Oxygen Gas and UV Barrier Properties of Nano-ZnO-Coated PET and PHBHHx Materials Fabricated by Ultrasonic Spray-Coating Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Ink Materials
2.1.2. Substrates
Commercial-Grade PET Foils
Compression-Molded PHBHHx Films
2.2. Methods
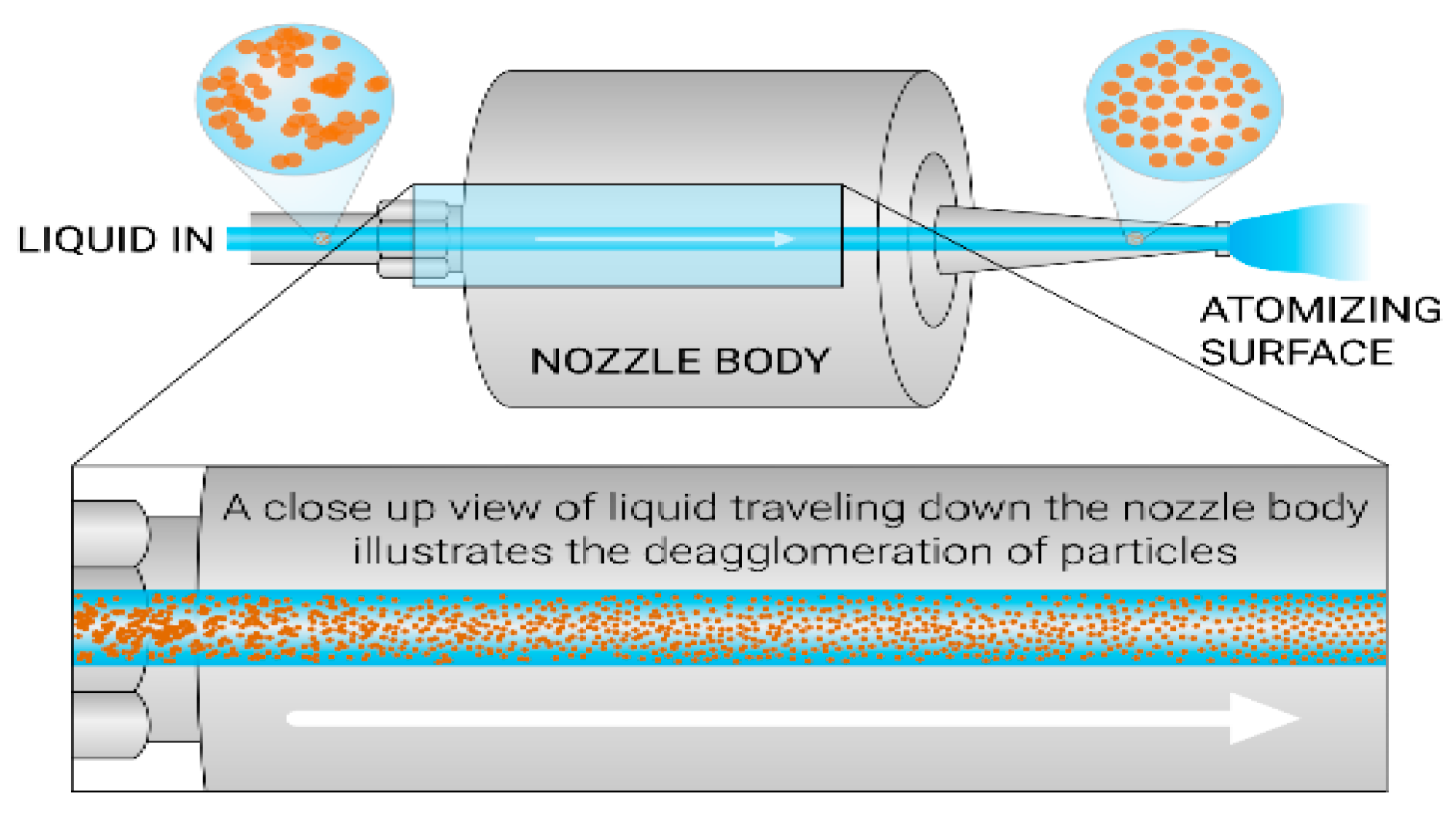
2.2.1. Ultrasonic Spray-Coating
- Ink composition: A solution prepared with nanoparticles along with chemicals, including solvents.
- Ink flow rate (mL/min): The volume of the ink sprayed per unit time [44].
- Path speed (mm/s): The distance traveled by the ultrasonic spray nozzle per unit time.
- Nozzle frequency (kHz): This is the frequency at which the nozzle vibrates [39].
- Generator power (W): This is the power required by the generator to operate the ultrasonic spray nozzle [45].
- Hot-plate temperature (°C): The value of the temperature set for the hot plate on which the substrate is placed.
- Nozzle to substrate distance (mm): This is the distance between the nozzle tip and the substrate.
- Nitrogen guiding pressure (kPa): This gas pressure helps move the sprayed droplets toward the substrate [46].
- The number of coated layers: This is the number of layers coated on the substrates.
2.2.2. Surface Morphology
2.2.3. Gas Barrier Characterization
2.2.4. UV Barrier Characterization
3. Results and Discussion
3.1. Coating Parameters
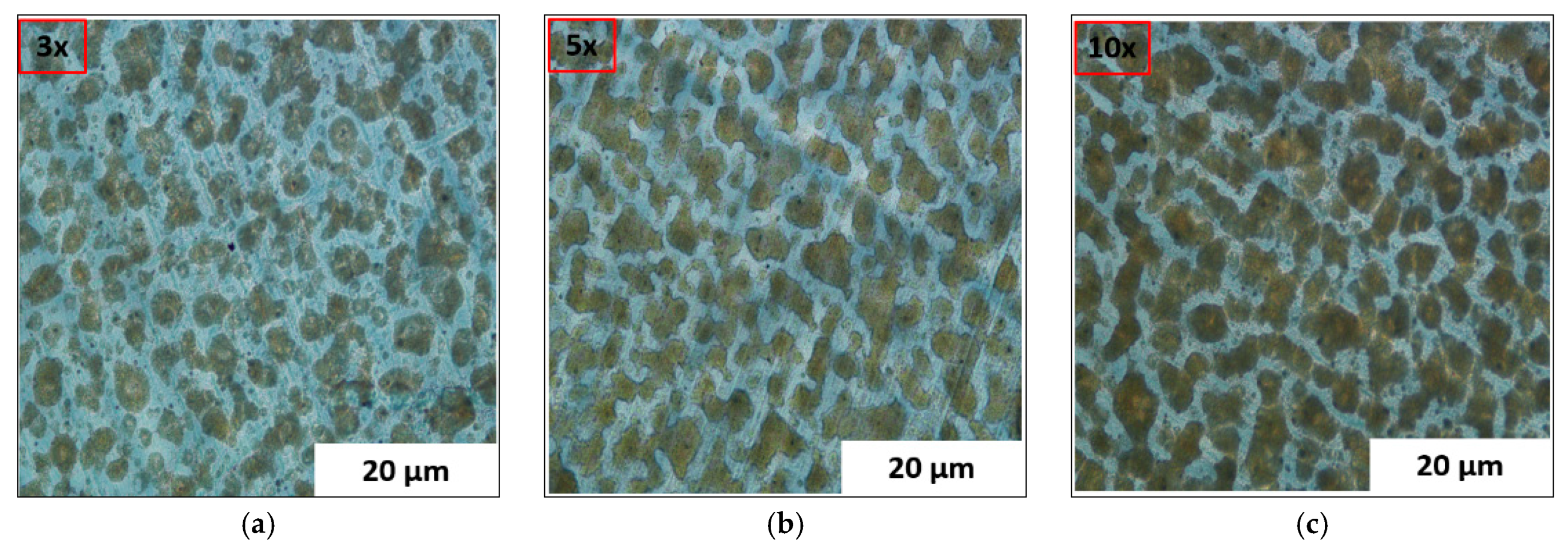
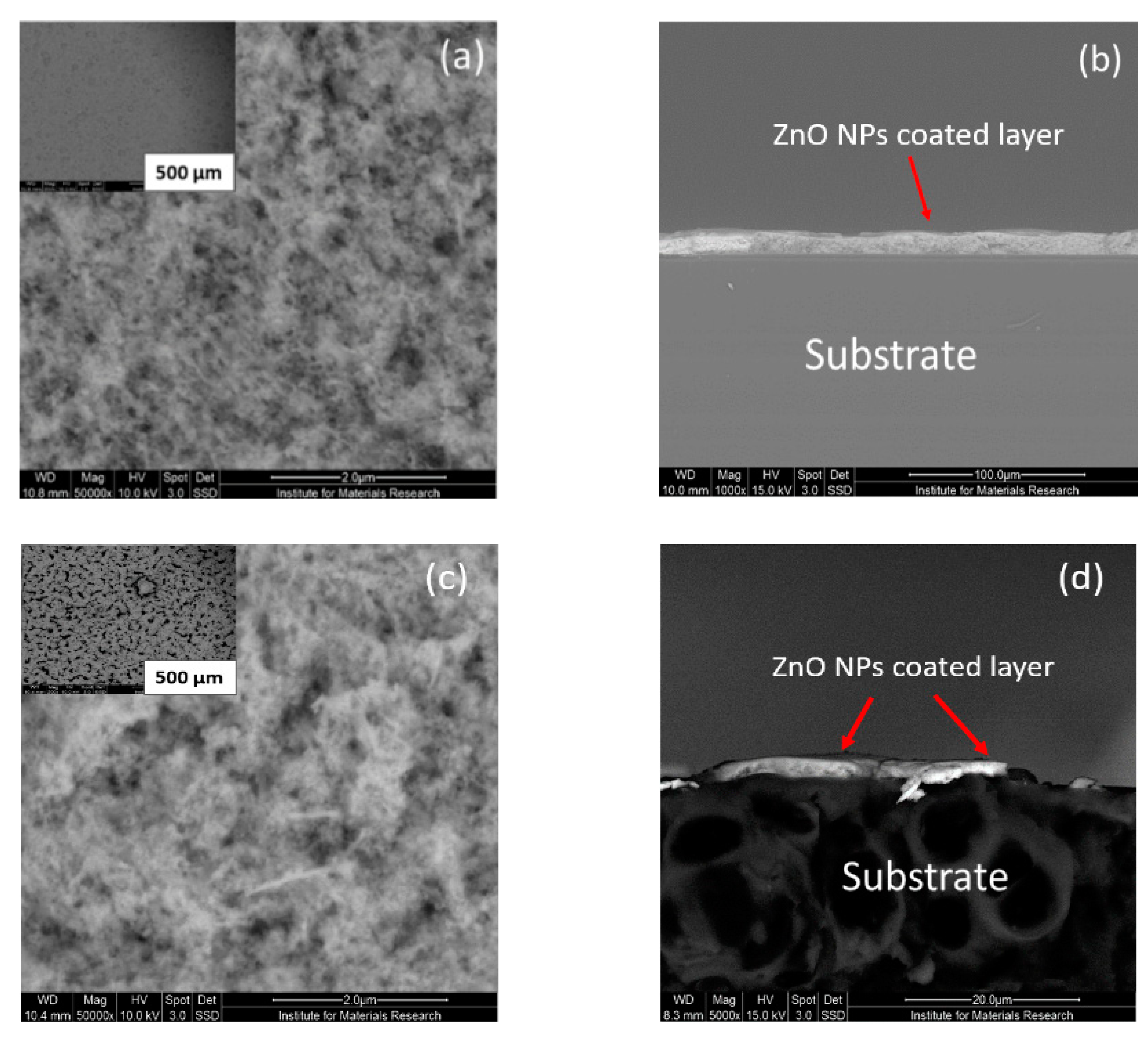
3.2. Optical Microscopic (OM) and Scanning Electron Microscopic (SEM) Characterizations
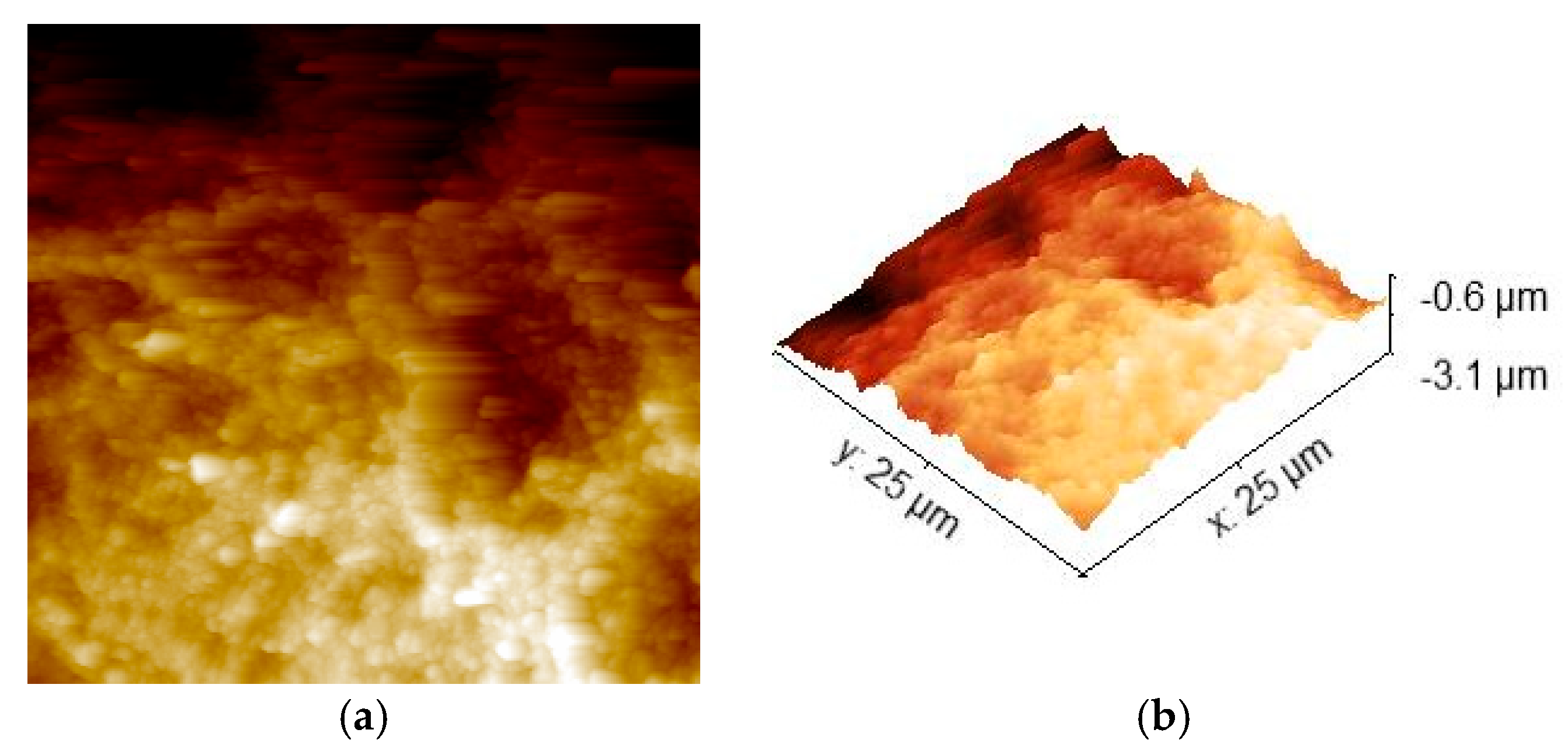
3.3. AFM Characterization
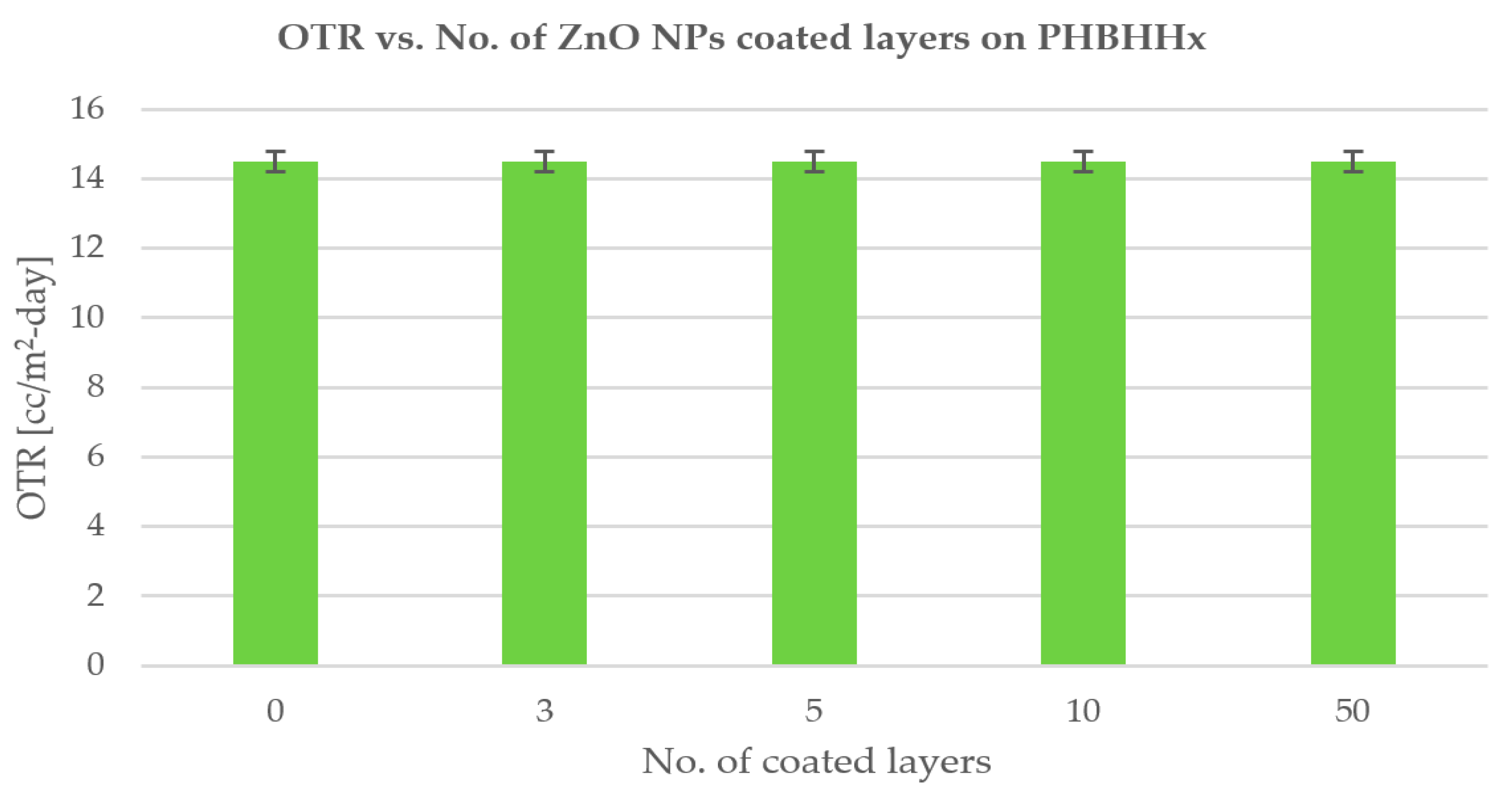
3.4. OTR
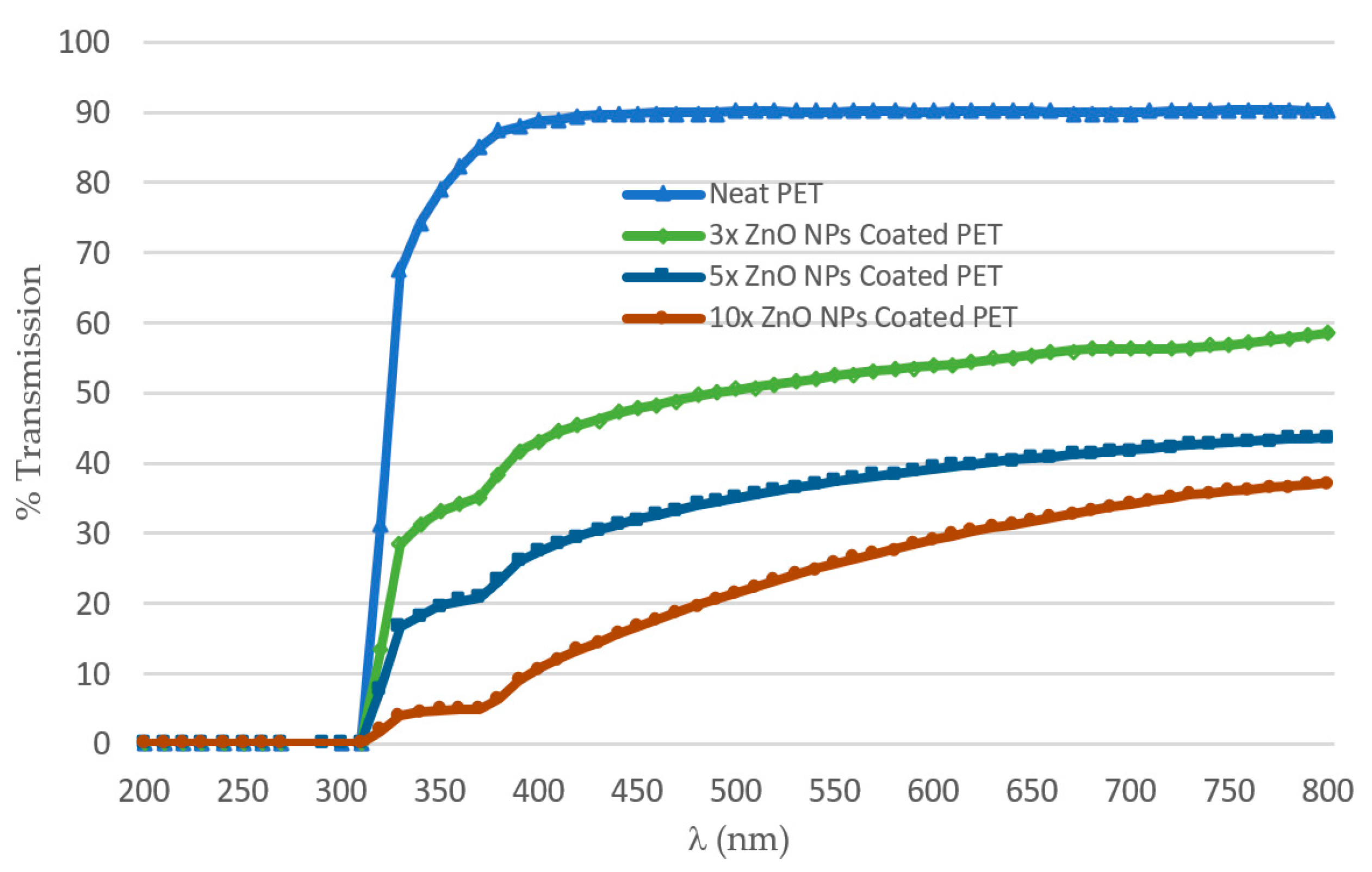
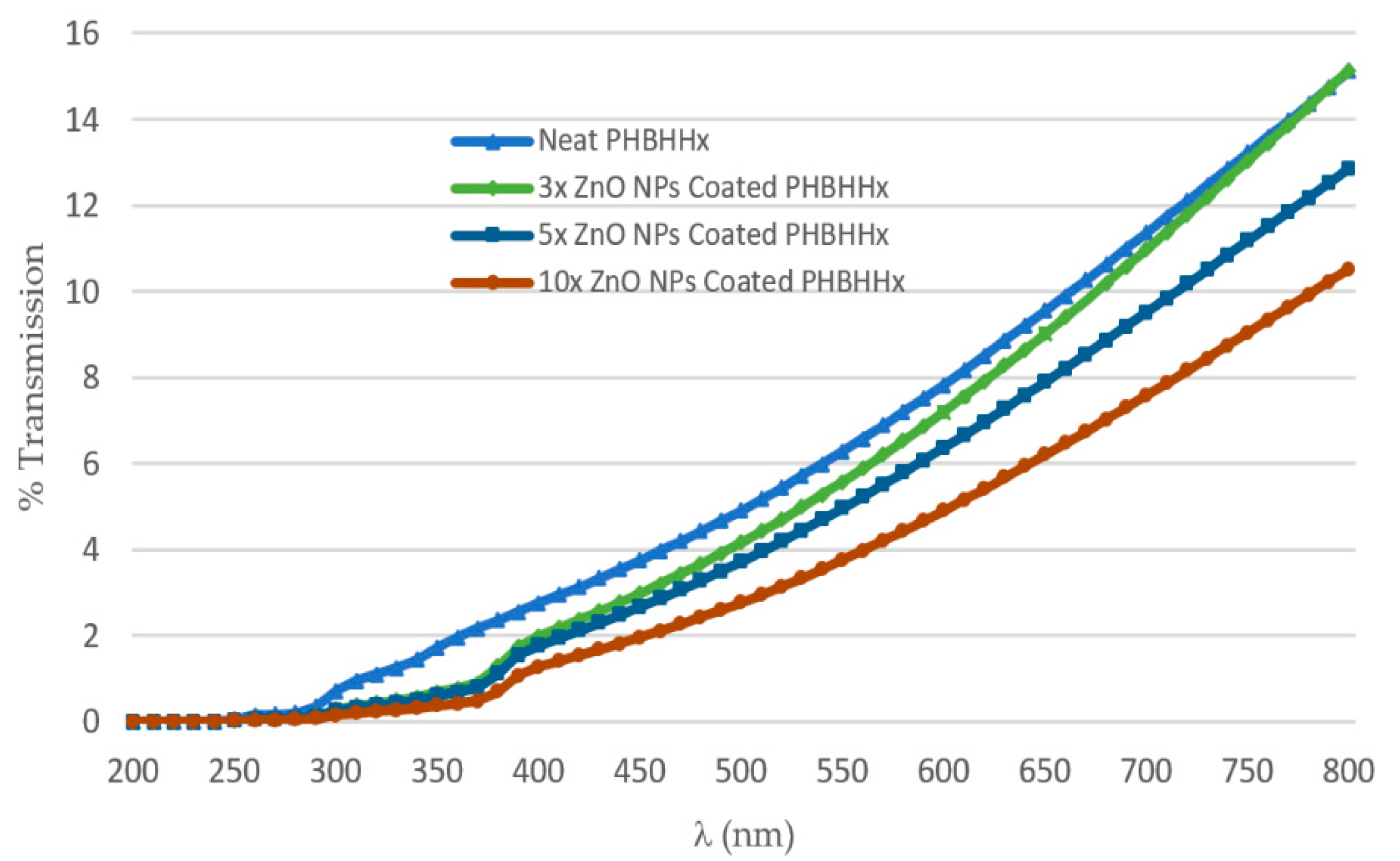
3.5. UV/Vis Transmission Measurements
- Self-made PHBHHx used was not a transparent material like PET material, i.e., commercial-grade foil.
- The thickness of the self-made PHBHHx material was approximately 0.5 mm, while the PET material thickness was 0.13 mm. It was revealed that increasing the path length traveled by incident light caused less UV transmission, as also evident from Beer–Lambert’s law [54].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, M.; Buntinx, M.; Deferme, W.; Peeters, R. (Bio) polymer/ZnO nanocomposites for packaging applications: A review of gas barrier and mechanical properties. Nanomaterials 2019, 9, 1494. [Google Scholar] [CrossRef] [Green Version]
- Wyser, Y.; Adams, M.; Avella, M.; Carlander, D.; Garcia, L.; Pieper, G.; Rennen, M.; Schuermans, J.; Weiss, J. Outlook and Challenges of Nanotechnologies for Food Packaging. Packag. Technol. Sci. 2016, 29, 615–648. [Google Scholar] [CrossRef] [Green Version]
- Ariyarathna, I.R.; Rajakaruna, R.; Karunaratne, D.N. The rise of inorganic nanomaterial implementation in food applications. Food Control. 2017, 77, 251–259. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Nanostructures: Current uses and future applications in food science. J. Food Drug Anal. 2017, 25, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [Green Version]
- Noruzi, M. Electrospun nanofibres in agriculture and the food industry: A review. J. Sci. Food Agric. 2016, 96, 4663–4678. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Fortunati, E.; Puglia, D.; Kenny, J.M.; Nanni, F. Effect of silver nanoparticles and cellulose nanocrystals on electrospun poly(lactic) acid mats: Morphology, thermal properties and mechanical behavior. Carbohydr. Polym. 2014, 103, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, A.; Padua, G. Nanocomposites in food packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Arshak, K.; Adley, C.; Moore, E.; Cunniffe, C.; Campion, M.; Harris, J. Characterisation of polymer nanocomposite sensors for quantification of bacterial cultures. Sens. Actuators B Chem. 2007, 126, 226–231. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Al Hazza, A.; Jacob, H.; Joseph, A. Comparative effects of untreated and 3-methacryloxypropyltrimethoxysilane treated ZnO nanoparticle reinforcement on properties of polylactide-based nanocomposite films. Int. J. Biol. Macromol. 2017, 101, 1041–1050. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Ariffin, F.; Mahmud, S.; Alias, A.K.; Hosseini, S.F.; Ahmad, M. Improving the physical and protective functions of semolina films by embedding a blend nanofillers (ZnO-nr and nano-kaolin). Food Packag. Shelf Life 2017, 12, 66–75. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(3-hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef] [Green Version]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, R.; Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Adv. Technol. 2016, 28, 20–27. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Al-Attar, H.; Auras, R.; Ejaz, M. Rheological, structural, ultraviolet protection and oxygen barrier properties of linear low- density polyethylene films reinforced with zinc oxide (ZnO) nanoparticles. Food Packag. Shelf Life 2017, 13, 20–26. [Google Scholar] [CrossRef]
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial Edible Films and Coatings. J. Food Prot. 2004, 67, 833–848. [Google Scholar] [CrossRef]
- Joshi, M.; Khanna, R.; Shekhar, R.; Jha, K. Chitosan nanocoating on cotton textile substrate using layer-by-layer self-assembly technique. J. Appl. Polym. Sci. 2011, 119, 2793–2799. [Google Scholar] [CrossRef]
- Caruso, R.A.; Antonietti, M. Sol−Gel Nanocoating: An Approach to the Preparation of Structured Materials. Chem. Mater. 2001, 13, 3272–3282. [Google Scholar] [CrossRef]
- Holder, K.M.; Spears, B.; Huff, M.E.; Priolo, M.A.; Harth, E.; Grunlan, J.C. Stretchable Gas Barrier Achieved with Partially Hydrogen-Bonded Multilayer Nanocoating. Macromol. Rapid Commun. 2014, 35, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xu, J.; Liu, X.; Hu, Q.; Huang, J. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J. Mater. Chem. B 2013, 1, 3477–3485. [Google Scholar] [CrossRef]
- Li, Y.-C.; Mannen, S.; Cain, A.C.; Grunlan, J.C. Flame retardant polymer/clay layer-by-layer assemblies on cotton fabric. Proc. Abstr. Papers Am. Chnem. Soc. 2015, 7, 23750–23759. [Google Scholar]
- Si, Y.; Zhu, H.; Chen, L.; Jiang, T.; Guo, Z. A multifunctional transparent superhydrophobic gel nanocoating with self-healing properties. Chem. Commun. 2015, 51, 16794–16797. [Google Scholar] [CrossRef] [PubMed]
- Guedri, L.; Ben-Amor, S.; Gardette, J.; Jacquet, M.; Rivaton, A. Lifetime improvement of poly(ethylene naphthalate) by ZnO adhesive coatings. Polym. Degrad. Stab. 2005, 88, 199–205. [Google Scholar] [CrossRef]
- Guedri-Knani, L.; Gardette, J.; Jacquet, M.; Rivaton, A. Photoprotection of poly (ethylene-naphthalate) by zinc oxide coating. Surf. Coat. Technol. 2004, 180, 71–75. [Google Scholar] [CrossRef]
- Peternel, I.T.; Koprivanac, N.; Božić, A.M.L.; Kušić, H.M. Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J. Hazard. Mater. 2007, 148, 477–484. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chaudhuri, B.; Bhattacharjee, S.; Das, P.; Dutta, B.K. Degradation mechanism and kinetic model for photocatalytic oxidation of PVC–ZnO composite film in presence of a sensitizing dye and UV radiation. J. Hazard. Mater. 2008, 154, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.; Parija, S. Photocatalytic degradation of phenol under solar radiation using microwave irradiated zinc oxide. Solar Energy 2006, 80, 1048–1054. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S. Preparation, characterization and antibacterial applications of ZnO-nanoparticles coated polyethylene films for food packaging. Colloids Surf. B Biointerfaces 2012, 90, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Faraj, M.; Ibrahim, K.; Ali, M. PET as a plastic substrate for the flexible optoelectronic applications. Optoelectron. Adv. Mater. 2011, 5, 879–882. [Google Scholar]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Zhang, S.; Tang, X.; Shang, G.; Peng, Q.; Wang, R.; Cai, X. The properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and its applications in tissue engineering. Curr. Stem Cell Res. Ther. 2014, 9, 215–222. [Google Scholar] [CrossRef]
- Vandewijngaarden, J.; Murariu, M.; Dubois, P.; Carleer, R.; Yperman, J.; Adriaensens, P.; Schreurs, S.; Lepot, N.; Peeters, R.; Buntinx, M. Gas Permeability Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J. Polym. Environ. 2014, 22, 501–507. [Google Scholar] [CrossRef]
- Ragaert, P.; Buntinx, M.; Maes, C.; Vanheusden, C.; Peeters, R.; Wang, S.; D’Hooge, D.R.; Cardon, L. Polyhydroxyalkanoates for Food Packaging Applications. In Reference Module in Food Science; Elsevier BV: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ultrasonic Spray Coating of Nanoparticles. Available online: http://www.sono-tek.com/wp-content/uploads/2018/07/Ultrasonic-Spray-of-Nanoparticles.pdf (accessed on 4 January 2021).
- Pham, N.P.; Burghartz, J.N.; Sarro, P.M. Spray coating of photoresist for pattern transfer on high topography surfaces. J. Micromech. Microeng. 2005, 15, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.P.; Boellaard, E.; Burghartz, J.N.; Sarro, P.M. Photoresist coating methods for the integration of novel 3-D RF micro-structures. J. Microelectromech. Systems 2004, 13, 491–499. [Google Scholar] [CrossRef]
- Lang, R.J. Ultrasonic Atomization of Liquids. J. Acoust. Soc. Am. 1962, 34, 6–8. [Google Scholar] [CrossRef]
- Alternative Energy & Nanomaterials. Available online: https://www.sono-tek.com/industry/alternative-energy-nanomaterials/cnt-nanowires-and-other-nanomaterials/ (accessed on 17 November 2020).
- Slegers, S.; Linzas, M.; Drijkoningen, J.; D’Haen, J.; Reddy, N.; Deferme, W. Surface Roughness Reduction of Additive Manufactured Products by Applying a Functional Coating Using Ultrasonic Spray Coating. Coatings 2017, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- How Ultrasonic Nozzles Work. Available online: https://www.sono-tek.com/ultrasonic-coating/how-ultrasonic-nozzles-work/ (accessed on 28 November 2020).
- Bose, S.; Keller, S.S.; Alstrøm, T.S.; Boisen, A.; Almdal, K. Process Optimization of Ultrasonic Spray Coating of Polymer Films. Langmuir 2013, 29, 6911–6919. [Google Scholar] [CrossRef] [PubMed]
- Scanning Electron Microscope A To Z. Available online: https://www.jeol.co.jp/en/applications/pdf/sm/sem_atoz_all.pdf (accessed on 25 November 2020).
- Abbas, M.; Buntinx, M.; Deferme, W.; Reddy, N.; Peeters, R. Comparison of the oxygen gas and UV barrier properties of nano ZnO coated PET and PHBHHx packaging films via ultrasonic spray coating. In Proceedings of the Nanotech France 2019 International Conference, Paris, France, 26–28 June 2019. [Google Scholar] [CrossRef]
- Marra, A.; Rollo, G.; Cimmino, S.; Silvestre, C. Assessment on the Effects of ZnO and Coated ZnO Particles on iPP and PLA Properties for Application in Food Packaging. Coatings 2017, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Wang, Z.; Luo, H.; Xu, M.; Ren, X.; Zheng, G.; Wu, B.; Zhang, X.; Lu, X.; Chen, F.; et al. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)-based scaffolds for tissue engineering. Braz. J. Med. Biol. Res. 2014, 47, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Spectrophotometry. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/02%3A_Reaction_Rates/2.01%3A_Experimental_Determination_of_Kinetics/2.1.05%3A_Spectrophotometry (accessed on 20 November 2020).
- Zak, A.K.; Razali, R.; Majid, W.H.B.A.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Sahare, S.; Veldurthi, N.; Singh, R.; Swarnkar, A.K.; Salunkhe, M.; Bhave, T. Enhancing the efficiency of flexible dye-sensitized solar cells utilizing natural dye extracted fromAzadirachta indica. Mater. Res. Express 2015, 2, 105903. [Google Scholar] [CrossRef]
- The Beer-Lambert Law. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Spectroscopy/Electronic_Spectroscopy/Electronic_Spectroscopy_Basics/The_Beer-Lambert_Law (accessed on 20 November 2020).


| Sr. No. | Parameters | Optimized Values |
|---|---|---|
| 1 | Ink composition | 2.5 wt.% ZnO NP solution in PVA, IPA, and DI water |
| 2 | Path speed (mm/sec) | 10 [44] |
| 3 | Nozzle frequency (kHz) | 120 |
| 4 | Ink flow rate (mL/min) | 0.1 |
| 5 | Generator power (W) | 2.5 [44] |
| 6 | Nozzle to substrate distance (mm) | 75 |
| 7 | Hot plate temperature (°C) | 30 |
| 8 | Nitrogen guiding pressure (kPa) | 0.34 |
| 9 | Number of coated layers | 3, 5, 10, and 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.; Buntinx, M.; Deferme, W.; Reddy, N.; Peeters, R. Oxygen Gas and UV Barrier Properties of Nano-ZnO-Coated PET and PHBHHx Materials Fabricated by Ultrasonic Spray-Coating Technique. Nanomaterials 2021, 11, 449. https://doi.org/10.3390/nano11020449
Abbas M, Buntinx M, Deferme W, Reddy N, Peeters R. Oxygen Gas and UV Barrier Properties of Nano-ZnO-Coated PET and PHBHHx Materials Fabricated by Ultrasonic Spray-Coating Technique. Nanomaterials. 2021; 11(2):449. https://doi.org/10.3390/nano11020449
Chicago/Turabian StyleAbbas, Mohsin, Mieke Buntinx, Wim Deferme, Naveen Reddy, and Roos Peeters. 2021. "Oxygen Gas and UV Barrier Properties of Nano-ZnO-Coated PET and PHBHHx Materials Fabricated by Ultrasonic Spray-Coating Technique" Nanomaterials 11, no. 2: 449. https://doi.org/10.3390/nano11020449







