Large-Area Nanocrystalline Caesium Lead Chloride Thin Films: A Focus on the Exciton Recombination Dynamics
Abstract
1. Introduction
2. Materials and Methods
3. Results
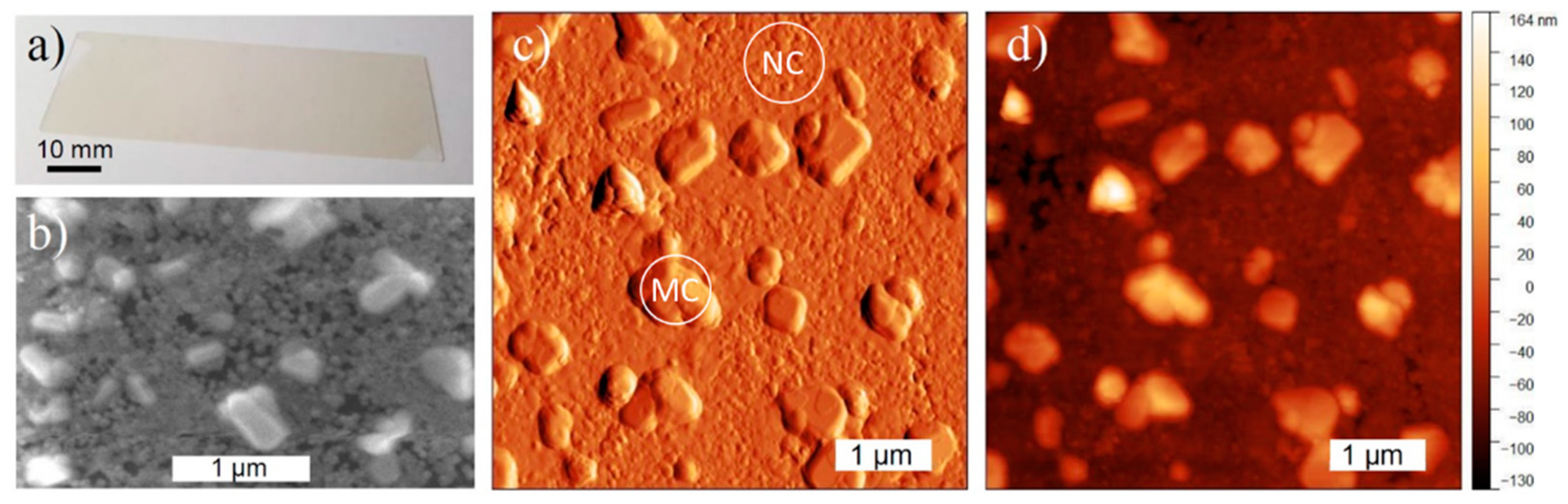
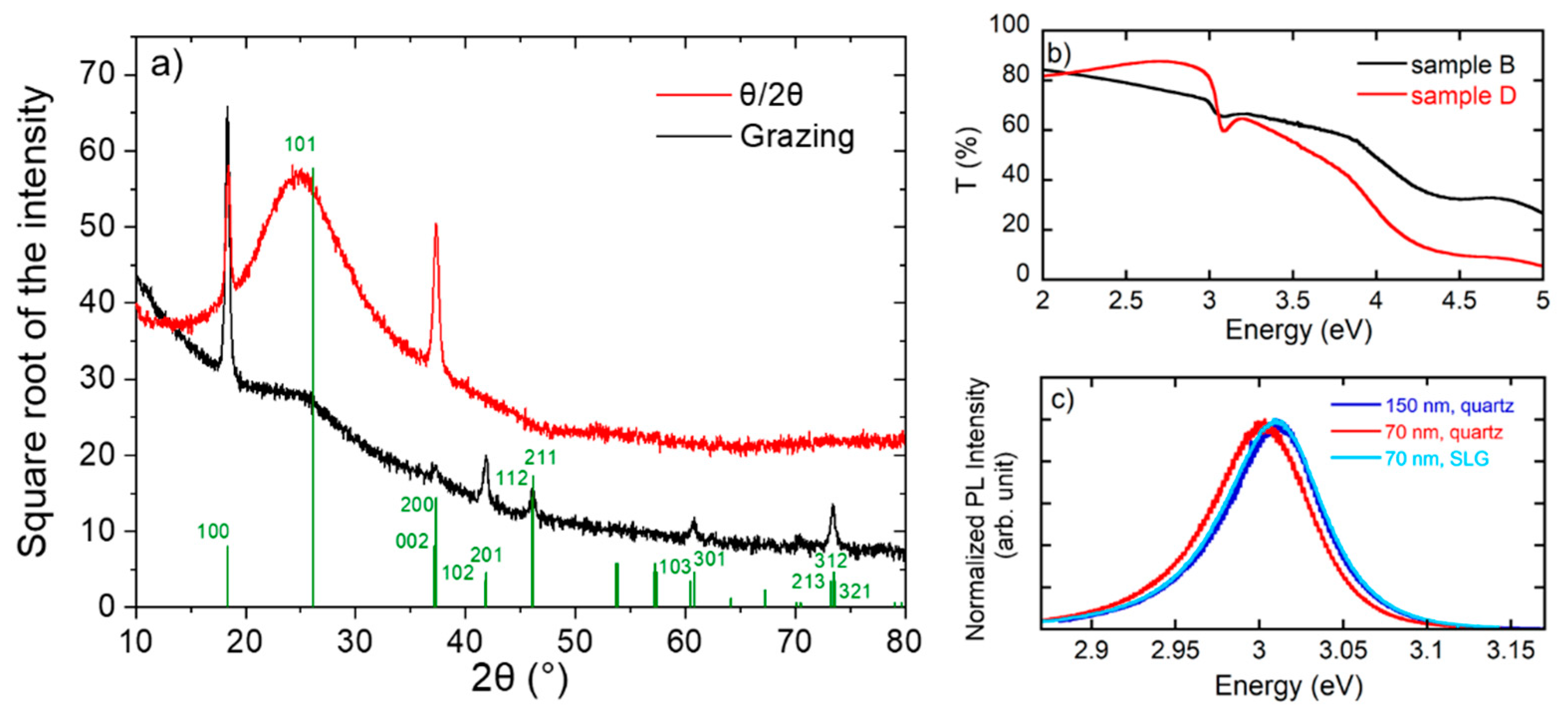
3.1. Samples Characterization at Room Temperature
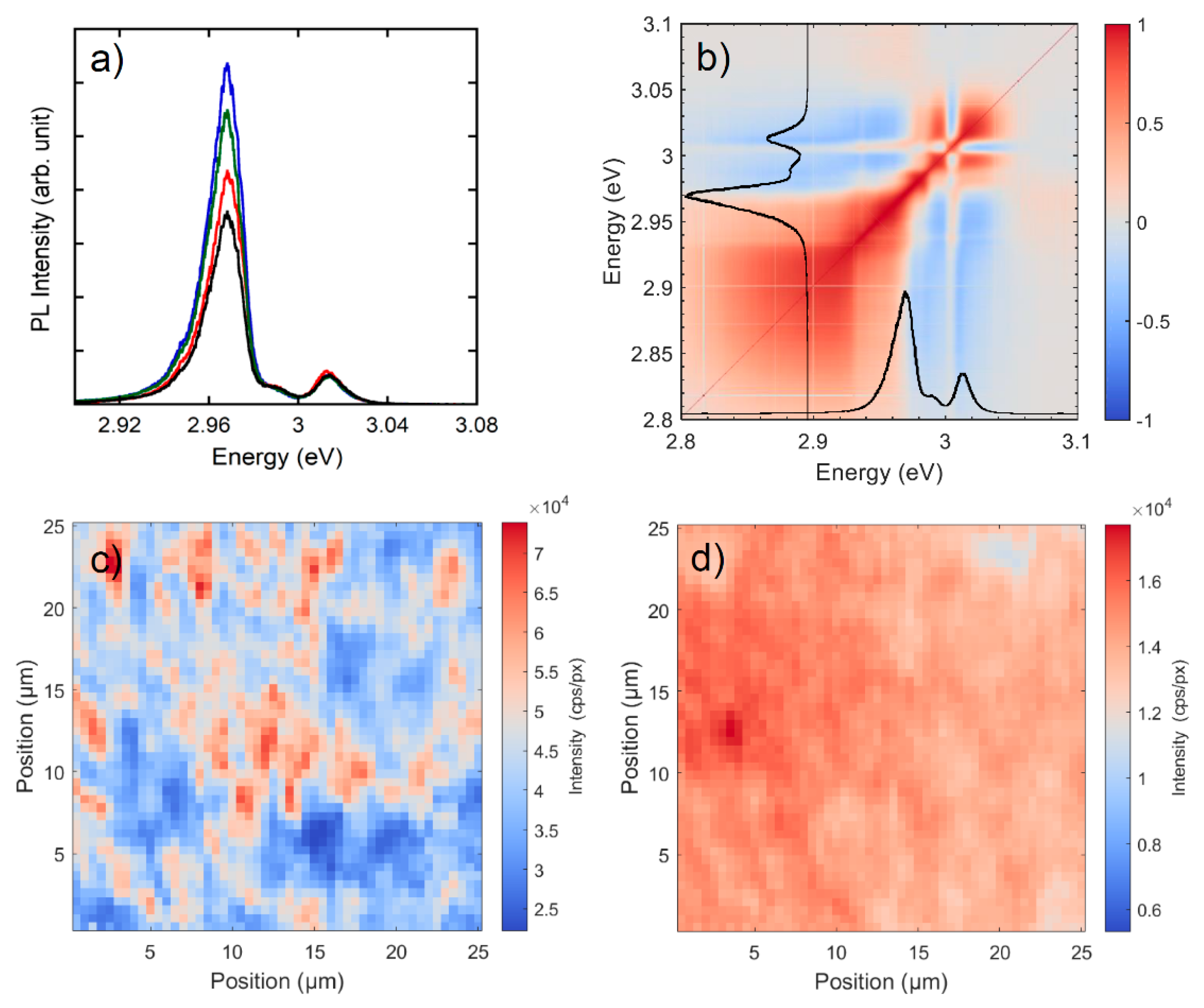
3.2. Photoluminescence Study
3.2.1. Photoluminescence at Macro and Micro-Scale
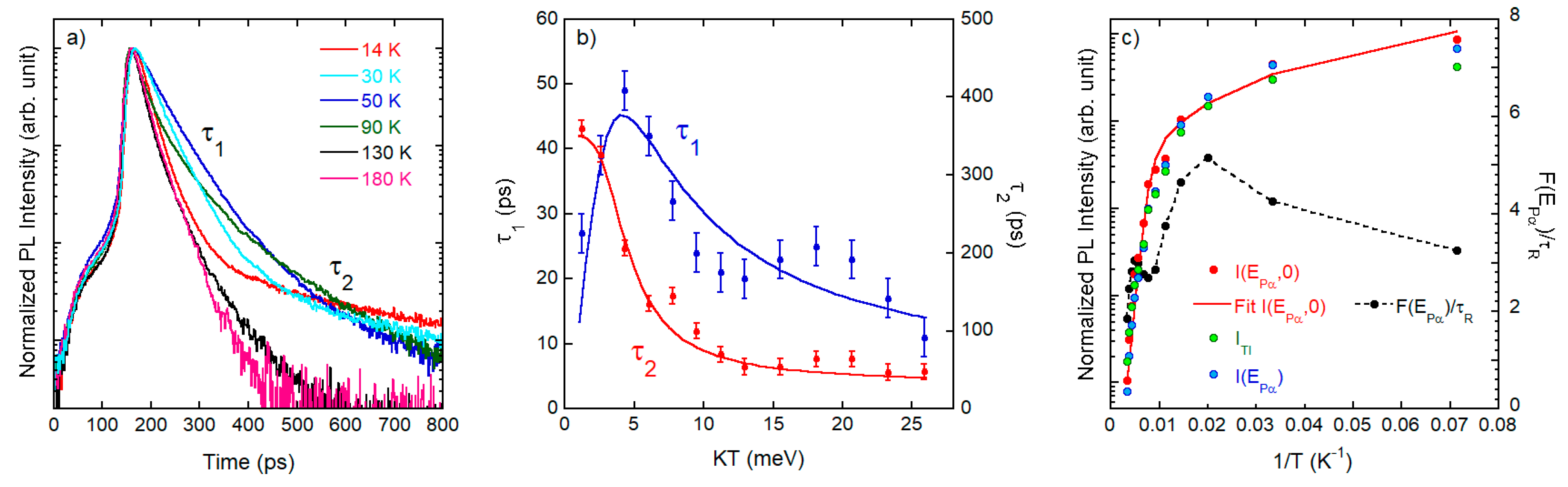
3.2.2. Time-Resolved Photoluminescence
4. Data Analysis and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PL | Photoluminescence |
| SLG | Soda Lime Glass |
| RF | Radio Frequency |
| SEM | Scanning Electron Microscopy |
| AFM | Atomic Force Microscopy |
| XPS | X-Ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
| TI | Time-integrated |
| TR | Time-resolved |
| NC | Network of nanocrystals |
| MCs | Sub-micrometer size larger crystals |
| FE | Free Exciton |
| IB | Inhomogeneous Broadening |
References
- Sutherland, B.R.; Sargent, E.H. Perovskite photonic sources. Nat. Photogr. 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.-K.; Dai, Z.; Yu, G.; Haus, J.W.; Zhang, H.; Prasad, P.N. Photonics and optoelectronics using nano-structured hybrid perovskite media and their optical cavities. Phys. Rep. 2019, 795, 1–51. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Li, J.; Xue, J.; Dong, Y.; Li, X.; Zeng, H. Monolayer and few-layer all-inorganic perovskites as a new family of two-dimensional semiconductors for printable optoelectronic devices. Adv. Mater. 2016, 28, 4861. [Google Scholar] [CrossRef]
- Zhang, J.; Hodes, G.; Jin, S.; Liu, S. All-inorganic CsPbX3 perovskite solar cells: Progress and prospects. Angew. Chem. Int. Ed. 2019, 58, 15596. [Google Scholar] [CrossRef] [PubMed]
- Bruzzi, M.; Talamonti, C.; Calisi, N.; Caporali, S.; Vinattieri, A. First proof-of-principle of inorganic perovskites clinical radiotherapy dosimeters. APL Mater. 2019, 7, 051101. [Google Scholar] [CrossRef]
- Li, D.; Liao, P.; Shai, X.; Huang, W.; Liu, S.; Li, H.; Shena, Y.; Wang, M. Recent progress on stability issues of organic–inorganic hybrid lead perovskite-based solar cells. RSC Adv. 2016, 6, 89356. [Google Scholar] [CrossRef]
- Mitzi, D.B. Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials, in Progress in Inorganic Chemistry; Karlin, K.D., Ed.; Wiley: Hoboken, NJ, USA, 1999; pp. 1–121. [Google Scholar]
- Biccari, F.; Falsini, N.; Bruzzi, M.; Gabelloni, F.; Calisi, N.; Vinattieri, A. Defects in Perovskites for Solar Cells and LEDs, Chapter 3 in “Defects in Functional Materials”; Ling, F.C., Zhou, S., Kuznetsov, A., Eds.; World Scientific Publishing: Singapore, 2020; ISBN 978-9811203169. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, S.; Li, L.; Chen, W. Research progress on large-area perovskite thin films and solar modules. J. Mater. 2017, 3, 231. [Google Scholar] [CrossRef]
- Pasquarelli, R.M.; Ginley, D.S.; O’Hayre, R. Solution processing of transparent conductors: From flask to film. Chem. Soc. Rev. 2011, 40, 5406. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, S.; Sawada, S. Crystal growth and phase transitions of CsPbCl3. Phys. Lett. 1969, 28, 762. [Google Scholar] [CrossRef]
- Yunakova, N.; Miloslavsky, V.K.; Kovalenko, E.N.; Kovalenko, V.V. Effect of structural phase transitions on the exciton absorption spectrum of thin CsPbCl3 films. J. Low Temp. Phys. 2014, 40, 690. [Google Scholar] [CrossRef]
- Heidrich, K.; Künzel, H.; Treusch, J. Optical properties and electronic structure of CsPbCl3 and CsPbBr3. Solid State Commun. 1978, 25, 887. [Google Scholar] [CrossRef]
- Somma, F.; Aloe, P.; Lo Mastro, S. Structural and optical properties of ternary Cs–Pb–Cl nanoaggregates in thin films. J. Vac. Sci. Technol. 2001, 19, 2237. [Google Scholar] [CrossRef]
- Nikl, M.; Nitsch, K.; Polak, K.; Pazzi, G.P.; Fabeni, P.; Citrin, D.S.; Gurioli, M. Optical properties of the Pb2+-based aggregated phase in a CsCl host crystal: Quantum-confinement effects. Phys. Rev. B 1995, 51, 5192. [Google Scholar] [CrossRef]
- Kondo, S.; Nakagawa, H.; Saito, T.; Asada, H. Photoluminescence of CsPbCl3 films prepared by quench deposition and subsequent heat treatments. J. Phys. Condens. Matt. 2003, 15, 1247. [Google Scholar] [CrossRef]
- Borri, C.; Calisi, N.; Galvanetto, E.; Falsini, N. First proof-of-principle of inorganic lead halide perovskites deposition by magnetron-sputtering. Nanomaterials 2020, 10, 60. [Google Scholar] [CrossRef]
- Scardi, P.; Setti, S.; Leoni, M. Multicapillary optics for materials science studies. Mat. Sci. Forum 2000, 321, 162. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Tozaki, K.; Kojima, A.; Iwasaki, H. In Smart Structures and Materials 2001: Active Materials: Behavior and Mechanics. In Proceedings of the SPIE 2001, Newport Beach, CA, USA, 4–8 March 2001; Volume 4333. [Google Scholar]
- Iwanaga, M. Photoacoustic detection of phase transitions at low temperatures in CsPbCl3 crystals. Phase Trans. 2005, 78, 377. [Google Scholar] [CrossRef]
- Chen, H.; Guo, A.; Gu, X.; Feng, M. Highly luminescent CsPbX3 (X = Cl, Br, I) perovskite nanocrystals with tunable photoluminescence properties. J. Alloys Comp. 2019, 789, 392. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MI, USA, 1992. [Google Scholar]
- Fröhlich, D.; Heidrich, K.; Künzel, H.; Trendel, G.; Treusch, J. Cesium-trihalogen-plumbates a new class of ionic semiconductors. J. Luminescence 1979, 18, 385. [Google Scholar] [CrossRef]
- Ito, H.; Onuki, H.; Onaka, R. Optical and photoelectronic studies of CsPbCl3 and CsPbBr3. J. Phys. Soc. Jpn. 1978, 45, 2043. [Google Scholar] [CrossRef]
- Sebastian, M.; Peters, J.A.; Stoumpos, C.C.; Kostina, S.S.; Liu, Z.; Kanatzidis, M.G.; Freeman, A.J.; Wessels, B.W. Excitonic emissions and above-band-gap luminescence in the single-crystal perovskite semiconductors CsPbBr3 and CsPbCl3. Phys. Rev. B 2015, 92, 235210. [Google Scholar] [CrossRef]
- Pashuk, P.; Pidzyrailo, N.S.; Matsko, M.G. Exciton absorption, luminescence, and resonant Raman scattering in CsPbCl3 and CsPbBr3 at low temperatures. Sov. Phys. Solid State 1981, 23, 1263. [Google Scholar]
- Lohar, A.; Shinde, A.; Gahlaut, R.; Sagdeo, A.; Mahamuni, S. Enhanced photoluminescence and stimulated emission in CsPbCl3 nanocrystals at low temperature. J. Phys. Chem. C 2018, 122, 25014. [Google Scholar] [CrossRef]
- Ito, H.; Nakahara, J.; Onaka, R. Magneto-optical study of the exciton states in CsPbCl3. J. Phys. Soc. Jpn. 1979, 47, 1927. [Google Scholar] [CrossRef]
- Becker, M.A.; Scarpelli, L.; Nedelcu, G.; Rainò, G.; Masia, F.; Borri, P.; Stöferle, T.; Kovalenko, M.V.; Langbein, W.; Mahrt, R.F. Long exciton dephasing time and coherent phonon coupling in CsPbBr2Cl perovskite nanocrystals. Nano Lett. 2018, 18, 7546. [Google Scholar] [CrossRef] [PubMed]
- Shah, J. Ultrafast Spectroscopy of Semiconductors and Semiconductor Nanostructures; Springer: Heidelberg, Germany, 1996. [Google Scholar]
- Gabelloni, F.; Biccari, F.; Falsini, N.; Calisi, N.; Caporali, S.; Vinattieri, A. Long-living nonlinear behavior in CsPbBr3 carrier recombination dynamics. Nanophotonics 2019, 8, 1447. [Google Scholar] [CrossRef]
- Cavigli, L.; Bogani, F.; Vinattieri, A.; Faso, V.; Baldi, G. Volume versus surface-mediated recombination in anatase TiO2 nanoparticles. J. Appl. Phys. 2009, 106, 053516. [Google Scholar] [CrossRef]
- Kondo, S.; Suzuki, K.; Saito, T.; Asada, H.; Nakagawa, H. Confinement-enhanced stimulated emission in microcrystalline CsPbCl3 films grown from the amorphous phase. J. Cryst. Growth 2005, 282, 94. [Google Scholar] [CrossRef]
- Nikl, M.; Birch, D.J.S.; Polak, K. Blue and violet emission of PbCl2. Phys. Solid State 1991, 165, 611. [Google Scholar] [CrossRef]
- Voloshinovskii, A.; Myagkota, S.; Gloskovskii, A.; Zazubovich, S. Luminescence of CsPbCl3 nanocrystals dispersed in a CsCl crystal under high-energy excitation. Phys. Solid State 2001, 225, 257. [Google Scholar] [CrossRef]
- Bai, K.; Tan, R.; Ke, B.; Xue, X.; Zhao, J.; Zouc, B.; Zeng, R. Room temperature synthesis of Mn-doped Cs3Pb6.48Cl16 perovskite nanocrystals with pure dopant emission and temperature-dependent photoluminescence. Cryst. Eng. Comm. 2019, 21, 3568–3575. [Google Scholar] [CrossRef]
- Biccari, F.; Gabelloni, F.; Burzi, E.; Gurioli, M.; Pescetelli, S.; Agresti, A.; Castillo, A.E.D.; Ansaldo, A.; Kymakis, E.; Bonaccorso, F.; et al. Graphene-based electron transport layers in perovskite solar cells: A step-up for an efficient carrier collection. Adv. Energy Mat. 2017, 7, 1701349. [Google Scholar] [CrossRef]
- Dobrovolsky, A.; Merdasa, A.; Unger, E.L.; Yartsev, A.; Scheblykin, I.G. Defect-induced local variation of crystal phase transition temperature in metal-halide perovskites. Nat. Commun. 2017, 8, 34. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, R.; Fu, Z.; Zhang, Q. Domain-dependent electronic structure and optical absorption property in hybrid organic–inorganic perovskite. Phys. Chem. Chem. Phys. 2016, 18, 27358. [Google Scholar] [CrossRef] [PubMed]
- Piveteau, L.; Aebli, M.; Yazdani, N.; Millen, M.; Korosec, L.; Krieg, F.; Benin, B.M.; Morad, V.; Piveteau, C.; Shiroka, T.; et al. Bulk and nanocrystalline cesium lead-halide perovskites as seen by halide magnetic resonance. ACS Cent. Sci. 2020, 6, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, X.; Sun, X.; Zhang, X. Phonon mode transformation in size-evolved solution-processed inorganic lead halide perovskite. Nanoscale 2018, 10, 9892. [Google Scholar] [CrossRef]
- Cape, J.A.; White, R.L.; Feigelson, R.S. EPR study of the structure of CsPbCl3. J. Appl. Phys. 1969, 40, 5001. [Google Scholar] [CrossRef]
- Carabatos-Nédelec, C.; Oussaїd, M.; Nitsch, K. Raman scattering investigation of cesium plumbochloride, CsPbCl3, phase transitions. J. Raman Spectrosc. 2003, 34, 388. [Google Scholar] [CrossRef]
- Yi, J.; Ge, X.; Liu, E.; Cai, T.; Zhao, C.; Wen, S.; Sanabria, H.; Chen, O.; Rao, A.M.; Gao, J. The correlation between phase transition and photoluminescence properties of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals. Nanoscale Adv. 2020, 2, 4390. [Google Scholar] [CrossRef]
- Becker, M.; Vaxenburg, R.; Nedelcu, G.; Sercel, P.; Shabaev, A.; Mehl, M.; Michopoulos, J.; Lambrakos, S.; Bernstein, N.; Lyons, J.; et al. Bright triplet excitons in caesium lead halide perovskites. Nature 2018, 553, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Andreani, L.C.; d’Andrea, A.; del Sole, R. Excitons in confined systems: From quantum well to bulk behaviour. Phys. Lett. A 1992, 168, 451. [Google Scholar] [CrossRef]
- Haug, R. (Ed.) Advances in Solid State Physics; Springer: New York, NY, USA, 2008; Volume 48. [Google Scholar]
- Chen, L.; Li, B.; Zhang, C.; Huang, X.; Wang, X.; Xiao, M. Composition-dependent energy splitting between bright and dark excitons in lead halide perovskite nanocrystals. Nano Lett. 2018, 18, 2074. [Google Scholar] [CrossRef]
- Mondal, N.; De Samanta, A. Achieving near-unity photoluminescence efficiency for blue-violet-emitting perovskite nanocrystals. ACS Energy Lett. 2019, 4, 32. [Google Scholar] [CrossRef]
- Ahmed, G.H.; El-Demellawi, J.K.; Yin, J.; Pan, J.; Velusamy, D.B.; Hedhili, M.N.; Alarousu, E.; Bakr, O.M.; Alshareef, H.N.; Mohammed, O.F. Giant photoluminescence enhancement in CsPbCl3 perovskite nanocrystals by simultaneous dual-surface passivation. ACS Energy Lett. 2018, 3, 2301. [Google Scholar] [CrossRef]
- Ahumada-Lazo, R.; Alanis, J.A.; Parkinson, P.; Binks, D.J.; Hardman, S.J.O.; Griffiths, J.T.; Rivarola, F.W.R.; Humphrey, C.J.; Ducati, C.; Davis, N.J.L.K. Emission properties and ultrafast carrier dynamics of CsPbCl3 perovskite nanocrystals. J. Phys. Chem. C 2019, 123, 2651. [Google Scholar] [CrossRef]
- Lai, R.; Wu, K. Picosecond electron trapping limits the emissivity of CsPbCl3 perovskite nanocrystals. J. Chem. Phys. 2019, 151, 194701. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The role of oxygen in the degradation of methylammonium lead trihalide perovskite photoactive layers. Angew. Chem. Int. Ed. 2015, 54, 8208–8212. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, Y.; Lai, M.; Xie, C.; Lin, J.; Lei, T.; Lin, Z.; Kley, C.S.; Huang, J.; Rabani, E.; et al. Giant light-emission enhancement in lead halide perovskites by surface oxygen passivation. Nano Lett. 2018, 18, 6967–6973. [Google Scholar] [CrossRef]
- Rodà, C.; Abdelhady, A.L.; Shamsi, J.; Lorenzon, M.; Pinchetti, V.; Gandini, M.; Meinardi, F.; Manna, L.; Brovelli, S. O2 as a molecular probe for nonradiative surface defects in CsPbBr3 perovskite nanostructures and single crystals. Nanoscale 2019, 11, 7613. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Mosconi, E.; De Angelis, F. Mechanism of reversible trap passivation by molecular oxygen in lead-halide perovskites. ACS Energy Lett. 2017, 2, 2794–2798. [Google Scholar] [CrossRef]


| Sample | Average Thickness (nm) | Substrate |
|---|---|---|
| A | 70 | SLG |
| B | 70 | Quartz |
| C | 150 | SLG |
| D | 150 | Quartz |
| Element | Glass (Sample C) | Quartz (Sample D) | Expected |
|---|---|---|---|
| Cs | 20% | 19% | 20% |
| Pb | 30% | 30% | 20% |
| Cl | 50% | 51% | 60% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falsini, N.; Calisi, N.; Roini, G.; Ristori, A.; Biccari, F.; Scardi, P.; Barri, C.; Bollani, M.; Caporali, S.; Vinattieri, A. Large-Area Nanocrystalline Caesium Lead Chloride Thin Films: A Focus on the Exciton Recombination Dynamics. Nanomaterials 2021, 11, 434. https://doi.org/10.3390/nano11020434
Falsini N, Calisi N, Roini G, Ristori A, Biccari F, Scardi P, Barri C, Bollani M, Caporali S, Vinattieri A. Large-Area Nanocrystalline Caesium Lead Chloride Thin Films: A Focus on the Exciton Recombination Dynamics. Nanomaterials. 2021; 11(2):434. https://doi.org/10.3390/nano11020434
Chicago/Turabian StyleFalsini, Naomi, Nicola Calisi, Giammarco Roini, Andrea Ristori, Francesco Biccari, Paolo Scardi, Chiara Barri, Monica Bollani, Stefano Caporali, and Anna Vinattieri. 2021. "Large-Area Nanocrystalline Caesium Lead Chloride Thin Films: A Focus on the Exciton Recombination Dynamics" Nanomaterials 11, no. 2: 434. https://doi.org/10.3390/nano11020434
APA StyleFalsini, N., Calisi, N., Roini, G., Ristori, A., Biccari, F., Scardi, P., Barri, C., Bollani, M., Caporali, S., & Vinattieri, A. (2021). Large-Area Nanocrystalline Caesium Lead Chloride Thin Films: A Focus on the Exciton Recombination Dynamics. Nanomaterials, 11(2), 434. https://doi.org/10.3390/nano11020434












