Excitation Wavelength and Intensity-Dependent Multiexciton Dynamics in CsPbBr3 Nanocrystals
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
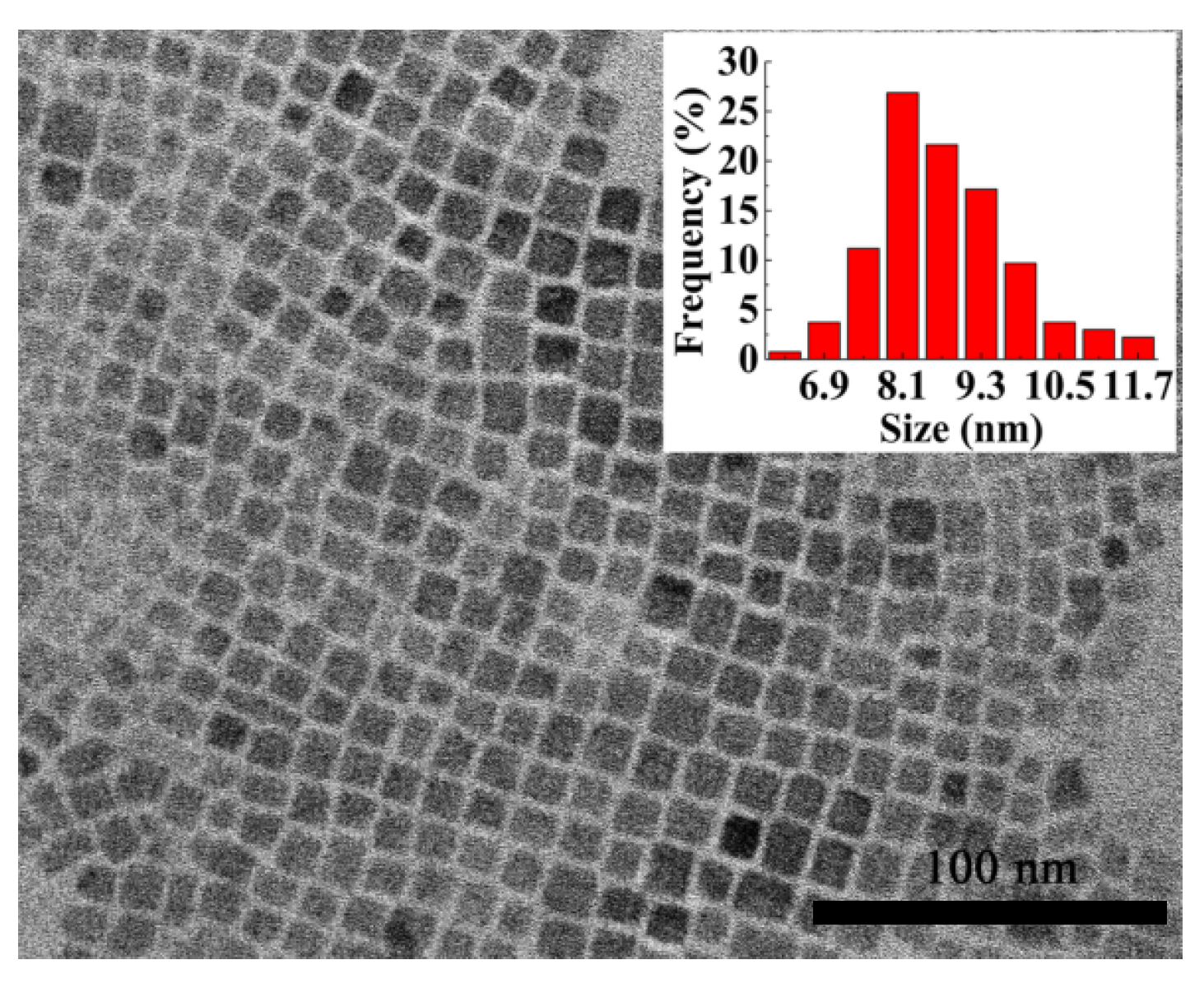
2.2. Size Characterization
2.3. Spectroscopic Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef]
- Kang, J.; Wang, L.-W. High defect tolerance in lead halide perovskite CsPbBr3. J. Phys. Chem. Lett. 2017, 8, 489–493. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; ten Brinck, S.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef]
- Yumoto, G.; Tahara, H.; Kawawaki, T.; Saruyama, M.; Sato, R.; Teranishi, T.; Kanemitsu, Y. Hot biexciton effect on optical gain in CsPbI3 perovskite nanocrystals. J. Phys. Chem. Lett. 2018, 9, 2222–2228. [Google Scholar] [CrossRef]
- Bi, C.; Wang, S.; Wen, W.; Yuan, J.; Cao, G.; Tian, J. Room-temperature construction of mixed-halide perovskite quantum dots with high photoluminescence quantum yield. J. Phys. Chem. C 2018, 122, 5151–5160. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.-H.; Kim, B.; Lee, S.-H.; Lee, M.-S.; Lee, J.-S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Li, Y.; Lai, R.; Luo, X.; Liu, X.; Ding, T.; Lu, X.; Wu, K. On the absence of a phonon bottleneck in strongly confined CsPbBr3 perovskite nanocrystals. Chem. Sci. 2019, 10, 5983–5989. [Google Scholar] [CrossRef]
- Soetan, N.; Puretzky, A.; Reid, K.; Boulesbaa, A.; Zarick, H.F.; Hunt, A.; Rose, O.; Rosenthal, S.; Geohegan, D.B.; Bardhan, R. Ultrafast Spectral Dynamics of CsPb(BrxCl1 − x)3 Mixed-Halide Nanocrystals. ACS Photonics 2018, 5, 3575–3583. [Google Scholar] [CrossRef]
- Damtie, F.A.; Karki, K.J.; Pullerits, T.; Wacker, A. Optimization schemes for efficient multiple exciton generation and extraction in colloidal quantum dots. J. Chem. Phys. 2016, 145, 064703. [Google Scholar] [CrossRef]
- Makarov, N.S.; Guo, S.; Isaienko, O.; Liu, W.; Robel, I.; Klimov, V.I. Spectral and dynamical properties of single excitons, biexcitons, and trions in cesium-lead-halide perovskite quantum dots. Nano Lett. 2016, 16, 2349–2362. [Google Scholar] [CrossRef]
- Robel, I.; Gresback, R.; Kortshagen, U.; Schaller, R.D.; Klimov, V.I. Universal size-dependent trend in Auger recombination in direct-gap and indirect-gap semiconductor nanocrystals. Phys. Rev. Lett. 2009, 102, 177404. [Google Scholar] [CrossRef]
- Aneesh, J.; Swarnkar, A.; Kumar Ravi, V.; Sharma, R.; Nag, A.; Adarsh, K.V. Ultrafast exciton dynamics in colloidal CsPbBr3 perovskite nanocrystals: Biexciton effect and Auger recombination. J. Phys. Chem. C 2017, 121, 4734–4739. [Google Scholar] [CrossRef]
- Mondal, N.; Samanta, A. Complete ultrafast charge carrier dynamics in photo-excited all-inorganic perovskite nanocrystals (CsPbX3). Nanoscale 2017, 9, 1878–1885. [Google Scholar] [CrossRef]
- Butkus, J.; Vashishtha, P.; Chen, K.; Gallaher, J.K.; Prasad, S.K.K.; Metin, D.Z.; Laufersky, G.; Gaston, N.; Halpert, J.E.; Hodgkiss, J.M. The evolution of quantum confinement in CsPbBr3 perovskite nanocrystals. Chem. Mater. 2017, 29, 3644–3652. [Google Scholar] [CrossRef]
- de Jong, E.M.L.D.; Yamashita, G.; Gomez, L.; Ashida, M.; Fujiwara, Y.; Gregorkiewicz, T. Multiexciton lifetime in all-inorganic CsPbBr3 perovskite nanocrystals. J. Phys. Chem. C 2017, 121, 1941–1947. [Google Scholar] [CrossRef]
- Mondal, N.; De, A.; Das, S.; Paul, S.; Samanta, A. Ultrafast carrier dynamics of metal halide perovskite nanocrystals and perovskite-composites. Nanoscale 2019, 11, 9796–9818. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- McGuire, J.A.; Joo, J.; Pietryga, J.M.; Schaller, R.D.; Klimov, V.I. New aspects of carrier multiplication in semiconductor nanocrystals. Accounts Chem. Res. 2008, 41, 1810–1819. [Google Scholar] [CrossRef]
- Wu, K.; Liang, G.; Shang, Q.; Ren, Y.; Kong, D.; Lian, T. Ultrafast interfacial electron and hole transfer from CsPbBr3 perovskite quantum dots. J. Am. Chem. Soc. 2015, 137, 12792–12795. [Google Scholar] [CrossRef]
- Han, Q.; Wu, W.; Liu, W.; Yang, Y. The peak shift and evolution of upconversion luminescence from CsPbBr3 nanocrystals under femtosecond laser excitation. RSC Adv. 2017, 7, 35757–35764. [Google Scholar] [CrossRef]
- Liu, X.; Han, J.; Li, Y.; Cao, B.; Sun, C.; Yin, H.; Shi, Y.; Jin, M.; Liu, C.; Sun, M.; et al. Ultrafast carrier dynamics in all-inorganic CsPbBr3 perovskite across the pressure-induced phase transition. Opt. Express 2019, 27, A995–A1003. [Google Scholar] [CrossRef]
- Wang, J.; Ding, T.; Leng, J.; Jin, S.; Wu, K. “Intact” carrier doping by pump-pump-probe spectroscopy in combination with interfacial charge transfer: A case study of CsPbBr3 nanocrystals. J. Phys. Chem. Lett. 2018, 9, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, P. Hot exciton relaxation dynamics in semiconductor quantum dots: Radiationless transitions on the nanoscale. J. Phys. Chem. C 2011, 115, 22089–22109. [Google Scholar] [CrossRef]
- Kambhampati, P. Unraveling the the structure and dynamics of excitons in semiconductor quantum dots. Accounts Chem. Res. 2011, 44, 1–13. [Google Scholar] [CrossRef]
- Klimov, V.; Hunsche, S.; Kurz, H. Biexciton effects in femtosecond nonlinear transmission of semiconductor quantum dots. Phys. Rev. B 1994, 50, 8110–8113. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, C.; Cui, M.; He, T.; Liu, K.; Huang, Y.; Luo, M.; Zhang, L.; Xu, H.; Li, S.; et al. Spectra stable blue perovskite light-emitting diodes. Nat. Commun. 2019, 10, 1868. [Google Scholar] [CrossRef]
- Qin, C.-C.; Cui, M.-H.; Song, D.-D.; He, W. Ultrafast multiexciton Auger recombination of CdSeS. Acta Phys. Sin. 2019, 68, 107801. [Google Scholar]
- Klimov, V.I.; McGuire, J.A.; Schaller, R.D.; Rupasov, V.I. Scaling of multiexciton lifetimes in semiconductor nanocrystals. Phys. Rev. B 2008, 77, 195324. [Google Scholar] [CrossRef]
- Yarita, N.; Tahara, H.; Ihara, T.; Kawawaki, T.; Sato, R.; Saruyama, M.; Teranishi, T.; Kanemitsu, Y. Dynamics of charged excitons and biexcitons in CsPbBr3 perovskite nanocrystals revealed by femtosecond transient-absorption and single-dot luminescence spectroscopy. J. Phys. Chem. Lett. 2017, 8, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Price, M.B.; Butkus, J.; Jellicoe, T.C.; Sadhanala, A.; Briane, A.; Halpert, J.E.; Broch, K.; Hodgkiss, J.M.; Friend, R.H.; Deschler, F. Hot-carrier cooling and photoinduced refractive index changes in organic-inorganic lead halide perovskites. Nat. Commun. 2015, 6, 8420. [Google Scholar]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Jing, P.; Li, J.; Wei, M.; Hua, J.; Zhao, J.; Tian, L. Temperature-dependent photoluminescence of inorganic perovskite nanocrystal films. RSC Adv. 2016, 6, 78311–78316. [Google Scholar] [CrossRef]
- Yang, L.; Wei, K.; Xu, Z.; Li, F.; Chen, R.; Zheng, X.; Cheng, X.; Jiang, T. Nonlinear absorption and temperature-dependent fluorescence of perovskite FAPbBr3 nanocrystal. Opt. Lett. 2018, 43, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, C.; Jiang, Z.; Zhou, Z.; Liu, Y.; Jiang, Y. Excitation Wavelength and Intensity-Dependent Multiexciton Dynamics in CsPbBr3 Nanocrystals. Nanomaterials 2021, 11, 463. https://doi.org/10.3390/nano11020463
Qin C, Jiang Z, Zhou Z, Liu Y, Jiang Y. Excitation Wavelength and Intensity-Dependent Multiexciton Dynamics in CsPbBr3 Nanocrystals. Nanomaterials. 2021; 11(2):463. https://doi.org/10.3390/nano11020463
Chicago/Turabian StyleQin, Chaochao, Zhinan Jiang, Zhongpo Zhou, Yufang Liu, and Yuhai Jiang. 2021. "Excitation Wavelength and Intensity-Dependent Multiexciton Dynamics in CsPbBr3 Nanocrystals" Nanomaterials 11, no. 2: 463. https://doi.org/10.3390/nano11020463
APA StyleQin, C., Jiang, Z., Zhou, Z., Liu, Y., & Jiang, Y. (2021). Excitation Wavelength and Intensity-Dependent Multiexciton Dynamics in CsPbBr3 Nanocrystals. Nanomaterials, 11(2), 463. https://doi.org/10.3390/nano11020463





