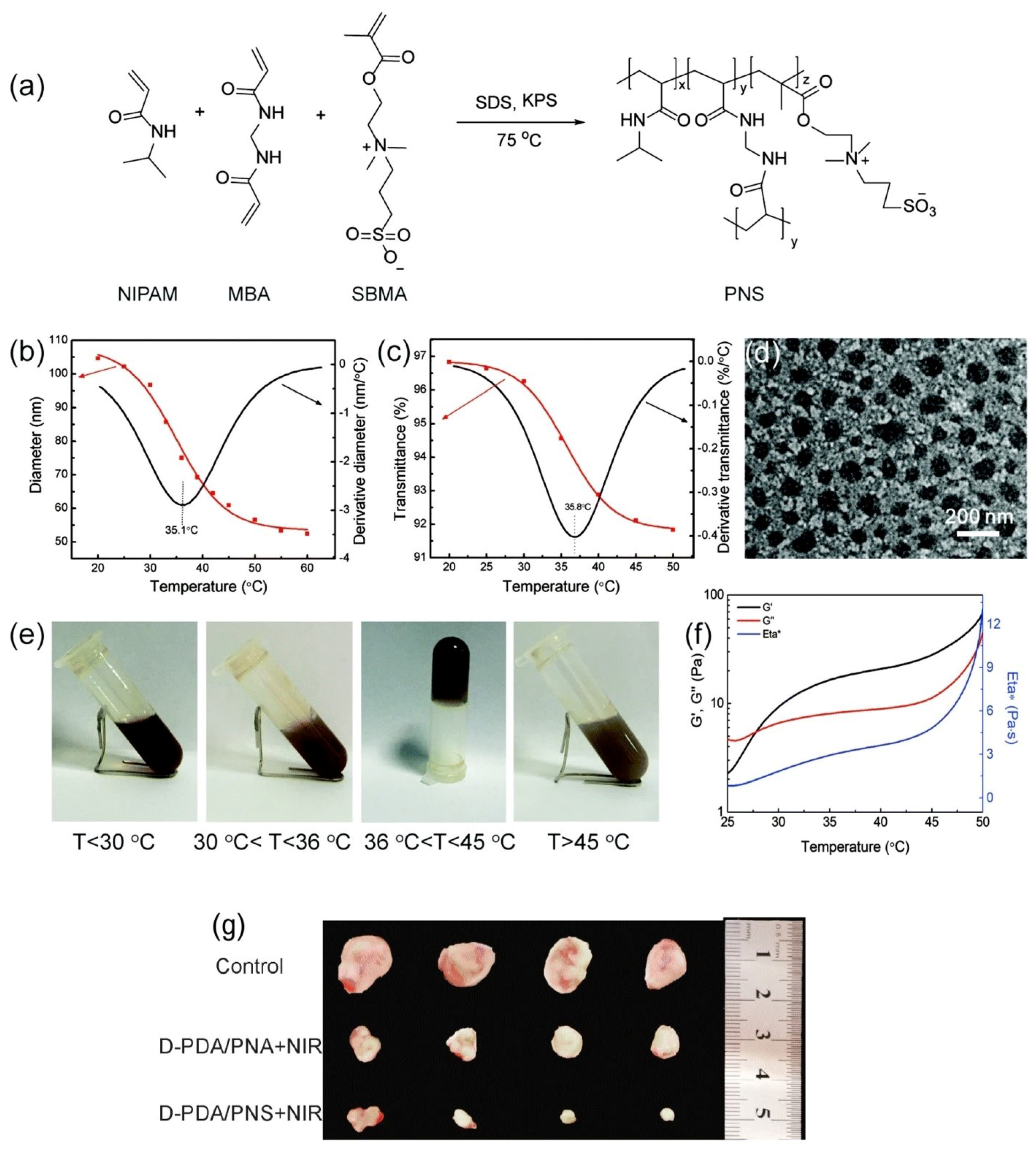
2.1.1. Temperature-Responsive Nanomaterials
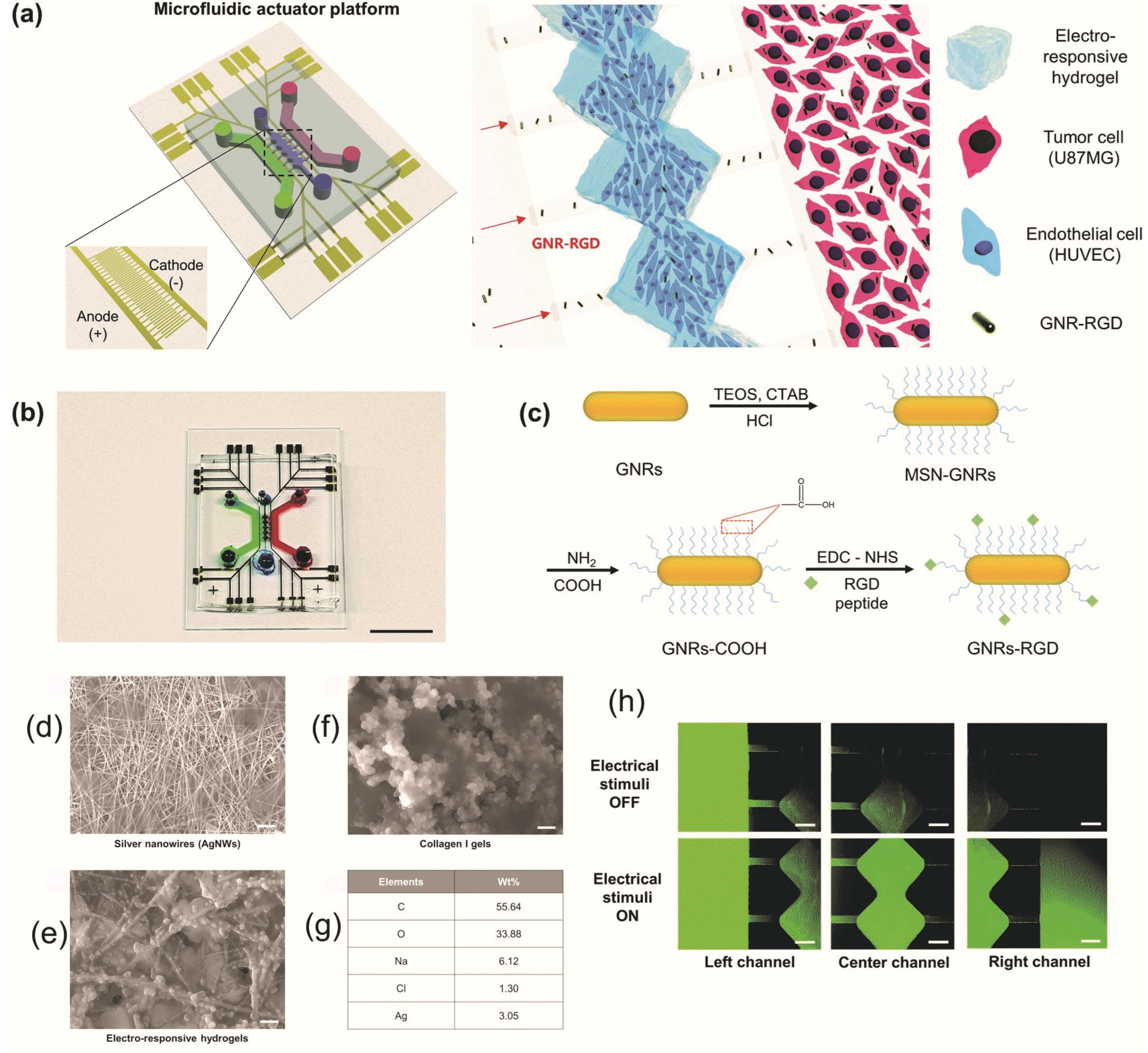
2.1.2. Electrical and Electrochemical Stimuli-Responsive Nanomaterials
2.1.3. Light-Responsive Nanomaterials
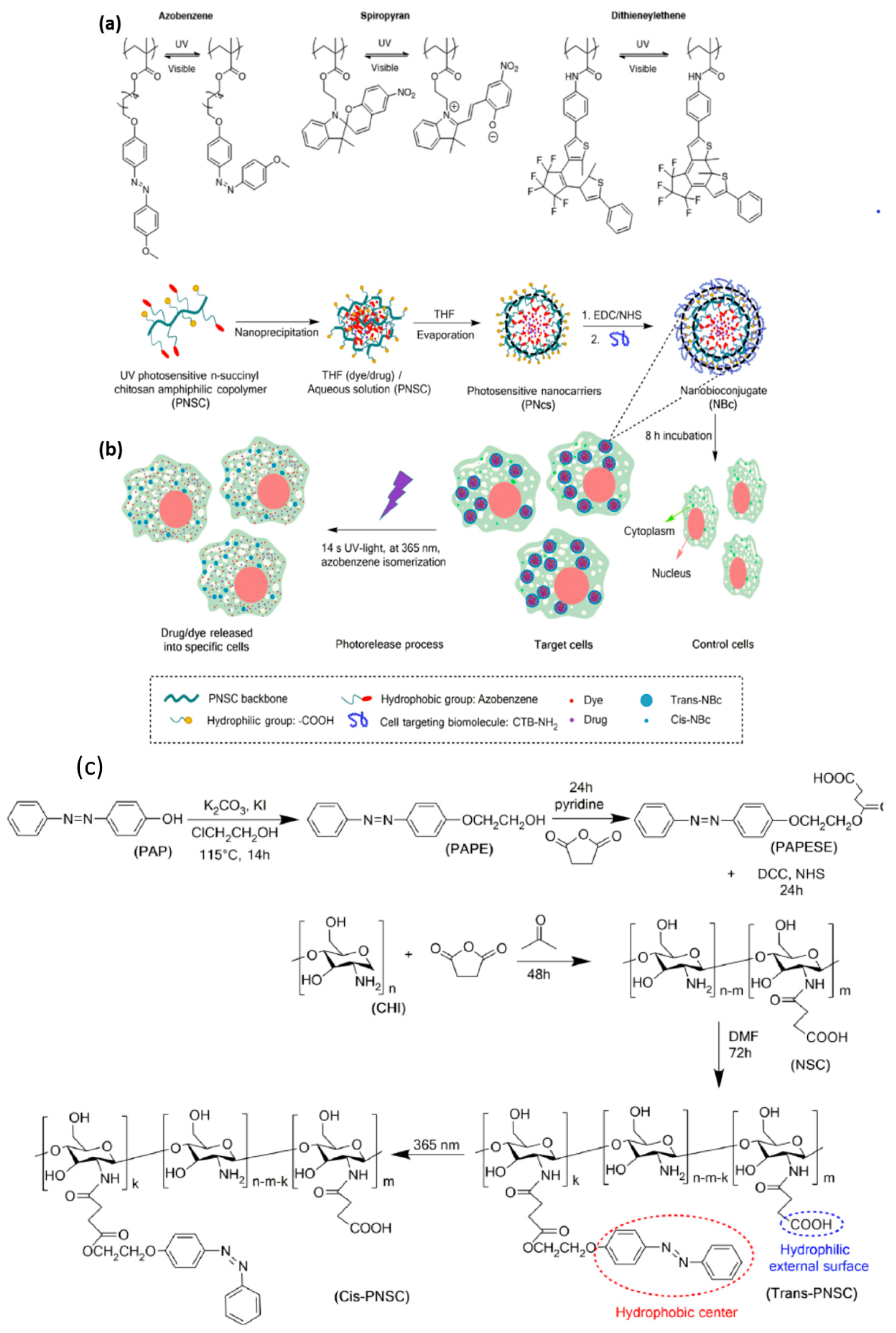
2.1.4. Magnetic-Responsive Nanomaterials
2.2. Chemical-Responsive Nanomaterials
2.2.1. pH-Responsive Nanomaterials
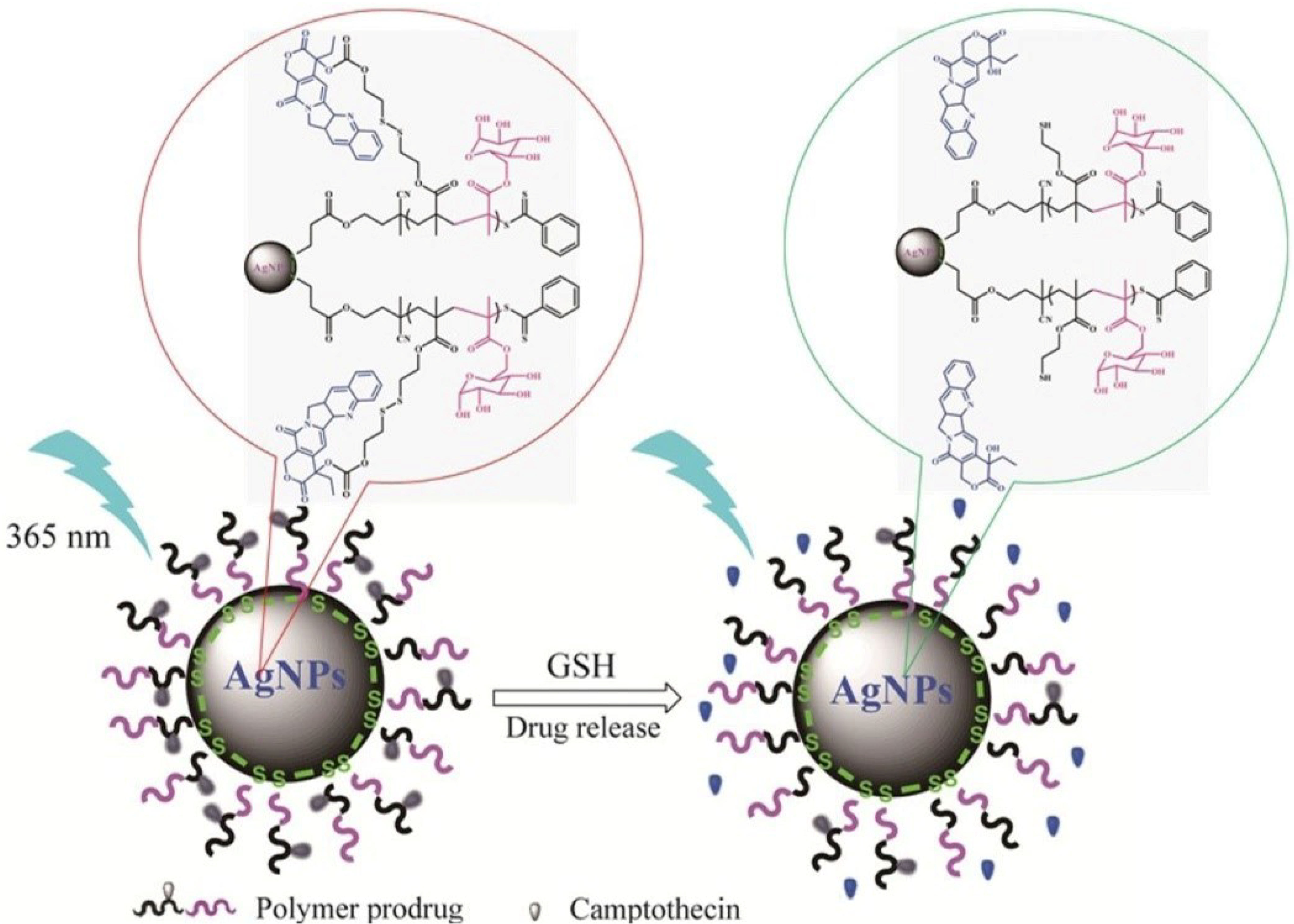
2.2.2. Redox-Responsive Nanomaterials
2.3. Biological-Responsive Nanomaterials
2.3.1. Glucose-Responsive Nanomaterials
2.3.2. Enzyme-Responsive Nanomaterials
2.4. Dual and Multi-Responsive Nanomaterials
3. Advances in Plasmonic Nanomaterials
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Amador-Vargas, S.; Dominguez, M.; León, G.; Maldonado, B.; Murillo, J.; Vides, G.L. Leaf-folding response of a sensitive plant shows context-dependent behavioral plasticity. Plant Ecol. 2014, 215, 1445–1454. [Google Scholar] [CrossRef]
- Reyssat, E.; Mahadevan, L. Hygromorphs: From pine cones to biomimetic bilayers. J. R. Soc. Interface 2009, 6, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbaum, R.; Zaltzman, L.; Burgert, I.; Fratzl, P. The role of wheat awns in the seed dispersal unit. Science 2007, 316, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Erb, R.M.; Sander, J.S.; Grisch, R.; Studart, A.R. Self-shaping composites with programmable bioinspired microstructures. Nat. Commun. 2013, 4, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forterre, Y.; Skotheim, J.M.; Dumais, J.; Mahadevan, L. How the Venus flytrap snaps. Nature 2005, 433, 421–425. [Google Scholar] [CrossRef]
- Fu, Q.; Li, C. Robotic modelling of snake traversing large, smooth obstacles reveals stability benefits of body compliance. R. Soc. Open Sci. 2020, 7, 191192. [Google Scholar] [CrossRef] [Green Version]
- Teyssier, J.; Saenko, S.V.; Van Der Marel, D.; Milinkovitch, M.C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 6368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totakagi, T. A Concept of intelligent materials. J. Intell. Mater. Syst. Struct. 1990, 1, 149–156. [Google Scholar] [CrossRef]
- Totakagi, T. Proceedings of the International Workshop on Intelligent Materials, Tsukuba Science City; The Society of Non-Traditional Technology: Tokyo, Japan, 1989. [Google Scholar]
- Feynman, R. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Taniguchi, N. On the basic concept of ‘nano-technology’. In International Conference on Production Engineering, Part II; Japan Society of Precision Engineering: Tokyo, Japan, 1974. [Google Scholar]
- Roco, M.C. International strategy for nanotechnology research and development. J. Nanoparticle Res. 2001, 3, 353–360. [Google Scholar] [CrossRef]
- Aflori, M.; Butnaru, M.; Tihauan, B.-M.; Doroftei, F. Eco-friendly method for tailoring biocompatible and antimicrobial surfaces of poly-L-lactic acid. Nanomaterials 2019, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Yang, C.; Zhang, P.; Zeng, J.; Li, Z.; Gao, M. Nanoparticles weaponized with built-in functions for imaging-guided cancer therapy. View 2020, 1, e19. [Google Scholar] [CrossRef]
- Genchi, G.G.; Marino, A.; Tapeinos, C.; Ciofani, G. Smart materials meet multifunctional biomedical devices: Current and prospective implications for nanomedicine. Front. Bioeng. Biotechnol. 2017, 5, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nano-medicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Thangudu, S. Next generation nanomaterials: Smart nanomaterials, significance, and biomedical applications. In Applications of Nanomaterials in Human Health; Khan, F.A., Ed.; Springer: Singapore, 2020; pp. 287–312. [Google Scholar]
- Pinteala, M.; Abadie, M.J.M.; Rusu, R.D. Smart supra-and macro-molecular tools for biomedical applications. Materials 2020, 13, 3343. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Aflori, M. (Ed.) Intelligent Polymers for Nanomedicine and Biotechnologies; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1941, 9, 660. [Google Scholar] [CrossRef]
- Huggins, M.L. Some properties of solutions of long-chain compounds. J. Phys. Chem. 1942, 46, 151–158. [Google Scholar] [CrossRef]
- Aguilar, M.R.; San Roman, J. Introduction to smart polymers and their applications. In Smart Polymers and their Applications; Woodhead Publish: Cambridge, UK, 2014; Volume 1, pp. 1–11. [Google Scholar]
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Debashish, R.W.L.; Brooks, A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Da Silva, L.P.; Pirraco, R.P.; Ma, M.; Yang, L.; Reis, R.L.; Chen, J. A thermo-/pH-responsive hydrogel (PNIPAM-PDMA-PAA) with diverse nanostructures and gel behaviors as a general drug carrier for drug release. Polym. Chem. 2018, 9, 4063–4072. [Google Scholar] [CrossRef]
- Zheng, A.; Wu, D.; Fan, M.; Wang, H.; Liao, Y.; Wang, Q.; Yang, Y. Injectable zwitterionic thermosensitive hydrogels with low-protein adsorption and combined effect of photothermal-chemotherapy. J. Mater. Chem. B 2020, 8, 10637–10649. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.-H.; Martin, D.C. Conducting-polymer nanotubes for controlled drug. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.K.; Minami, H.; Hoque, S.M.; Rahman, M.M.; Sharafat, M.K.; Begum, M.F.; Islam, M.E.; Ahmad, H. Mesoporous electromagnetic composite particles: Electric current responsive release of biologically active molecules and antibacterial properties. Colloids Surf. B 2019, 181, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.O.; Srikanth, B.; Zhu, P.-Y.; Chuang, C.-H. Impedimetric immunosensor utilizing polyaniline/Gold nanocomposite-modified screen-printed electrodes for early detection of chronic kidney disease. Sensors 2019, 19, 3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, K.J.; Schmidt, C.E. Neuron-targeted electrical modulation. Science 2020, 367, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kim, Y.S.; Joubert, L.-M.; Jiang, Y.; Wang, H.; Fenno, L.E.; Tok, J.B.-H.; Paşca, S.P.; Shen, K.; Bao, Z.; et al. Genetically targeted chemical assembly of functional materials in living cells, tissues, and animals. Science 2020, 367, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Barazesh, B.; Khoshroo, A.; Moshtaghiun, M.; Sheikhha, M.H. A new composite consisting of electro-synthesized conducting polymers, graphene sheets and biosynthesized gold nanoparticles for biosensing acute lymphoblastic leukemia. Bioelectrochemistry 2018, 121, 38–45. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Li, S.; Haupler, B.; Liu, J.; Jin, S.; Steffen, W.; Schubert, U.S.; Butt, H.-J.; Liang, X.-J.; Wu, S. An amphiphilic ruthenium polymetallodrug for combined photodynamic therapy and potochemotherapy in vivo. Adv. Mater. 2017, 29, 1603702. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dou, H.; Tao, K.; Sun, K.; Ding, J.; Shi, W.; Guo, X.; Li, J.; Zhang, D.; Sun, K. Two-in-one fabrication of Fe3O4/MePEG-PLA composite nanocapsules as a potential ultrasonic/MRI dual contrast agent. Langmuir 2011, 27, 12134–12142. [Google Scholar] [CrossRef]
- Chiang, C.; Shen, Y.-S.; Liu, J.-J.; Shyu, W.-C.; Chen, S.-Y. Synergistic combination of multistage magnetic guidance and optimized ligand density in targeting a nanoplatform for enhanced cancer therapy. Adv. Health Mater. 2016, 5, 2131–2141. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Shefi, O. Remote magnetic orientation of 3D collagen hydrogels for directed neuronal regeneration. Nano Lett. 2016, 16, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D. Fever, fever patterns and diseases called ‘fever’—A review. J. Infect. Public Health 2011, 4, 108–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Fraylich, M.; Saunders, B.R. Thermoresponsive copolymers: From fundamental studies to applications. Colloid Polym. Sci. 2009, 287, 627–643. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horizons 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Niebuur, B.-J.; Chiappisi, L.; Jung, F.; Zhang, X.; Schulte, A.; Papadakis, C.M. Kinetics of mesoglobule formation and growth in aqueous poly(N-isopropylacrylamide) Solutions: Pressure jumps at low and at high pressure. Macromolecules 2019, 52, 6416–6427. [Google Scholar] [CrossRef] [Green Version]
- Sahle, F.F.; Gulfam, M.; Lowe, T.L. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov. Today 2018, 23, 992–1006. [Google Scholar] [CrossRef]
- Marcelo, G.; Areias, L.R.; Viciosa, M.T.; Gaspar-Martinho, J.; Farinha, J.P.S. PNIPAm-based microgels with a UCST response. Polymer 2017, 116, 261–267. [Google Scholar] [CrossRef]
- Bischofberger, I.; Calzolari, D.C.E.; Rios, P.D.L.; Jelezarov, I.; Trappe, V. Hydrophobic hydration of poly-N-isopropyl acrylamide: A matter of the mean energetic state of water. Sci. Rep. 2014, 4, 4377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotsuchibashi, Y. Recent advances in multi-temperature-responsive polymeric materials. Polym. J. 2020, 52, 681–689. [Google Scholar] [CrossRef]
- Alejo, T.; Uson, L.; Arruebo, M. Reversible stimuli-responsive nanomaterials with on-off switching ability for biomedical ap-plications. J. Control. Release 2019, 314, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, M.; Kojima, C. pH-Switchable LCST/UCST-type thermosensitive behaviors of phenylalanine-modified zwitterionic dendrimers. RSC Adv. 2020, 10, 10452–10460. [Google Scholar] [CrossRef] [Green Version]
- Qiao, S.; Wang, H. Temperature-responsive polymers: Synthesis, properties, and biomedical applications. Nano Res. 2018, 11, 5400–5423. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-responsive nanocomposite hydrogels for biomedical applications. Adv. Funct. Mater. 2020, 2005941. [Google Scholar] [CrossRef]
- Custodio, C.A.; Reis, R.L.; Mano, J.F.; Del Campo, A. Smart instructive polymer substrates for tissue engineering. In Smart Polymers and Their Applications, 2nd ed.; Aguilar, M.R., Roman, J.S., Eds.; Woodhead Publishing in Materials: Cambridge, UK, 2014; Volume 10, pp. 10301–10326. [Google Scholar]
- Sun, H.; Feng, M.; Chen, S.; Wang, R.; Luo, Y.; Yin, B.; Li, J.; Wang, X.J. Near-infrared photothermal liposomal nanoantagonists for amplified cancer photodynamic therapy. Mater. Chem. B 2020, 8, 7149–7159. [Google Scholar] [CrossRef]
- Ha, J.H.; Shin, H.H.; Choi, H.W.; Lim, J.H.; Mo, S.J.; Ahrberg, C.D.; Lee, J.M.; Chung, B.G. Electro-responsive hydrogel-based microfluidic actuator platform for photothermal therapy. Lab Chip 2020, 20. [Google Scholar] [CrossRef]
- Hosseini-Nassab, N.; Samanta, D.; Abdolazimi, Y.; Annes, J.P.; Zare, R.N. Electrically controlled release of insulin using polypyrrole nanoparticles. Nanoscale 2017, 9, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Groenendaal, B.L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Kotov, N.A.; Winter, J.O.; Clements, I.P.; Jan, E.; Timko, B.P.; Campidelli, S.; Pathak, S.; Mazzatenta, A.; Lieber, C.M.; Prato, M.; et al. Nanomaterials for Neural Interfaces. Adv. Mater. 2009, 21, 3970–4004. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Liang, S.; Xu, W.-C.; Xu, G.; Wu, S. Photoresponsive polymers with multi-azobenzene groups. Polym. Chem. 2019, 10, 4389–4401. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, O.; Gohy, J. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2016, 8, 52–73. [Google Scholar] [CrossRef]
- Pierini, F.; Nakielski, P.; Urbanek, O.; Pawłowska, S.; Lanzi, M.; De Sio, L.; Kowalewski, T.A. Polymer-based nanomaterials for photothermal therapy: From light-responsive to multifunctional nanoplatforms for synergistically combined technologies. Biomacromolecules 2018, 19, 4147–4167. [Google Scholar] [CrossRef]
- Thangudu, S.; Kalluru, P.; Vankayala, R. Preparation, cytotoxicity, and in vitro bioimaging of water soluble and highly fluorescent palladium nanoclusters. Bioengineering 2020, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.; Li, S.; Sun, Z.; Li, W.; Zhou, L.; Zhang, J.; Gong, P.; Cai, L. Near-infrared fluorescence imaging in the largely unexplored window of 900–1000 nm. Theranostics 2018, 8, 4116–4128. [Google Scholar] [CrossRef]
- Molla, M.R.; Rangadurai, P.; Antony, L.; Swaminathan, S.; De Pablo, J.J.; Thayumanavan, S. Dynamic actuation of glassy polymerases through isomerization of a single azobenzene unit at the block copolymer interface. Nat. Chem. 2018, 10, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Mena-Giraldo, P.; Perez-Buitrago, S.; Londono-Berrío, M.; Ortiz-Trujillo, I.C.; Hoyos-Palacio, L.M.; Orozco, J. Photosensitive nanocarriers for specific delivery of cargo into cells. Sci. Rep. 2020, 10, 2110. [Google Scholar] [CrossRef] [Green Version]
- Pei, C.; Liu, C.; Wang, Y.; Cheng, D.; Li, R.; Shu, W.; Zhang, C.; Hu, W.; Jin, A.; Yang, Y.; et al. FeOOH@metal–organic framework core–satellite nanocomposites for the serum metabolic fingerprinting of gynecological cancers. Angew. Chem. Int. Ed. 2020, 59, 10831–10835. [Google Scholar] [CrossRef] [PubMed]
- Habash, R.W.Y.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part III: Ablation techniques. Crit. Rev. Biomed. Eng. 2007, 35, 37–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zborowski, M.; Dutz, S.; Häfeli, U.; Schütt, W. JMMM special issue “scientific and clinical applications of magnetic carriers”. J. Magn. Magn. Mater. 2020, 167667. [Google Scholar] [CrossRef]
- Kostevsek, N. A review on the optimal design of magnetic nanoparticle-based T2 MRI contrast agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Goerner, F.L.; Snyder, C.; Morelli, J.; Hao, D.; Hu, D.; Li, X.; Runge, V.M. T1 relativities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 t. Investig. Radiol. 2015, 50, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Perlman, O.; Azhari, H. MRI and Ultrasound Imaging of Nanoparticles for Medical Diagnosis. In Nanotechnology Characterization Tools for Biosensing and Medical Diagnosis; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 333–365. [Google Scholar]
- Xie, X.; Sun, T.C.; Xue, J.; Miao, Z.; Yan, X.; Fang, W.; Li, Q.; Tang, R.; Lu, Y.; Tang, L.; et al. AG nanoparticles cluster with PH-triggered reassembly in targeting antimicrobial applications. Adv. Funct. Mater. 2020, 30, 2000511. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, Y.; Huang, G.; Zhao, T.; Ma, X.; Wang, Z.; Sumer, B.D.; White, M.A.; Gao, J. Digitization of endocytic PH by hybrid ultra-PH-sensitive nanoprobes at single-organelle resolution. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Li, X.; Zheng, B.-Y.; Ke, M.-R.; Zhang, Y.; Huang, J.-D.; Yoon, J. A tumor-pH-responsive supramolecular photosensitizer for Activatable photodynamic therapy with minimal in vivo skin Phototoxicity. Theranostics 2017, 7, 2746–2756. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Kang, J.; Pyo, J.; Lim, J.; Chang, J.H.; Lee, J.-K. pH-induced aggregated melanin nanoparticles for photoacoustic signal amplification. Nanoscale 2016, 8, 14448–14456. [Google Scholar] [CrossRef] [Green Version]
- Bonadies, I.; Di Cristo, F.; Peluso, A.V.G.; Calarco, A.; Salle, A.D. Resveratrol-loaded electrospun membranes for the prevention of implant-associated infections. Nanomaterials 2020, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, J.; Lu, B.; Wu, W.; Zhang, L.; Liang, J.; Yi, J.; Li, X. Novel polyzwitterion shell with adaptable surface chemistry engineered to enhance anti-fouling and intracellular imaging of detonation nanodiamonds under tumor pHe. Front. Mater. Sci. 2020, 14, 402–412. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Facile fabrication of redox-responsive covalent organic framework nanocarriers for efficiently loading and delivering doxorubicin. Macromol. Rapid Commun. 2020, 41, e1900570. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-sensitive and hyaluronic acid-functionalized nanoparticles for improving breast cancer treatment by cytoplasmic 17α-methyltestosterone delivery. Molecules 2020, 25, 1181. [Google Scholar] [CrossRef] [Green Version]
- Mazzotta, E.; De Benedittis, S.; Qualtieri, A.; Muzzalupo, R. Actively targeted and redox responsive delivery of anticancer drug by chitosan nanoparticles. Pharmaceutics 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Mutlu-Agardan, N.B.; Sarisozen, C.; Torchilin, V.P. Cytotoxicity of novel redox sensitive PEG2000-S-S-PTX micelles against drug-resistant ovarian and breast cancer cells. Pharm. Res. 2020, 37, 1–8. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent advances in the redox-responsive drug delivery nanoplatforms: A chemical structure and physical property perspective. Mater. Sci. Eng. C 2021, 118, 111536. [Google Scholar] [CrossRef]
- Qiu, L.; Zhao, L.; Xing, C.; Zhan, Y. Redox-responsive polymer prodrug/AgNPs hybrid nanoparticles for drug delivery. Chin. Chem. Lett. 2018, 29, 301–304. [Google Scholar] [CrossRef]
- Abhijit, A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar]
- Lund, P.A.; De Biase, D.; O’Byrne, C.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; et al. Understanding how microorganisms respond to acid PH is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, L.; Mora-Boza, A.; Reyes-Ortega, F. pH-responsive polymers: Properties, synthesis and applications. In Smart Polymers and their Applications, 2nd ed.; Aguilar, M.R., Roman, J.S., Eds.; Woodhead Publishing in Materials: Cambridge, UK, 2019; Volume 3, pp. 45–86. [Google Scholar]
- Constantin, M.; Bucatariu, S.; Stoica, I.; Fundueanu, G. Smart nanoparticles based onpullulan-g-poly(N-isopropylacrylamide) for controlled delivery of indomethacin. Int. J. Biol. Macromol. 2017, 94, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, D.; Little, S.; Langer, R.; Amiji, M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: Part 2. In vivo distribution and tumor localization studies. Pharm Res. 2005, 22, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, S.; Velluto, D.; Hubbell, J.A. PEG-SS-PPS: Reduction-Sensitive Disulfide Block Copolymer Vesicles for Intracellular Drug Delivery. Biomacromolecules 2007, 8, 1966–1972. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Wen, Q.; Ni, C. Synthesis of core-crosslinked zwitterionic polymer nano aggregates and pH/Redox responsiveness in drug controlled release. Mater. Sci. Eng. C 2020, 106, 110288. [Google Scholar] [CrossRef]
- Ling, X.; Tu, J.; Aljaeid, B.M.; Shi, B.; Tao, W.; Farokhzad, O.C.; Wang, J.; Shajii, A.; Kong, N.; Feng, C.; et al. Glutathione-responsive prodrug nanoparticles for effective drug delivery and cancer therapy. ACS Nano 2019, 13, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Xu, M.; Zhu, J.; Liu, Y.; He, Z.; Zhang, Y.; Xu, Q.; Niu, Y. Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors. J. Mater. Chem. B 2020, 8, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Volpatti, L.R.; Matranga, M.A.; Cortinas, A.B.; Delcassian, D.; Daniel, K.B.; Langer, R.; Anderson, D.G. Glucose-responsive nanoparticles for rapid and extended self-regulated insulin delivery. ACS Nano 2019, 14, 488–497. [Google Scholar] [CrossRef]
- Tang, W.; Chen, C. Hydrogel-based colloidal photonic crystal devices for glucose sensing. Polymers 2020, 12, 625. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; He, J.; Bai, M.; Huang, C.; Wang, K.; Zhang, H.; Yang, S.-M.; Zhang, W.; Yang, S.-M. Engineering synthetic artificial pancreas using chitosan hydrogels integrated with glucose-responsive microspheres for insulin delivery. Mater. Sci. Eng. C 2019, 96, 374–382. [Google Scholar] [CrossRef]
- Liu, C.; Kou, Y.; Zhang, X.; Dong, W.; Cheng, H.; Mao, S. Enhanced oral insulin delivery via surface hydrophilic modification of chitosan copolymer based self-assembly polyelectrolyte nanocomplex. Int. J. Pharm. 2019, 554, 36–47. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, G.; Yu, W.; Li, L.; Tong, Z.; Kong, X.; Yao, J. Oral delivery of insulin using CaCO3-based composite nanocarriers with hyaluronic acid coatings. Mater. Lett. 2017, 188, 263–266. [Google Scholar] [CrossRef]
- Sonaje, K.; Chen, Y.J.; Chen, H.L.; Wey, S.-P.; Juang, J.-H.; Nguyen, H.-N.; Hsu, C.-W.; Lin, K.J.; Sung, H.W. Enteric-coated capsules filled with freeze-dried chitosan/poly(g-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials 2010, 31, 3384–3394. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Tong, Z.; Kong, X.; Yao, J. Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater. Sci. Eng. C 2017, 70, 278–286. [Google Scholar] [CrossRef]
- Ding, Y.; Hao, Y.; Yuan, Z.; Tao, B.; Chen, M.; Lin, C.; Liu, P.; Caib, K. A dual-functional implant with an enzyme-responsive effect for bacterial infection therapy and tissue regeneration. Biomater. Sci. 2020, 8, 1840–1854. [Google Scholar] [CrossRef]
- Datta, L.P.; Chatterjee, A.; Acharya, K.; De, P.; Das, M. Enzyme responsive nucleotide functionalized silver nanoparticles with effective antimicrobial and anticancer activity. New J. Chem. 2017, 41, 1538–1548. [Google Scholar] [CrossRef]
- Gu, G.; Xia, H.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; et al. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials 2013, 34, 196–208. [Google Scholar] [CrossRef]
- Gunawan, S.T.; Kempe, K.; Hagemeyer, C.E.; Caruso, F.; Bonnard, T.; Cui, J.; Alt, K.; Law, L.S.; Wang, X.; Westein, E.; et al. Multifunctional thrombin-activatable polymer capsules for specific targeting to activated platelets. Adv. Mater. 2015, 27, 5153–5157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, E.; Sunni, M.; Bellin, M.D. Secretion of Insulin in Response to Diet and Hormones. Pancreapedia 2020, 1–16. [Google Scholar] [CrossRef]
- Mansoor, S.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Polymer-based nanoparticle strategies for insulin delivery. Polymers 2019, 11, 1380. [Google Scholar] [CrossRef] [Green Version]
- Jamwal, S.; Ram, B.; Ranote, S.; Dharela, R.; Chauhan, G.S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int. J. Biol. Macromol. 2019, 123, 968–978. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ajiro, H.; Asoh, T.-A.; Akashi, M. Fabrication of surface-modified hydrogels with polyion complex for controlled release. Chem. Mater. 2010, 22, 2923–2929. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Wu, H.; Neish, A.S.; Champion, J.A. Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles. J. Control. Release 2019, 295, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Choi, N.; Kim, K.; Koo, H.-J.; Choi, J.; Kim, H.N. Chemoresistance of cancer cells: Requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics 2018, 8, 5259–5275. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cai, H.; Jiang, L.; Hu, J.; Bains, A.; Hu, J.; Gong, Q.; Luo, K.; Gu, Z. Enzyme-sensitive and amphiphilic pegylated dendrimer-paclitaxel prodrug-based nanoparticles for enhanced stability and anticancer efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef]
- Basel, M.T.; Shrestha, T.B.; Troyer, D.L.; Bossmann, S.H. Protease-sensitive, polymer-caged liposomes: A method for making highly targeted liposomes using triggered release. ACS Nano 2011, 5, 2162–2175. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Gao, W.; Pornpattananangkul, D.; Zhang, Q.; Fu, V.; Li, J.; Li, J.; Obonyo, M.; Zhang, L. Phospholipase A2-responsive antibiotic delivery via nanoparticle-stabilized liposomes for the treatment of bacterial infection. J. Mater. Chem. B 2014, 2, 8201–8207. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Wang, X.; Zhang, H.; Sun, L.; Pan, D.; Gong, Q.; Gu, Z.; Luo, K. Enzyme-sensitive biodegradable and multifunctional polymeric conjugate as theranostic nanomedicine. Appl. Mater. Today 2018, 11, 207–218. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Yu, B.; Song, N.; Hu, H.; Chen, G.; Shen, Y.; Cong, H. A degradable triple temperature-, pH-, and redox-responsive drug system for cancer chemotherapy. J. Biomed. Mater. Res. Part A 2018, 106, 3203–3210. [Google Scholar] [CrossRef]
- Chen, W.; Du, J. Ultrasound and PH dually responsive polymer vesicles for anticancer drug delivery. Sci. Rep. 2013, 3, srep02162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.J.; Hoffman, J.M.; Ebara, M.; Hoffman, A.S.; Estournès, C.; Wattiaux, A.; Stayton, P.S. Dual magnetic-/Temperature-responsive nanoparticles for microfluidic separations and assays. Langmuir 2007, 23, 7385–7391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mo, F.; Lin, Y.; Wang, X.; Xu, L.; Fu, F. Tumor targeting dual stimuli responsive controllable release nanoplatform based on DNA-conjugated reduced graphene oxide for chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2018, 6, 4360–4367. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Behera, B.; Sahu, S.K.; Ananthakrishnan, R.; Maiti, T.K.; Pramanik, P. Design of dual stimuli responsive polymer modified magnetic nanoparticles for targeted anti-cancer drug delivery and enhanced MR imaging. New J. Chem. 2015, 40, 545–557. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, W.; Zhu, Y.; Luo, J.; Liu, R.; Liu, X. Synthesis of temperature/PH dual-stimuli-response multicompartmental microcapsules via pickering emulsion for preprogrammable payload release. ACS Appl. Mater. Interfaces 2020, 12, 4821–4832. [Google Scholar] [CrossRef]
- Poudel, K.; Gautam, M.; Jin, S.G.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Copper sulfide: An emerging adaptable nanoplatform in cancer theranostics. Int. J. Pharm. 2019, 562, 135–150. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Cui, J.; Hao, J. Dual-stimuli-responsive polypeptide nanoparticles for photothermal and photodynamic therapy. ACS Appl. Bio Mater. 2020, 3, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhao, Q.; Wang, X.; Mao, Y.; Chen, C.; Gao, Y.; Sun, C.; Wang, S. Multi-stimuli responsive mesoporous silica-coated carbon nanoparticles for chemo-photothermal therapy of tumor. Colloids Surf. B 2020, 190, 110941. [Google Scholar] [CrossRef]
- Shi, Y.; Shan, S.; Li, C.; Song, X.; Zhang, C.; Chen, J.; You, J.; Xiong, J. Application of the tumor site recognizable and dual-responsive nanoparticles for combinational treatment of the drug-resistant colorectal cancer. Pharm. Res. 2020, 37, 1–14. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Y.; Xu, L.; Chen, D.; Cheng, L. Transferrin conjugated PH- and redox-responsive poly(Amidoamine) Dendrimer conjugate as an efficient drug delivery carrier for cancer therapy. Int. J. Nanomed. 2020, 15, 2751–2764. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhang, J.; Tian, B.; Wu, Z.; Svirskis, D.; Han, J. A NAG-guided nano-delivery system for redox-and pH-triggered intracellularly sequential drug release in cancer cells. Int. J. Nanomed. 2020, 15, 841. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Tang, Z.; Peng, S.; Zhang, J.; Wang, W.; Wang, Q.; Lin, W.; Lin, X.; Zu, X.; Luo, H.; et al. pH/redox/UV irradiation multi-stimuli responsive nanogels from star copolymer micelles and Fe3+ complexation for “on-demand” anticancer drug delivery. React. Funct. Polym. 2020, 149, 104532. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Khamooshi, N.; Nasri, S.; Vancaeyzeele, C. Multi-stimuli responsive nanogel/hydrogel nanocomposites based on κ-carrageenan for prolonged release of levodopa as model drug. Int. J. Biol. Macromol. 2020, 153, 180–189. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Luo, W.; Qian, Q.; Li, Q.; Han, B.; Liu, C. Triple cell-responsive nanogels for delivery of drug into cancer cells. Colloids Surf. B 2018, 163, 362–368. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, Y.H.; Liu, Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 2020, 32, 1806158. [Google Scholar] [CrossRef]
- Lu, J.; Luo, B.; Chen, Z.; Yuan, Y.; Kuang, Y.; Wan, L.; Yao, L.; Chen, X.; Jiang, B.; Liu, J.; et al. Host-guest fabrication of dualresponsive hyaluronic acid/mesoporous silica nanoparticle based drug delivery system for targeted cancer therapy. Int. J. Biol. Macromol. 2020, 146, 363–373. [Google Scholar] [CrossRef]
- Xiong, D.; Wen, L.; Peng, S.; Xu, J.; Zhang, L. Reversible cross-linked mixed micelles for PH triggered swelling and redox triggered degradation for enhanced and controlled drug release. Pharmaceutics 2020, 12, 258. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Fan, X.; Zhao, Y.; Zhi, D.; Cui, S.; Zhang, E.; Lan, H.; Du, J.; Zhang, Z.; Zhang, S.; et al. Stimuli-responsive polysaccharide enveloped liposome for targeting and penetrating delivery of survivin-shRNA into breast tumor. ACS Appl. Mater. Interfaces 2020, 12, 22074–22087. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Poudel, B.K.; Ruttala, R.R.T.; Jin, S.G.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Multi-responsive albumin-lonidamine conjugated hybridized gold nanoparticle as a combined photothermal-chemotherapy for synergistic tumor ablation. Acta Biomater. 2020, 101, 531–543. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Gatti, G.; Picchio, M.L.; Maccioni, M.; Gugliotta, L.M.; Igarzabal, C.I.A. Dually responsive nanogels as smart carriers for improving the therapeutic index of doxorubicin for breast cancer. Eur. Polym. J. 2019, 116, 445–452. [Google Scholar] [CrossRef]
- Yang, J.; Wei-Yahng, Y. Metal-organic framework-based cancer theranostic nanoplatforms. View 2020, 1, e20. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Li, T.; Yang, X.; Li, Z.; Yang, X.; Chen, J.; Wan, J.; Xiao, C.; Guan, J.; et al. A simple glutathione-responsive turn-on theranostic nanoparticle for dual-modal imaging and chemo-photothermal combination therapy. Nano Lett. 2019, 19, 5806–5817. [Google Scholar] [CrossRef]
- Halas, N.J. Connecting the dots: Reinventing optics for nanoscale dimensions. Proc. Natl. Acad. Sci. USA 2009, 106, 3643–3644. [Google Scholar] [CrossRef] [Green Version]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Accounts Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; Ghosh, D.; Do, B.H.; Chen, K.; Dawson, M.R.; Fang, N.; Sulchek, T.A.; El-Sayed, M.A. Nuclear membrane-targeted gold nanoparticles inhibit cancer cell migration and invasion. ACS Nano 2017, 11, 3716–3726. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Mackey, M.A.; Huang, X.; Kangy, B.; El-Sayed, M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391–3404. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Shi, X.; Gurav, D.D.; Huang, L.; Su, H.; Li, K.; Niu, J.; Zhang, M.; Wang, Q.; Jiang, M.; et al. Metabolic fingerprinting on synthetic alloys for medulloblastoma diagnosis and radiotherapy evaluation. Adv. Mater. 2020, 32, 2000906. [Google Scholar] [CrossRef]
- Xu, W.; Wang, L.; Dai, H.; Sun, X.; Huang, L.; Su, H.; Wei, X.; Chen, C.-C.; Lou, J.; Dai, H.; et al. Diagnosis and prognosis of myocardial infarction on a plasmonic chip. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Gurav, D.D.; Price, C.-A.H.; Velliou, E.; Liu, J.; Qian, K.; Wu, S.; Xu, W.; Vedarethinam, V.; Yang, J.; et al. A multifunctional platinum nanoreactor for point-of-care metabolic analysis. Matter 2019, 1, 1669–1680. [Google Scholar] [CrossRef] [Green Version]
- Shu, W.; Wang, Y.; Liu, C.; Li, R.; Pei, C.; Lou, W.; Lin, S.; Di, W.; Wan, J. Construction of a plasmonic chip for metabolic analysis in cervical cancer screening and evaluation. Small Methods 2019, 4, 190046. [Google Scholar] [CrossRef]
- Baffou, G.; Cichos, F.; Quidant, R. Applications and challenges of thermoplasmonics. Nat. Mater. 2020, 19, 946–958. [Google Scholar] [CrossRef]
- Annesi, F.; Pane, A.; Bartolino, R.; De Sio, L.; Losso, M.A.; Guglielmelli, A.; Lucente, F.; Petronella, F.; Placido, T.; Comparelli, R.; et al. Thermo-plasmonic killing of Escherichia coli tg1 bacteria. Materials 2019, 12, 1530. [Google Scholar] [CrossRef] [Green Version]
- Guglielmelli, A.; Rosa, P.; Perotto, G.; De Sio, L.; Contardi, M.; Prato, M.; Mangino, G.; Miglietta, S.; Petrozza, V.; Pani, R.; et al. Biomimetic keratin gold nanoparticle-mediated in vitro photothermal therapy on glioblastoma multiforme. Nanomedicine 2021, 4. [Google Scholar] [CrossRef]
- De Angelis, B.; DePalo, N.; Petronella, F.; Quintarelli, C.; Curri, M.L.; Pani, R.; Calogero, A.; Locatelli, F.; De Sio, L. Stimuli-responsive nanoparticle-assisted immunotherapy: A new weapon against solid tumours. J. Mater. Chem. B 2020, 8, 1823–1840. [Google Scholar] [CrossRef]
- Renard, D.; Tian, S.; Lou, M.; Neumann, O.; Yang, J.; Bayles, A.; Solti, D.; Nordlander, P.; Halas, N.J. Uv-resonant al nanocrystals: Synthesis, silica coating, and broadband photothermal response. Nano Lett. 2020, 21. [Google Scholar] [CrossRef]



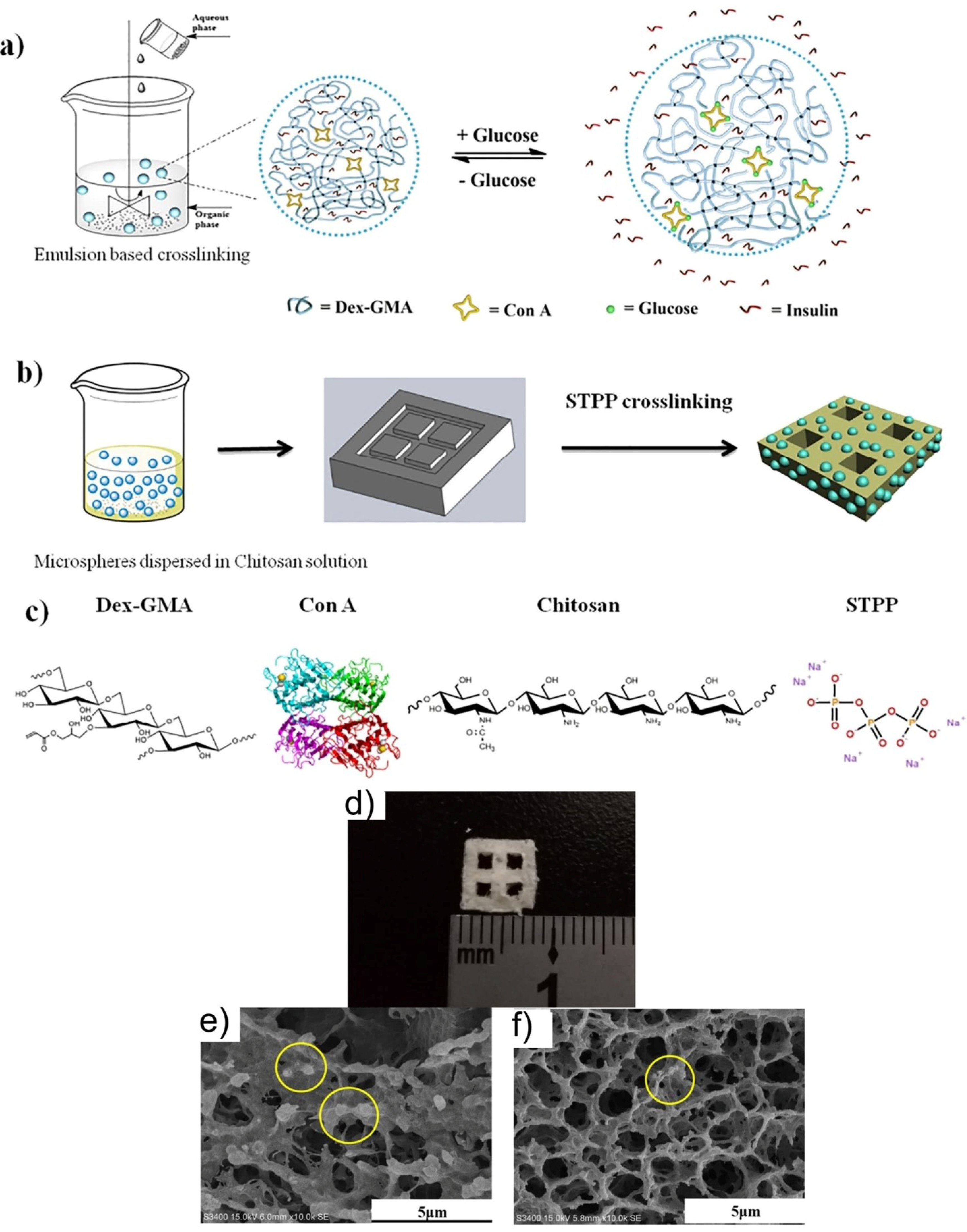

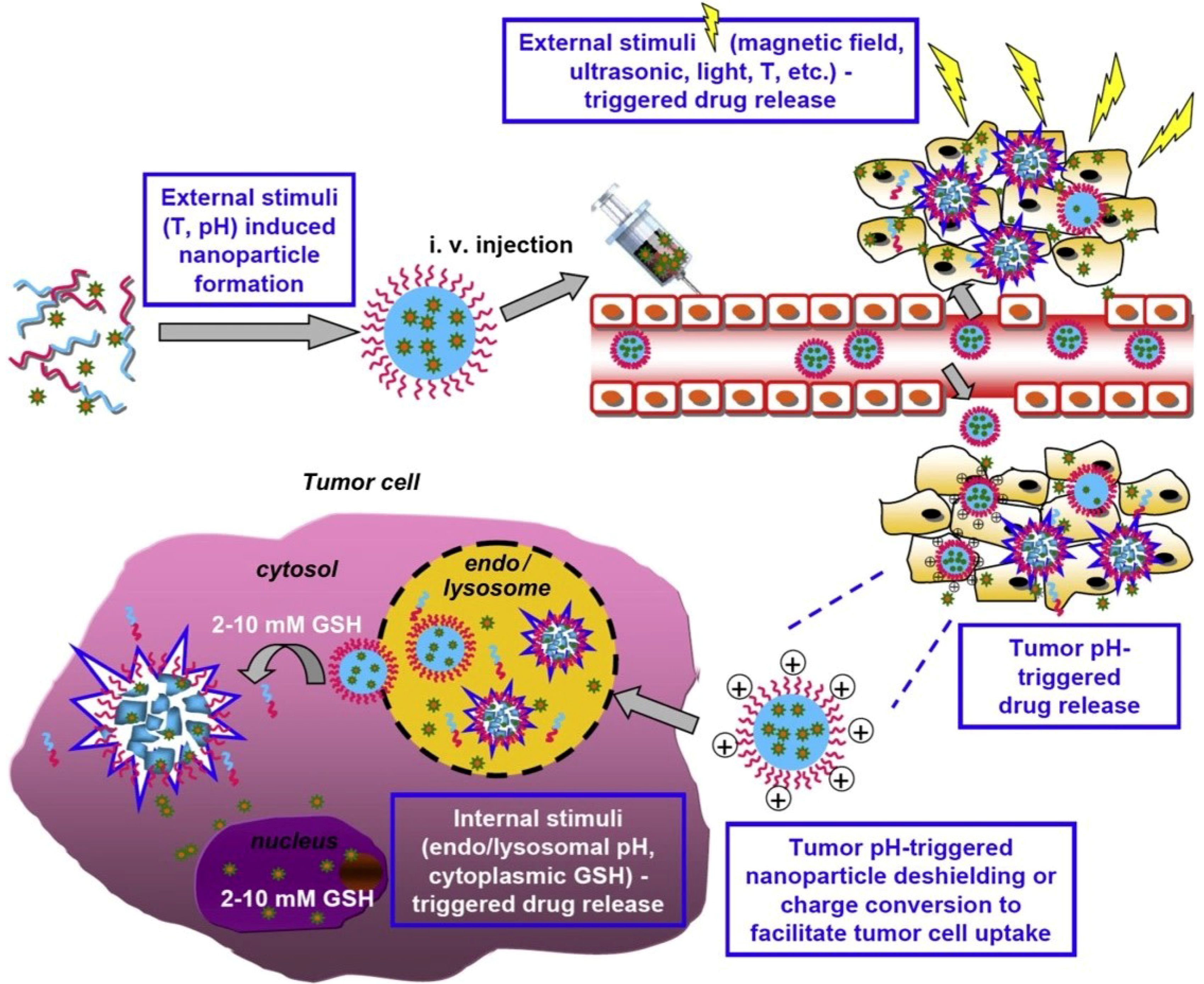
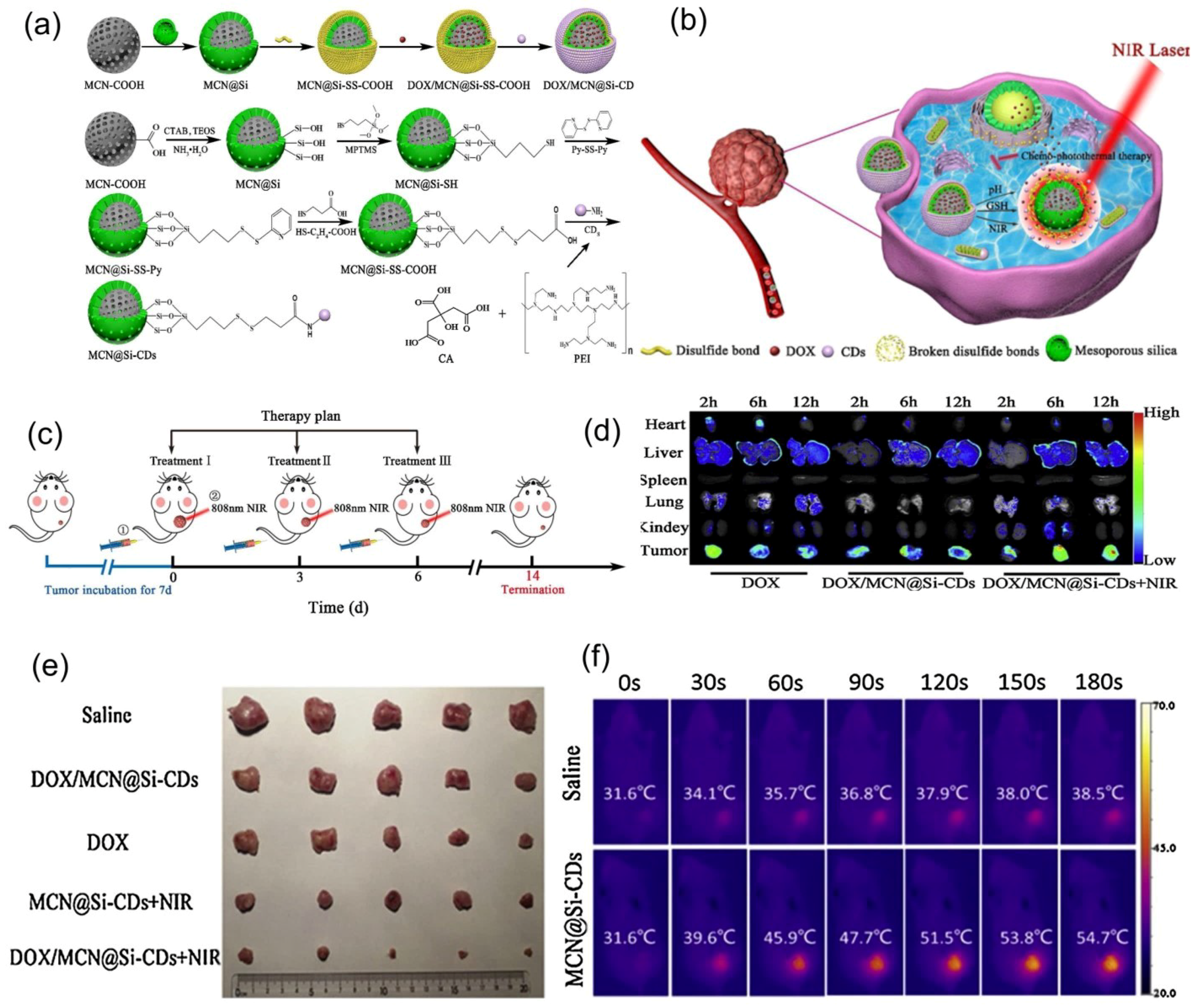
| Nr. Crt. | Stimuli | Nanomaterial | Application | Reference |
|---|---|---|---|---|
| 1. | Temperature | Poly(ethylene oxide)a-poly(propylene oxide)b- poly(ethylene oxide)a PEO-PPO-PEO | Oral drug delivery, wound healing | [24] |
| 2. | Temperature | Gold nanoparticles—Pluronic®F127-Hydroxypropyl methylcellulose AuNPs-PF127-HPMC | Drug delivery, photothermal platform, skin wound healing | [25] |
| 3. | Temperature | Poly(oligo(ethylene glycol) methacrylate –co-poly(glycidal methacrylate) copolymers/poly(lactic acid-co-glycolic acid) P(OEGMA-co-PGMA) copolymers/PLGA | Tissue engineering | [26] |
| 4. | Temperature | Collagen- or chitosan-based | Drug delivery | [27] |
| 5. | Temperature | Poly(N-isopropylacrylamide)- poly(N,N-dimethylacrylamide)- poly(acrylic acid) PNIPAM-PDMA-PAA | Drug delivery | [28] |
| 6. | Temperature | Poly(Nisopropylacrylamide-co-sulfobetaine methacrylate) nanogel PNS nanogels | Diagnosis/chemotherapy | [29] |
| 7. | Electrical | Poly(3,4-ethylenedioxythiophene)-coated poly(lactic acid-co-glycolic acid) nanofiber PEDOT-coated PLGA nanofiber | Drug delivery | [30] |
| 8. | Electrical | Fe3O4/Polyaniline Fe3O4/PANI | Antimicrobial, drug delivery | [31] |
| 9. | Electrical | Polyaniline/gold nanocomposite PANI/AuNCs | Immunosensor detection of chronic kidney disease | [32] |
| 10. | Electrical | Polyaniline, poly(3,4-ethylenedioxythiophene) PANIP, PEDOT | Neural prostheses | [33,34] |
| 11. | Electrochemical | Biosynthesized gold nanoparticles/ poly(catechol)/graphene sheets/glassy carbon electrode Bio AuNP/Pol/Gr/GCE | Biosensor, DNA mutation and acute lymphoblastic leukemia detection | [35] |
| 12. | Light | poly(ethylene glycol) PEG | Switchable fluorescent probes | [36] |
| 13. | Light | Ruthenium-containing block copolymer Poly-Ru nanoparticles | In vivo photodynamic therapy and photochemotherapy | [37] |
| 14. | Magnetic | Fe3O4/methoxy poly(ethylene glycol)-poly- (lactide) composite nanocapsules Fe3O4/MePEG-PLA composite nanocapsules | MRI | [38] |
| 15. | Magnetic | Trastuzumab (Tra, a humanized monoclonal antibody that specifically recognizes HER2)- doxorubicin poly(vinyl alcohol)/ single-component thiol-functionalized poly (methacrylic acid) T-DOX PVA/PMASH magnetic nanocapsules | Tumor therapy | [39] |
| 16. | Magnetic | 3D collagen hydrogel | Directed neuronal regeneration | [40] |
| Nr. Crt. | Stimuli | Nanomaterial | Application | Reference. |
|---|---|---|---|---|
| 1. | pH | Ppoly (ethylene glycol)-Ag nanoparticle PEG-Ag NPs | Antibacterial, wound healing | [77] |
| 2. | pH | Hybrid ultra-pH-sensitive (HyUPS) nanotransistor HyUPS nanotransistors | Receptor-mediated endocytosis in tumor cells | [78] |
| 3. | pH | Layered double hydroxides-zinc (II) phthalocyanine containing octasulfonate nanohybrid LDH-ZnPcS8 nanohybrid | Theranostics | [79] |
| 4. | pH | Melanin-like nanoparticles | Photoacoustic imaging of tumors | [80] |
| 5. | pH | polylactic acid-Resveratrol PLA-RSV | Drug delivery | [81] |
| 6. | pH | Poly(carboxybetaine methacrylate)-nanodiamonds PCBSA-@-NDs | Theranostics | [82] |
| 7. | Redox | Poly (ethylene glycol)-Pluronic F68-nanoscale covalent organic frameworks F68@SS-COFs | Cancer therapy | [83] |
| 8. | Redox | Hyaluronic acid–chitosan–lipoic acid nanoparticles (HACSLA-NPs) | Breast cancer therapy | [84] |
| 9. | Redox | Folate redox-responsive chitosan nanoparticles FTC-NPs | Anticancer drug delivery | [85] |
| 10. | Redox | Poly (ethylene glycol) conjugated to paclitaxel via disulfide linkage PEG2000-S-S-PTX | Prodrug for breast cancer cells | [86] |
| 11. | Redox | Prodrug/AgNPs hybrid nanoparticles | Drug delivery | [87] |
| 12. | Redox | P[(2-((2- ((camptothecin)-oxy)ethyl)disulfanyl)ethylmethacrylate) -co- (2-(D-galactose)methylmethacryl-ate)] and silver nanoparticles P(MACPTS-co-MAGP)@AgNPs nanoparticles | Drug release | [88] |
| Nr. Crt. | Stimuli | Nanomaterial | Application | Reference. |
|---|---|---|---|---|
| 1. | Glucose | Acetalated dextran nanoparticles Ac-Dex Nps | Glycemic control | [103] |
| 2. | Glucose | Boronic acid-derived polymers | Drug delivery | [104] |
| 3. | Glucose | Glycidyl methacrylated dextran/Concanavalin A Dex-GMA/Con A ConA micro/nanospheres | Insulin treatment | [105] |
| 4. | Glucose | Chitosan-g-polyethylene glycol monomethyl ether nanocomplex CS-g-(mPEG) NP | Oral insulin delivery | [106] |
| 5. | Glucose | Hyaluronic Acid (HA)-coated calcium carbonate NPs | Oral insulin delivery | [107] |
| 6. | Glucose | Chitosan/poly(gamma-glutamic acid) nanoparticles | Oral insulin delivery | [108] |
| 7. | Glucose | Carboxymethyl chitosan-phenylboronic acid-Lvaline nanoparticles (CMCS-PBA-LV) NPs | Oral administration of insulin | [109] |
| 8. | Enzyme | Nanoplatform formed from Ti substrates modified with layer-by layer mesoporous silica nanoparticles-silver nanoparticles LBL@MSN-Ag nanoparticles | Tissue growth in vivo and, simultaneously, treat implant-associated bacterial infection | [110] |
| 9. | Enzyme | Adenosine triphosphate coated with silver nanoparticles ATP-Ag nanoparticles | Participate in signal transduction and protein activity | [111] |
| 10. | Enzyme | Activatable low-molecular weight protamine—poly(ethylene glycol) poly(ε-caprolactone) nanoparticles—loaded with paclitaxel ALMWP-NP-PTX | Glioblastoma therapy | [112] |
| 11. | Enzyme | Layer-by-layer assembly of poly(2-oxazoline)-based materials | Therapeutic delivery | [113] |
| Nr. Crt. | Stimuli | Nanomaterial | Application | Ref. |
|---|---|---|---|---|
| 1. | pH/redox/temperature | N,N0 -bis(acryloyl)cystamine, Poly(N-isopropylacrylamide), 2-hydroxyethylmethacrylate, Methacrylic acid, a disulfide bond contained cross-linker, and doxorubicin SS-NPs@DOX | Drug delivery | [128] |
| 2. | Ultrasound/pH | Poly(ethylene oxide, 2-(diethylamino)ethyl methacrylate, (2-tetrahydrofuranyloxy)ethyl methacrylate PEO43-b-P(DEA33-stat-TMA47) | Drug release | [129] |
| 3. | Temperature/magnetic field | Poly(N-isopropylacrylamide)- Magnetic nanoparticles b-PNIPAM-mNPs | The isolation of diagnostic targets that can be used in point-of-care devices | [130] |
| 4. | Light/pH | rGO-PDA nanosheets | Drug delivery, phototherapy | [131] |
| 5. | pH/magnetic field | Magnetic nanoparticles MFNPs | Targeting, drug delivery, MRI | [132] |
| 6. | Temperature/pH | Poly(N-isopropylacrylamide) pNIPAM | Drug release | [133] |
| 7. | pH/light/enzyme | Copper sulfide nanoparticles CuS NPs | Theranostics | [134] |
| 8. | pH/redox | Thiol-modified polylysine- indocyanine green/ poly(ethylene glycol) nanoparticles PLL-ICG/DPEG Nps | Photothermal and photodynamic therapy | [135] |
| 9. | pH/redox | Poly (ethylene glycol) –polylacticacid-thioketal groups-Paclitaxel-(Maleimide thioether) Chlorin e6 mPEG-PLA-TKI-PTX nanoparticles and Ce6-(SS-mal-)-Ce6 (PNPCe6) | Chemotherapy, drug release | [136] |
| 10. | pH/redox | Histidine -4 polyamidoamine dendrimer -Disulfide bonds- (poly (ethylene glycol)- Transferrin (His-PAMAM-ss-PEG-Tf, HP-ss-PEG-Tf) nanocarrier | Anticancer drug delivery | [137] |
| 11. | pH/redox | Lipoic acid ethylenediamine- Polyethylene glycol diglycidyl ether- Llysine poly(LAE-co-PGDE-co-Lys) core-crosslinked nano aggregate | Anticancer drug delivery | [138] |
| 12. | pH/redox | Paclitaxel- poly(6-O-methacryloyl-d-galactopyranose)- gemcitabine/ N-acetyl-d-glucosamine(NAG)-poly(styrene-alt-maleic anhydride)-b-polystyrene PTXL-ss-PMAGP-GEM/NAG NLCs | Anticancer drug delivery | [139] |
| 13. | UV light/redox/pH | Six-arm star-shaped amphiphilic copolymer with poly (caprolactone) -bpoly (acrylic acid) -b-poly (poly (ethylene glycol) methyl ether methacrylate) | Anticancer drug delivery | [140] |
| 14. | pH/temperature | Poly(NIPAM)nanogel @ Fe3O4 NPs/poly(acrylic acid) -graft—κ—carrageenan | Drug delivery | [141] |
| 15. | Redox/pH/temperature | Nanogels based on alginate and cystamine | Anticancer drug delivery | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aflori, M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials 2021, 11, 396. https://doi.org/10.3390/nano11020396
Aflori M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials. 2021; 11(2):396. https://doi.org/10.3390/nano11020396
Chicago/Turabian StyleAflori, Magdalena. 2021. "Smart Nanomaterials for Biomedical Applications—A Review" Nanomaterials 11, no. 2: 396. https://doi.org/10.3390/nano11020396






