Air-Filled Bubbles Stabilized by Gold Nanoparticle/Photodynamic Dye Hybrid Structures for Theranostics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. AuNPs Synthesis
2.2.2. BSA-ZnPc Preparation
2.2.3. BSA-ICG Preparation
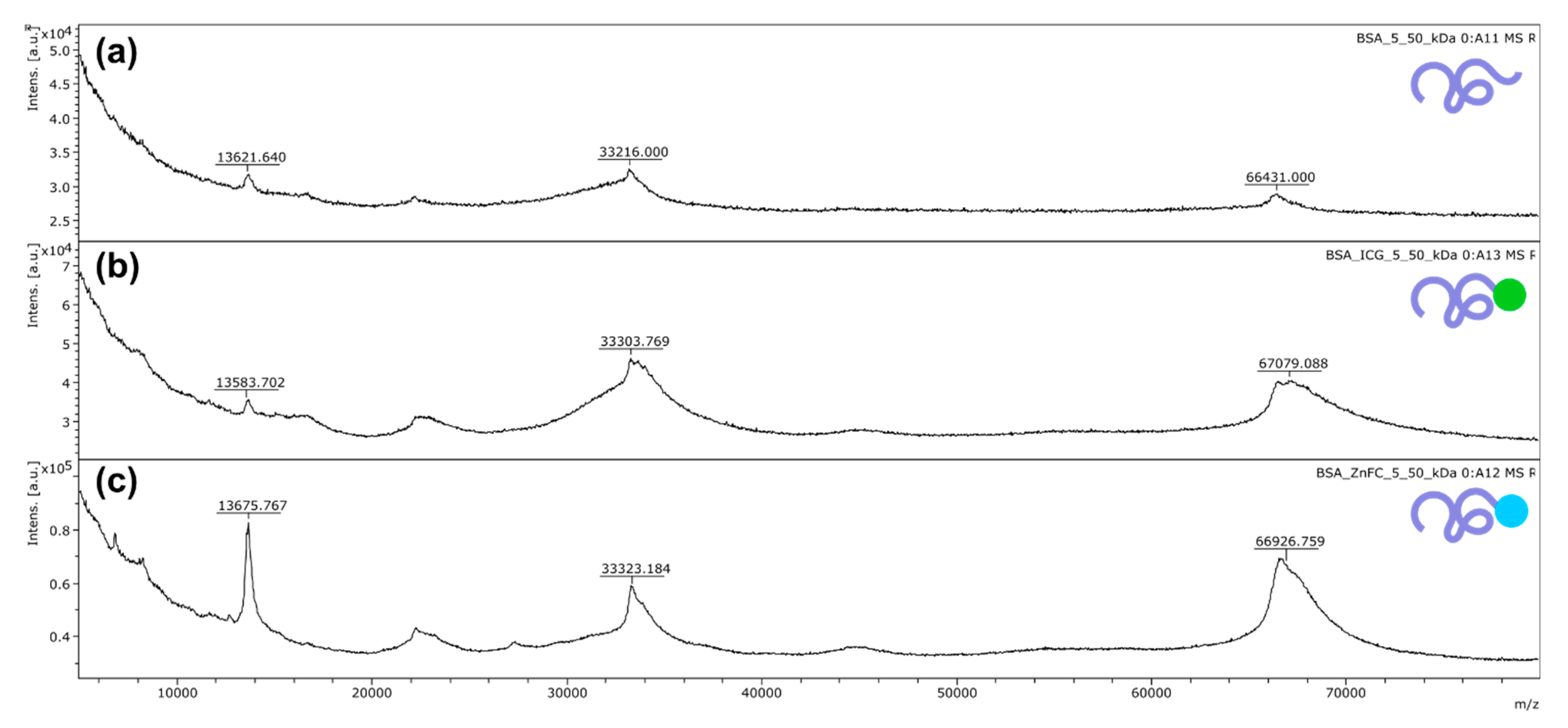
2.2.4. Mass Spectrometry Measurements
2.2.5. Surface Tension Measurements
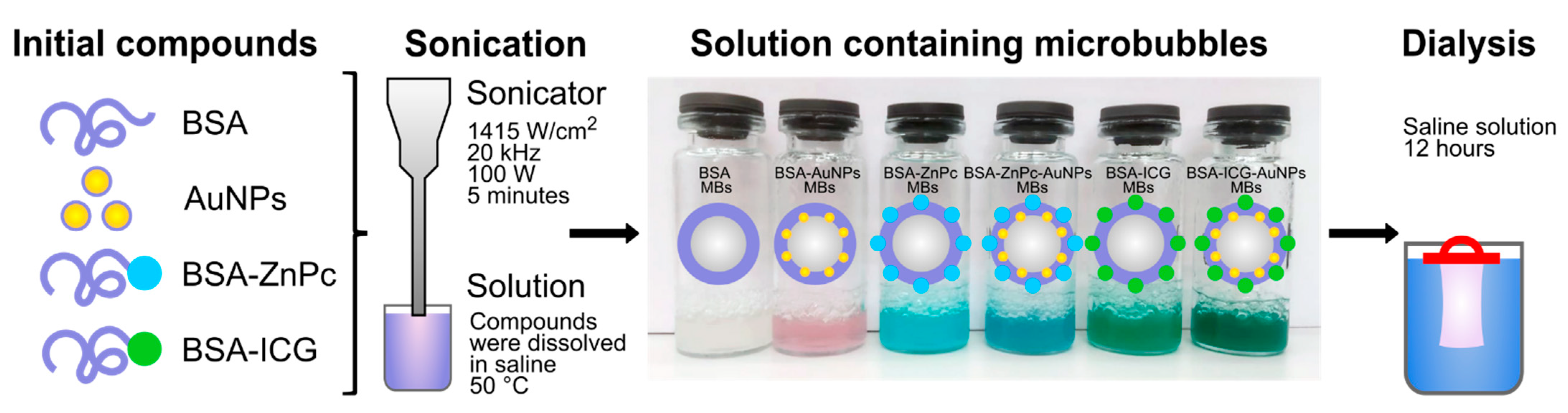
2.2.6. Microbubbles Preparation
2.2.7. Optical Microscopy
2.2.8. Microbubbles Concentration Measurements
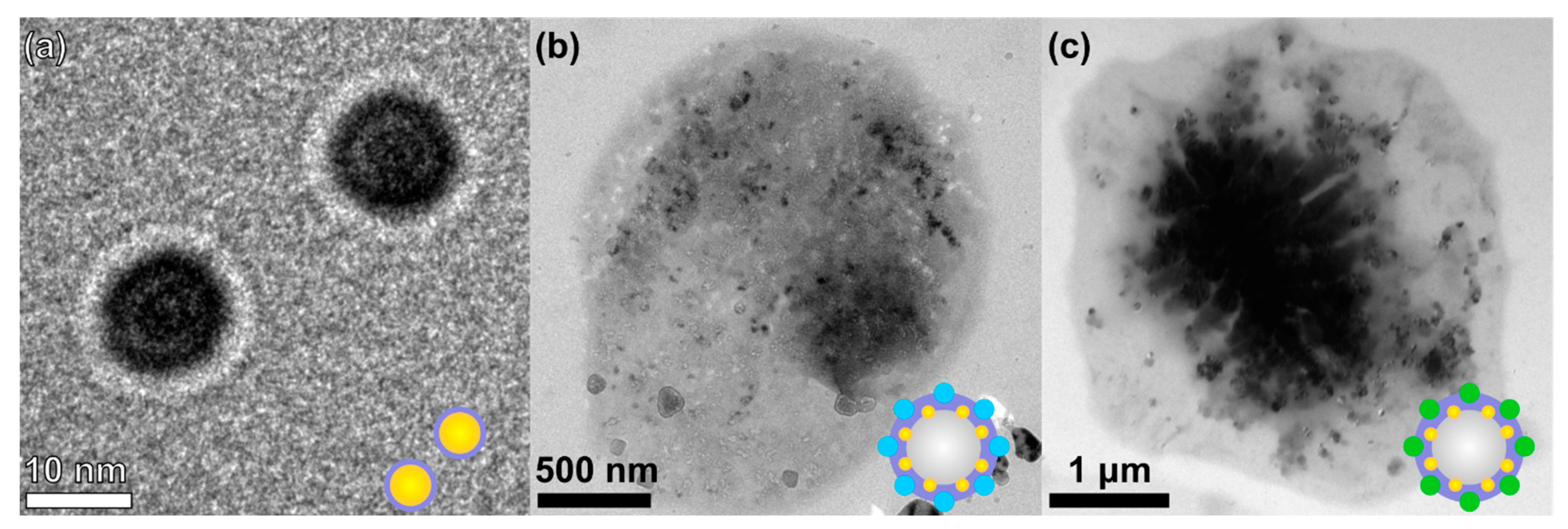
2.2.9. Transition Electron Microscopy
2.2.10. Zeta-Potential Measurements
2.2.11. Extinction Spectra Measurements
2.2.12. Fluorescence Tomography Measurements
2.2.13. Raster-Scanning Optoacoustic Mesoscopy Measurements
2.2.14. Ultrasound Characterization
3. Results and Discussion
3.1. Optimization of the Experiment and Microbubbles’ Stabilization with Hybrid Structures
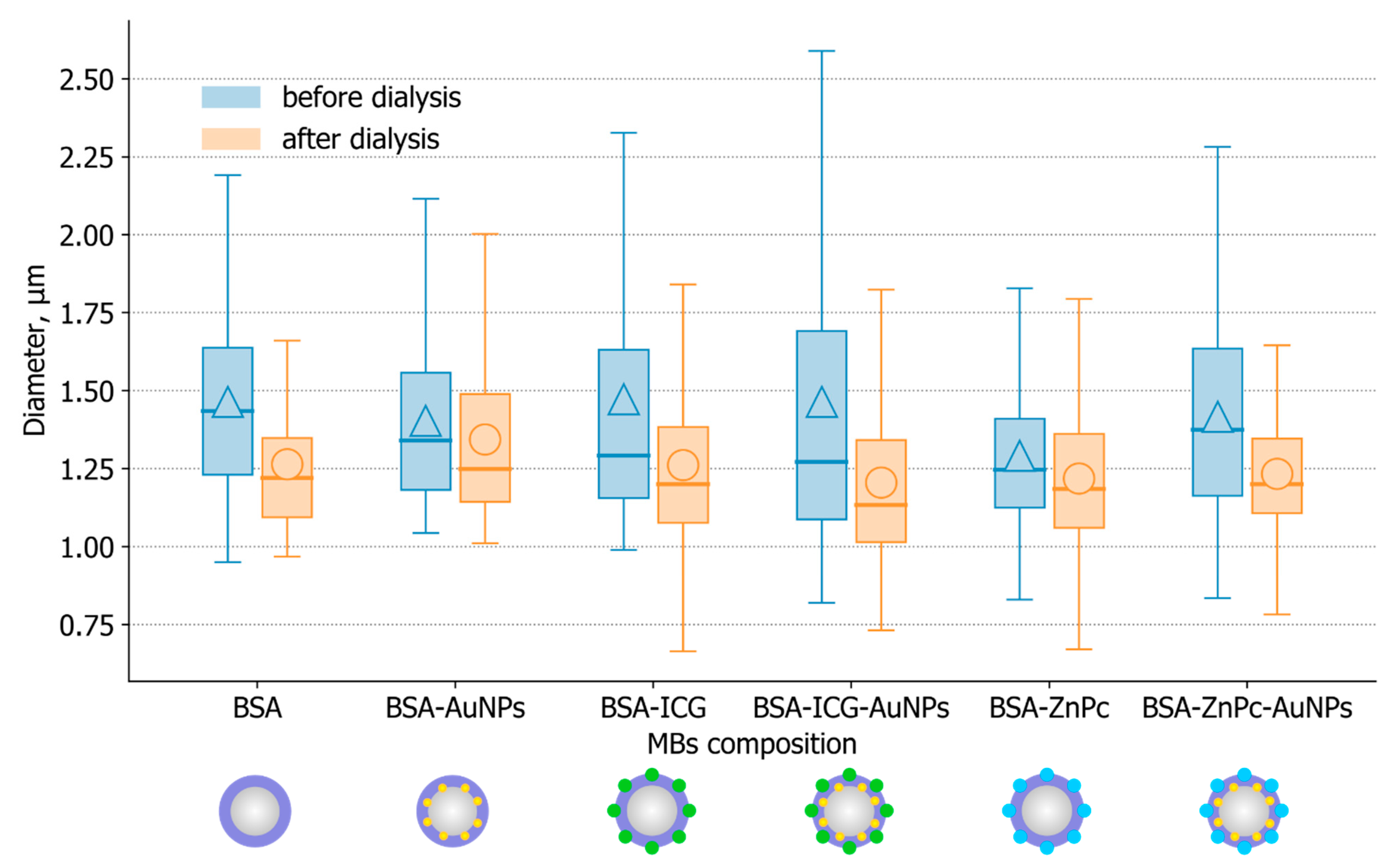
3.2. Samples Characterization: Concentration and Mean Size of Stabilized Microbubbles
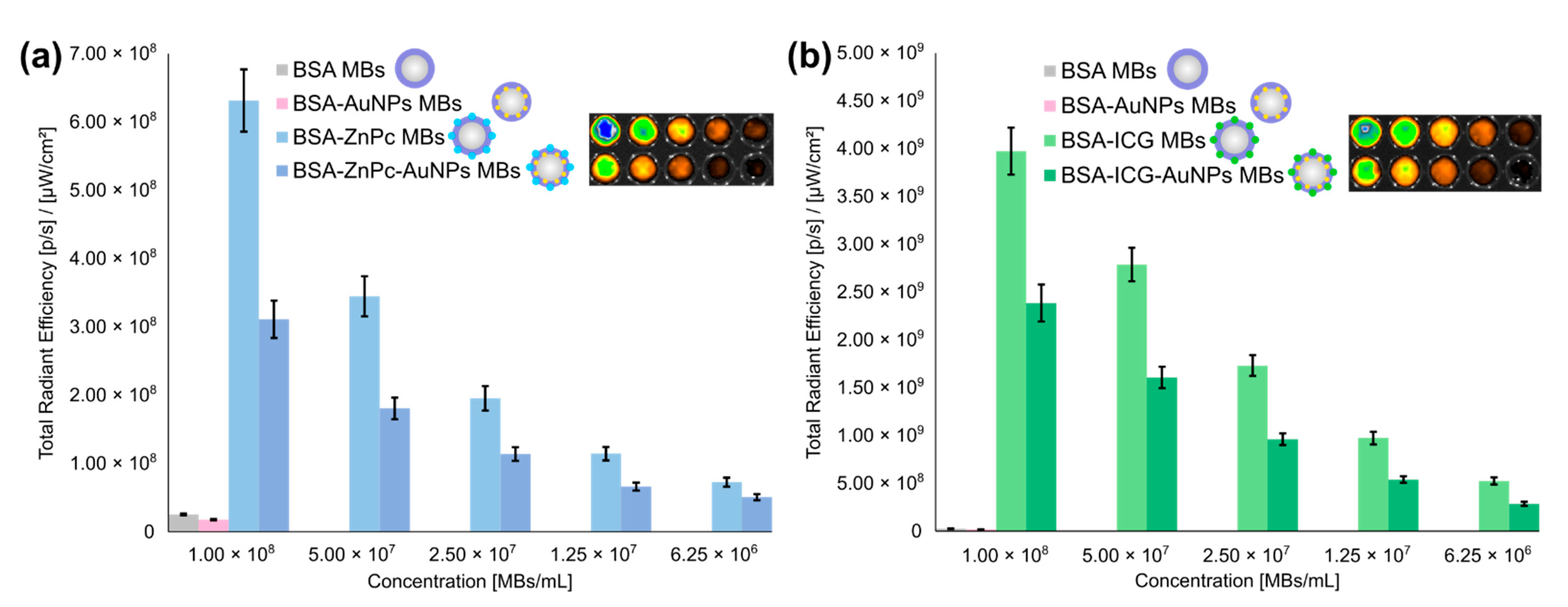
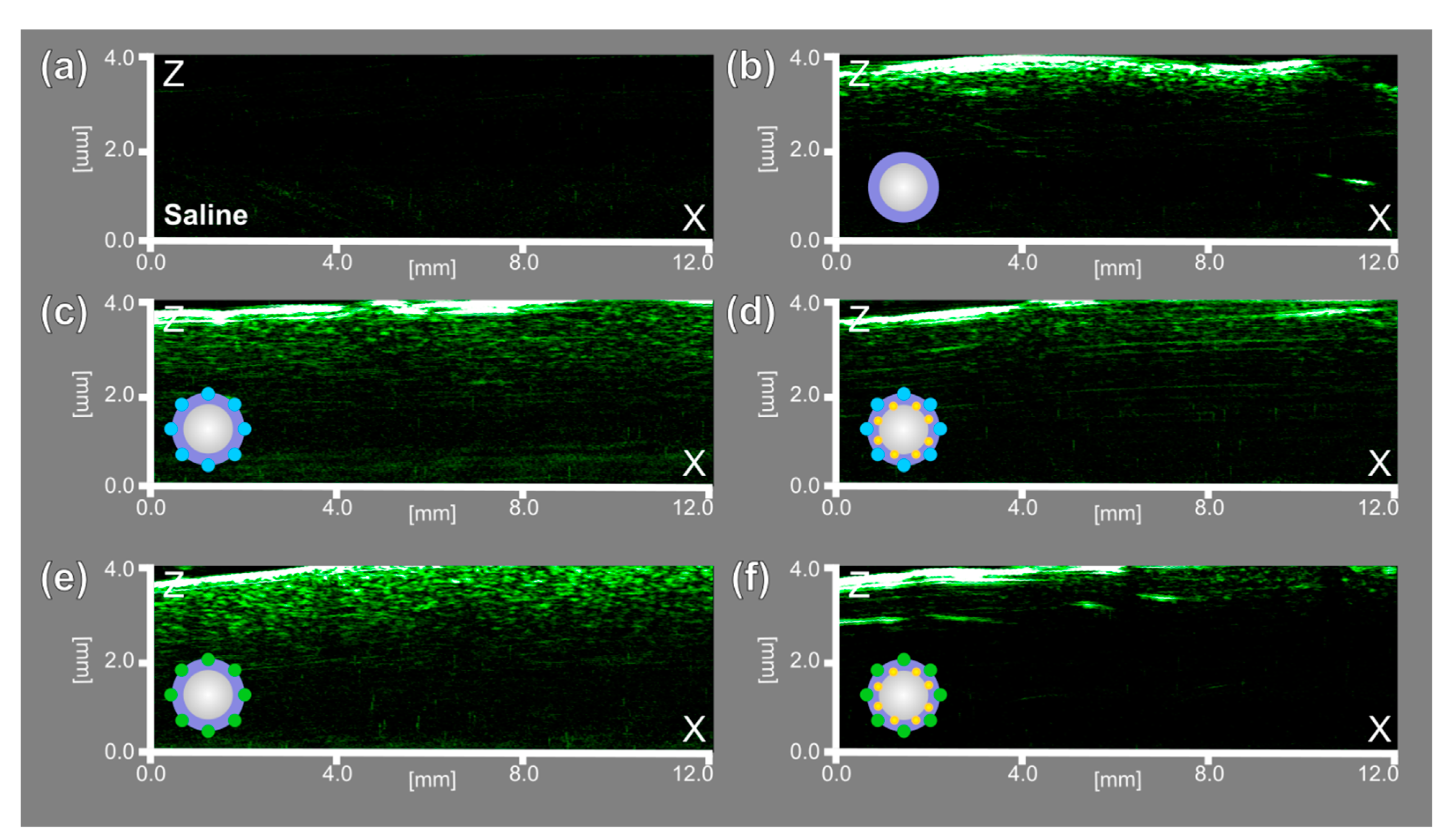
3.3. Characterization of Fluorescent, Photoacoustic and Ultrasound Imaging Properties of Microbubbles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Kim, H.; Han, H.; Lee, M.; Lee, S.; Yoo, H.; Chang, J.H.; Kim, H. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed. Eng. Lett. 2017, 7, 59–69. [Google Scholar] [CrossRef]
- Rovers, T.A.M.; Sala, G.; van der Linden, E.; Meinders, M.B.J. Temperature is key to yield and stability of BSA stabilized microbubbles. Food Hydrocoll. 2016, 52, 106–115. [Google Scholar] [CrossRef]
- Stride, E.; Saffari, N. Microbubble ultrasound contrast agents: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2003, 217, 429–447. [Google Scholar] [CrossRef]
- Orozco, J.; Jurado-Sánchez, B.; Wagner, G.; Gao, W.; Vazquez-Duhalt, R.; Sattayasamitsathit, S.; Galarnyk, M.; Cortés, A.; Saintillan, D.; Wang, J. Bubble-propelled micromotors for enhanced transport of passive tracers. Langmuir 2014, 30, 5082–5087. [Google Scholar] [CrossRef] [Green Version]
- Shchukin, D.G.; Möhwald, H. Sonochemical nanosynthesis at the engineered interface of a cavitation microbubble. Phys. Chem. Chem. Phys. 2006, 8, 3496–3506. [Google Scholar] [CrossRef]
- Himuro, S. Physicochemical characteristics of microbubbles. Chemical Engineering of Japan 2007, 71, 165–169. [Google Scholar] [CrossRef]
- Sirsi, S.; Feshitan, J.; Kwan, J.; Homma, S.; Borden, M. Effect of microbubble size on fundamental mode high frequency ultrasound imaging in mice. Ultrasound Med. Biol. 2010, 36, 935–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, A.; Dalvi, S.V. Synthesis, characterization and stability of BSA-encapsulated microbubbles. RSC Adv. 2016, 6, 15016–15026. [Google Scholar] [CrossRef]
- Stride, E.; Edirisinghe, M. Novel microbubble preparation technologies. Soft Matter 2008, 4, 2350–2359. [Google Scholar] [CrossRef]
- Hettiarachchi, K.; Talu, E.; Longo, M.L.; Dayton, P.A.; Lee, A.P. On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging. Lab Chip 2007, 7, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suslick, K.S.; Grinstaff, M.W.; Kolbeck, K.J.; Wong, M. Characterization of sonochemically prepared proteinaceous microspheres. Ultrason. Sonochem. 1994, 1, 65–68. [Google Scholar] [CrossRef]
- Murayama, K.; Tomida, M. Heat-induced secondary structure and conformation change of bovine serum albumin investigated by Fourier transform infrared spectroscopy. Biochemistry 2004, 43, 11526–11532. [Google Scholar] [CrossRef]
- Borrelli, M.J.; O’Brien, W.D.; Bernock, L.J.; Williams, H.R.; Hamilton, E.; Wu, J.; Oelze, M.L.; Culp, W.C. Production of uniformly sized serum albumin and dextrose microbubbles. Ultrason. Sonochem. 2012, 19, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Grinstaff, M.W.; Suslick, K.S. Air-filled proteinaceous microbubbles: Synthesis of an echo-contrast agent. Proc. Natl. Acad. Sci. USA 1991, 88, 7708–7710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalieri, F.; Ashokkumar, M.; Grieser, F.; Caruso, F. Ultrasonic synthesis of stable, functional lysozyme microbubbles. Langmuir 2008, 24, 10078–10083. [Google Scholar] [CrossRef]
- Avivi, S.; Gedanken, A. Proteinaceous Microspheres: The Case of Streptavidin. Biochem. J. 2002, 366, 705–707. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, B.A.; Sanders, J.M.; Davis, C.; Xie, A.; Aldred, P.; Sarembock, I.J.; Lindner, J.R. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 2007, 116, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Klibanov, A.L. Ultrasound molecular imaging with targeted microbubble contrast agents. J. Nucl. Cardiol. 2007, 14, 876–884. [Google Scholar] [CrossRef]
- Borden, M.A.; Zhang, H.; Gillies, R.J.; Dayton, P.A.; Ferrara, K.W. A stimulus-responsive contrast agent for ultrasound molecular imaging. Biomaterials 2008, 29, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, Y.; Fitzgerald, P.J. Frontiers in intravascular imaging technologies. Circulation 2008, 117, 2024–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkard, M.E.; Van Liew, H.D. Oxygen transport to tissue by persistent bubbles: Theory and simulations. J. Appl. Physiol. 1994, 77, 2874–2878. [Google Scholar] [CrossRef]
- Swanson, E.J.; Borden, M.A. Injectable Oxygen Delivery Based on Protein-Shelled Microbubbles. Nano Life 2010, 1, 215–218. [Google Scholar] [CrossRef]
- Swanson, E.J.; Mohan, V.; Kheir, J.; Borden, M.A. Phospholipid-stabilized microbubble foam for injectable oxygen delivery. Langmuir 2010, 26, 15726–15729. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.H.; Wan, M.; Dayton, P.A.; Ferrara, K.W. Optical observation of lipid- and polymer-shelled ultrasound microbubble contrast agents. Appl. Phys. Lett. 2004, 84, 631–633. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-H.; Liao, A.-H.; Chen, J.-H.; Chris Wang, C.-R.; Li, P.-C. Photoacoustic/ultrasound dual-modality contrast agent and its application to thermotherapy. J. Biomed. Opt. 2012, 17, 045001. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liao, A.-H.; Lin, J.-Y.; Lee, C.-R.; Wu, C.-H.; Liu, T.-M.; Wang, C.-R.; Li, P.-C. Enhanced Delivery of Gold Nanoparticles by Acoustic Cavitation for Photoacoustic Imaging and Photothermal Therapy. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2013, San Francisco, CA, USA, 4 March 2013; Volume 8581, p. 858123. [Google Scholar] [CrossRef]
- Dumur, F.; Dumas, E.; Mayer, C.R. Functionalization of gold nanoparticles by inorganic entities. Nanomaterials 2020, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Chen, S.P.; Liao, A.H.; Yang, Y.C.; Lee, C.R.; Wu, C.H.; Wu, P.C.; Liu, T.M.; Wang, C.R.C.; Li, P.C. Synergistic delivery of gold nanorods using multifunctional microbubbles for enhanced plasmonic photothermal therapy. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Pang, X.; Jung, S.; Zhang, G.; Kong, M.; Liu, G.; Chen, X. Smart gold nanoparticle-stabilized ultrasound microbubbles as cancer theranostics. J. Mater. Chem. B 2018, 6, 3235–3239. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Yakubovskaya, R.I.; Morozova, N.B.; Pankratov, A.A.; Kazachkina, N.I.; Plyutinskaya, A.D.; Karmakova, T.A.; Andreeva, T.N.; Venediktova, Y.B.; Plotnikova, E.A.; Nemtsova, E.R.; et al. Experimental photodynamic therapy: 15 years of development. Russ. J. Gen. Chem. 2015, 85, 217–239. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.J.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. J. Photochem. Photobiol. B Biol. 2019, 191, 128–134. [Google Scholar] [CrossRef]
- Li, X.; De Zheng, B.; Peng, X.H.; Li, S.Z.; Ying, J.W.; Zhao, Y.; Huang, J.D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef]
- Kolarova, H.; Tomankova, K.; Bajgar, R.; Kolar, P.; Kubinek, R. Photodynamic and Sonodynamic Treatment by Phthalocyanine on Cancer Cell Lines. Ultrasound Med. Biol. 2009, 35, 1397–1404. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Sun, J.; Zhu, S.; Chen, C.; Xie, W.; Zheng, J.; Zhu, Y.; Xiao, L.; Hao, L.; et al. Folate-Targeted and Oxygen/Indocyanine Green-Loaded Lipid Nanoparticles for Dual-Mode Imaging and Photo-sonodynamic/Photothermal Therapy of Ovarian Cancer in Vitro and in Vivo. Mol. Pharm. 2019, 16, 4104–4120. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wu, Q.; Si, T.; Chang, S.; Xu, R.X. Oxygen and Indocyanine Green loaded microparticles for dual-mode imaging and sonodynamic treatment of cancer cells. Ultrason. Sonochem. 2017, 39, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tchuenbou-Magaia, F.L.; Al-Rifai, N.; Ishak, N.E.M.; Norton, I.T.; Cox, P.W. Suspensions of air cells with cysteine-rich protein coats: Air-filled emulsions. J. Cell. Plast. 2011, 47, 217–232. [Google Scholar] [CrossRef]
- Tchuenbou-Magaia, F.L.; Cox, P.W. Tribological study of suspensions of cysteine-rich protein stabilized microbubbles and subsequent triphasic A/O/W emulsions. J. Texture Stud. 2011, 42, 185–196. [Google Scholar] [CrossRef]
- Zhou, M.; Cavalieri, F.; Ashokkumar, M. Tailoring the properties of ultrasonically synthesised microbubbles. Soft Matter 2011, 7, 623–630. [Google Scholar] [CrossRef]
- Makarov, D.A.; Yuzhakova, O.A.; Slivka, L.K.; Kuznetsova, N.A.; Negrimovsky, V.M.; Kaliya, O.L.; Lukyanets, E.A. Cationic Zn and Al phthalocyanines: Synthesis, spectroscopy and photosensitizing properties. J. Porphyr. Phthalocyanines 2007, 11, 586–595. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Tebbe, M.; Kuttner, C.; Männel, M.; Fery, A.; Chanana, M. Colloidally Stable and Surfactant-Free Protein-Coated Gold Nanorods in Biological Media. ACS Appl. Mater. Interfaces 2015, 7, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.S.; Skliar, M. Surface tension of water in the presence of perfluorocarbon vapors. Soft Matter 2014, 10, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.S.; Skliar, M. Diffusivity measurements of solutes impacting interfacial tension. Ind. Eng. Chem. Res. 2015, 54, 4535–4544. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Ljung, L. System Identification: Theory for the User, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1999. [Google Scholar]
- Rovers, T.A.M.; Sala, G.; Van Der Linden, E.; Meinders, M.B.J. Effect of Temperature and Pressure on the Stability of Protein Microbubbles. ACS Appl. Mater. Interfaces 2016, 8, 333–340. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V. Microbubble Formulations: Synthesis, Stability, Modeling and Biomedical Applications. Ultrasound Med. Biol. 2019, 45, 301–343. [Google Scholar] [CrossRef]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef]
- Köse, G.; Darguzyte, M.; Kiessling, F. Molecular ultrasound imaging. Nanomaterials 2020, 10, 1935. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallidi, S.; Larson, T.; Tam, J.; Joshi, P.P.; Karpiouk, A.; Sokolov, K.; Emelianov, S. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009, 9, 2825–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.Y.; Ricles, L.M.; Suggs, L.J.; Emelianov, S.Y. Nonlinear photoacoustic signal increase from endocytosis of gold nanoparticles. Opt. Lett. 2012, 37, 4708. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barmin, R.A.; Rudakovskaya, P.G.; Gusliakova, O.I.; Sindeeva, O.A.; Prikhozhdenko, E.S.; Maksimova, E.A.; Obukhova, E.N.; Chernyshev, V.S.; Khlebtsov, B.N.; Solovev, A.A.; et al. Air-Filled Bubbles Stabilized by Gold Nanoparticle/Photodynamic Dye Hybrid Structures for Theranostics. Nanomaterials 2021, 11, 415. https://doi.org/10.3390/nano11020415
Barmin RA, Rudakovskaya PG, Gusliakova OI, Sindeeva OA, Prikhozhdenko ES, Maksimova EA, Obukhova EN, Chernyshev VS, Khlebtsov BN, Solovev AA, et al. Air-Filled Bubbles Stabilized by Gold Nanoparticle/Photodynamic Dye Hybrid Structures for Theranostics. Nanomaterials. 2021; 11(2):415. https://doi.org/10.3390/nano11020415
Chicago/Turabian StyleBarmin, Roman A., Polina G. Rudakovskaya, Olga I. Gusliakova, Olga A. Sindeeva, Ekaterina S. Prikhozhdenko, Elizaveta A. Maksimova, Ekaterina N. Obukhova, Vasiliy S. Chernyshev, Boris N. Khlebtsov, Alexander A. Solovev, and et al. 2021. "Air-Filled Bubbles Stabilized by Gold Nanoparticle/Photodynamic Dye Hybrid Structures for Theranostics" Nanomaterials 11, no. 2: 415. https://doi.org/10.3390/nano11020415
APA StyleBarmin, R. A., Rudakovskaya, P. G., Gusliakova, O. I., Sindeeva, O. A., Prikhozhdenko, E. S., Maksimova, E. A., Obukhova, E. N., Chernyshev, V. S., Khlebtsov, B. N., Solovev, A. A., Sukhorukov, G. B., & Gorin, D. A. (2021). Air-Filled Bubbles Stabilized by Gold Nanoparticle/Photodynamic Dye Hybrid Structures for Theranostics. Nanomaterials, 11(2), 415. https://doi.org/10.3390/nano11020415










