Optimization of the Obtaining of Cellulose Nanocrystals from Agave tequilana Weber Var. Azul Bagasse by Acid Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Preparation of Cellulose Nanocrystals
2.4. Determination of Sulfate Groups in CNC-S
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. X-ray Diffraction (XRD)
2.8. Atomic Force Microscope (AFM)
2.9. Dynamic Light Scattering (DLS) and Z-Potential
3. Results
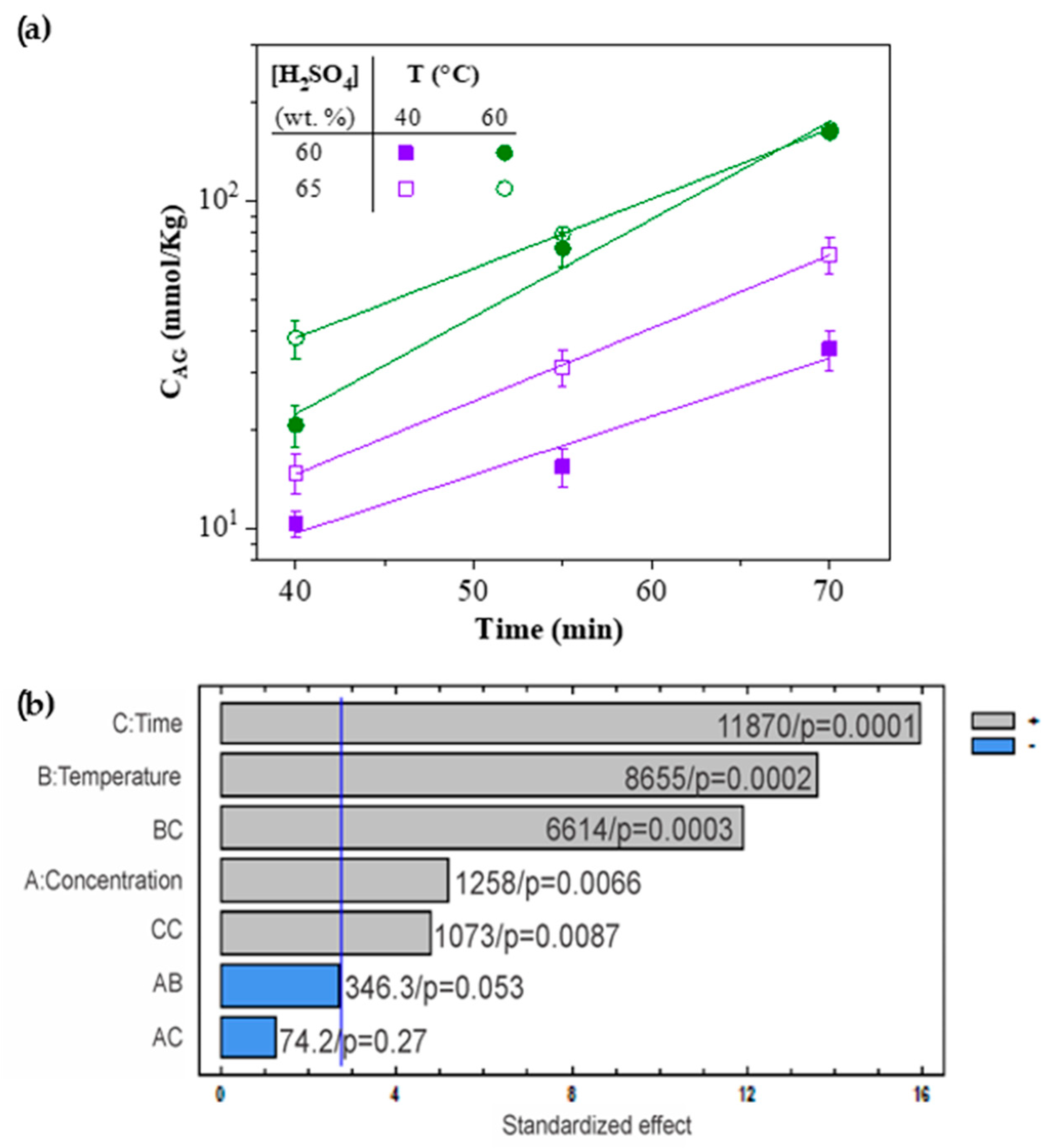
3.1. Yield and Factorial Design
3.2. Determination of Sulfate Groups in CNC-S
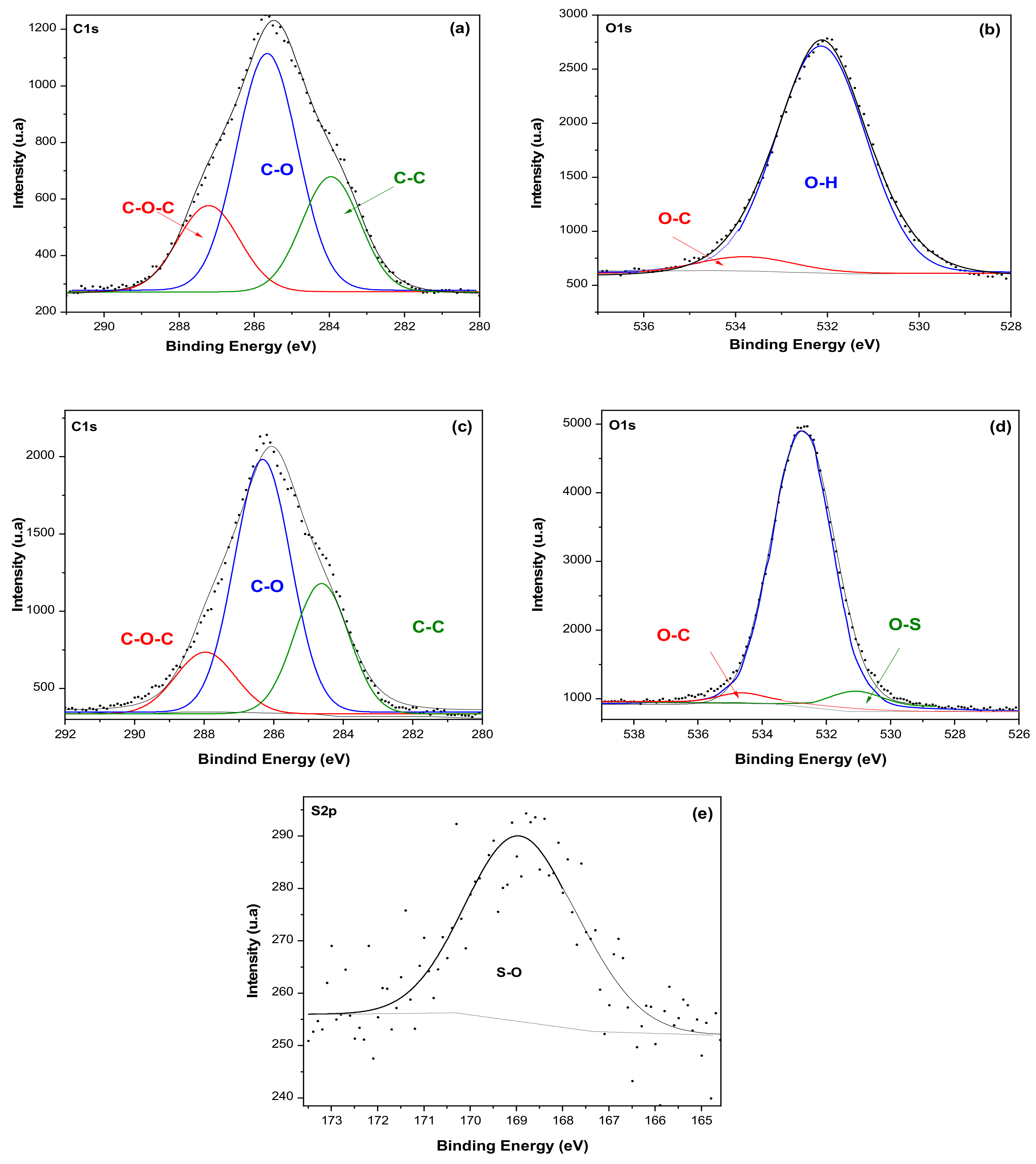
3.3. X-ray Photoelectron Spectroscopy (XPS)
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
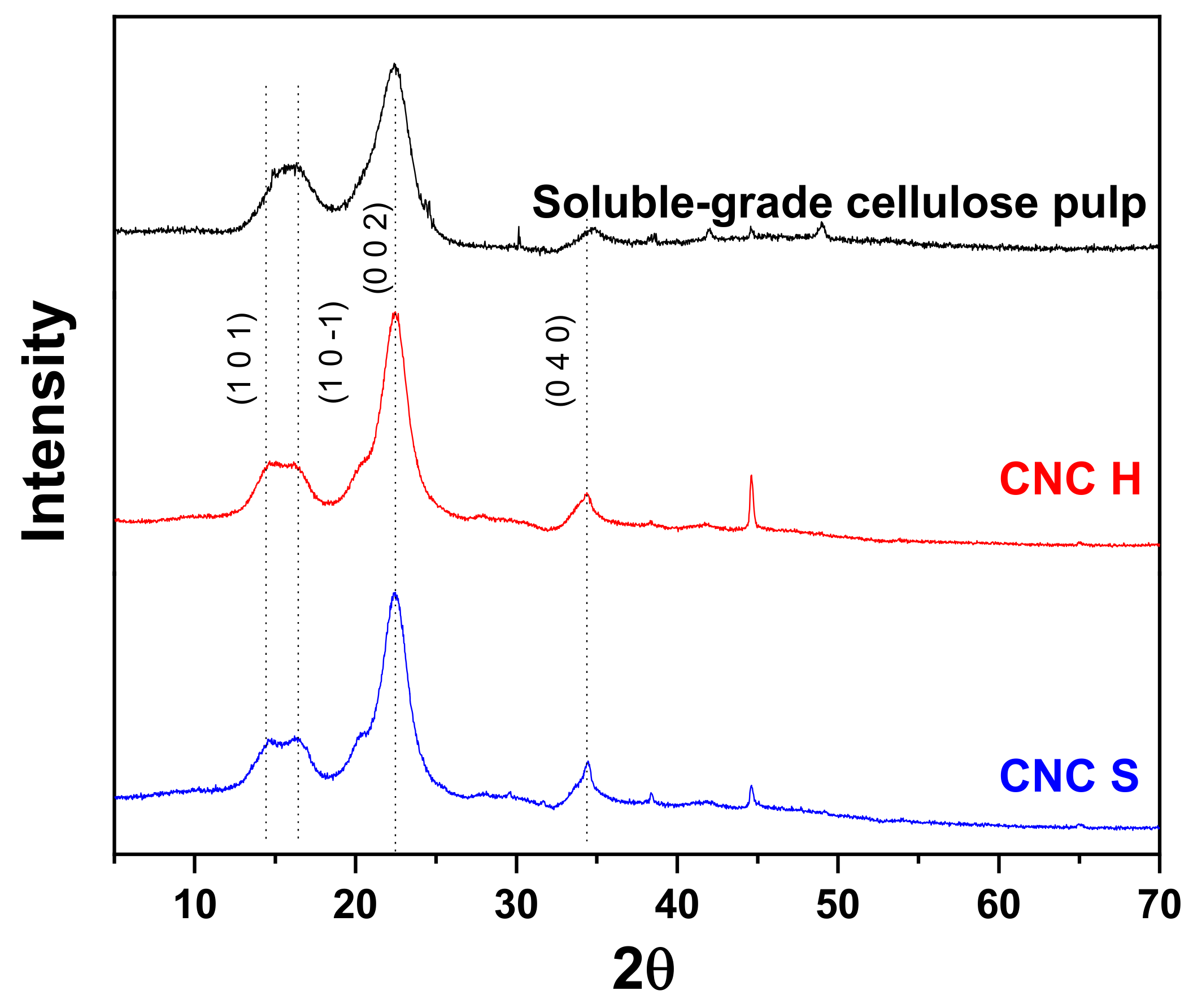
3.5. X-ray Diffraction (XRD)
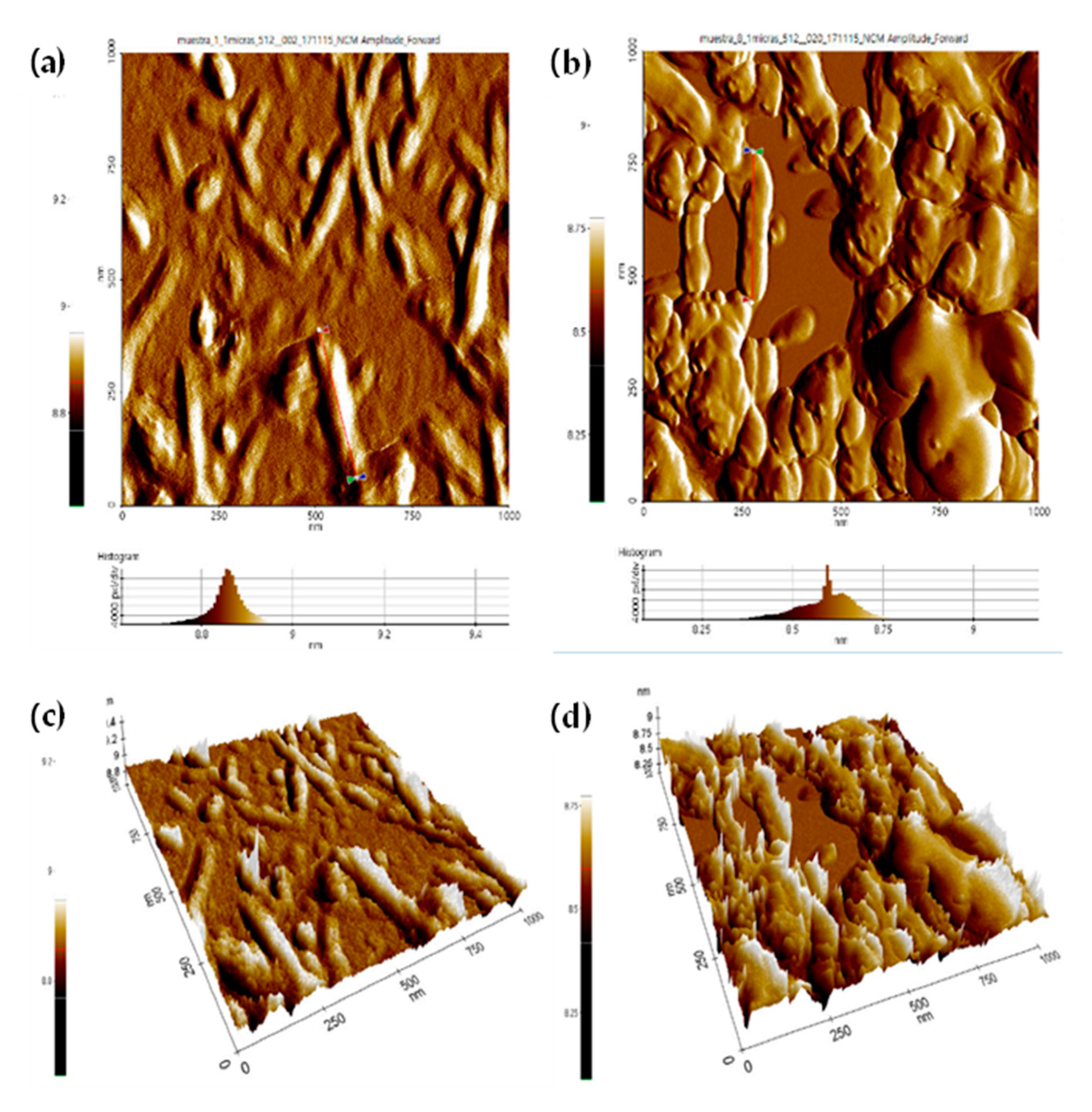
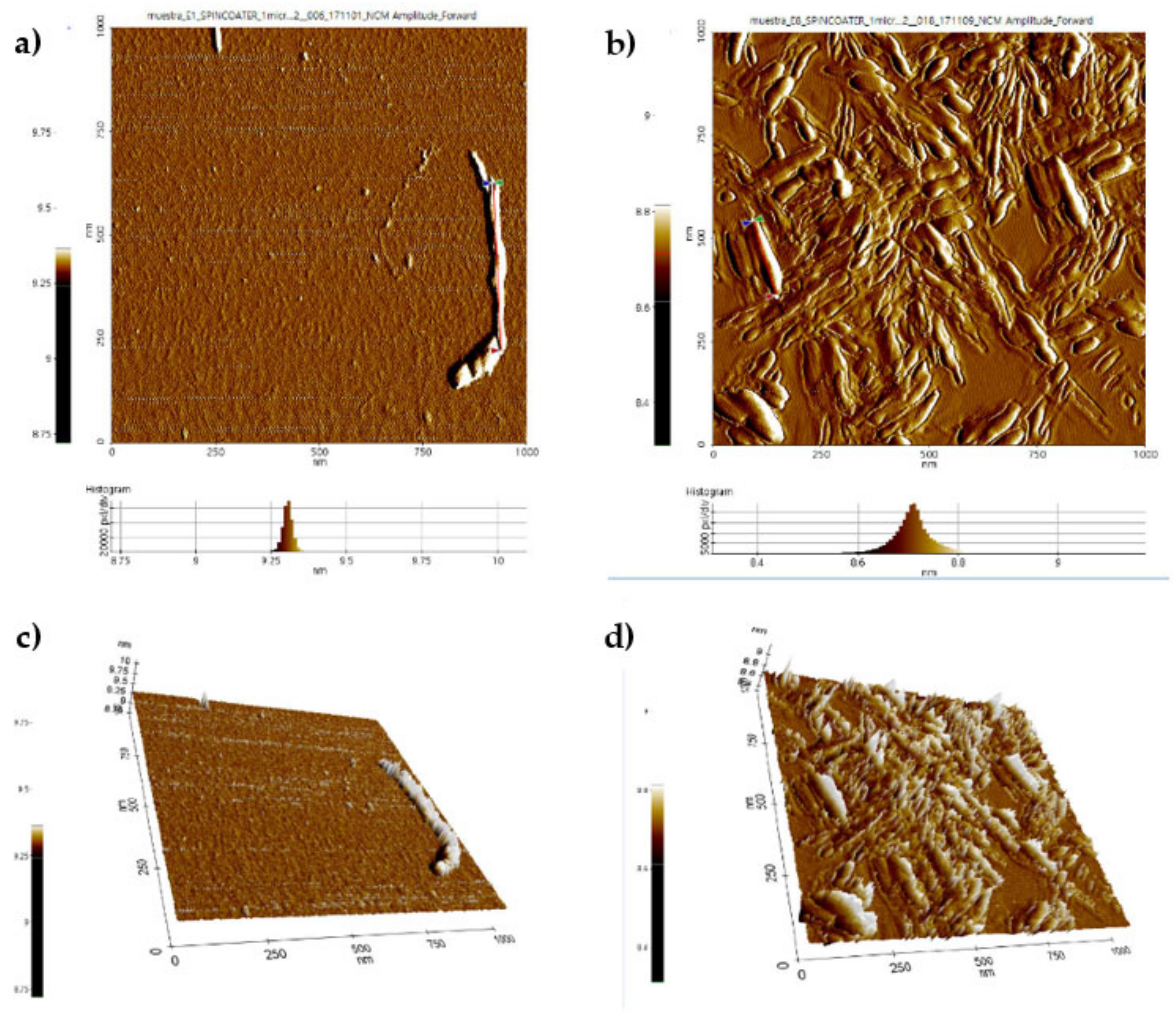
3.6. Atomic Force Microscopy (AFM)
3.7. Zeta Potential, Dynamic Light Scattering (DLS), and Atomic Force Microscopy (AFM) Comparison
4. Future Research Direction
5. Conclusions
- CNC were successfully obtained from soluble-grade cellulose pulp from A. tequilana Weber var. Azul bagasse using an experimental design 23 for two acids, H2SO4 and HCL.
- The maximum CNC yield was 90 and 96% for CNC-H and CNC-S, respectively, for the most severe hydrolysis conditions tested.
- For CNC-S, the total sulfate group content measured followed an exponential behavior as a function of time: . The CSG increased with hydrolysis time, temperature, and H2SO4 concentration. The CSG values ranged from 10 to 150 mmol/kg, depending on the hydrolysis conditions.
- The insertion of sulfate groups on CNC-S was also corroborated using XPS, and the results showed 4.92% of sulfate groups for E8S. The presence of sulfate groups on CNC-S was also detected by FTIR.
- The FTIR spectra showed LOI and TOI similar to those of soluble-grade cellulose pulp, near to 1 in both cases (1.03 ± 0.17 and 1.00 ± 0.13 respectively), while HBI increased about 30%.
- The CNC crystallinity was obtained by XRD using the Rietveld method. For all the analyzed samples, the crystallinity values ranged from 88.4 to 91.3%, while the value for soluble grade cellulose pulp was 79.2%
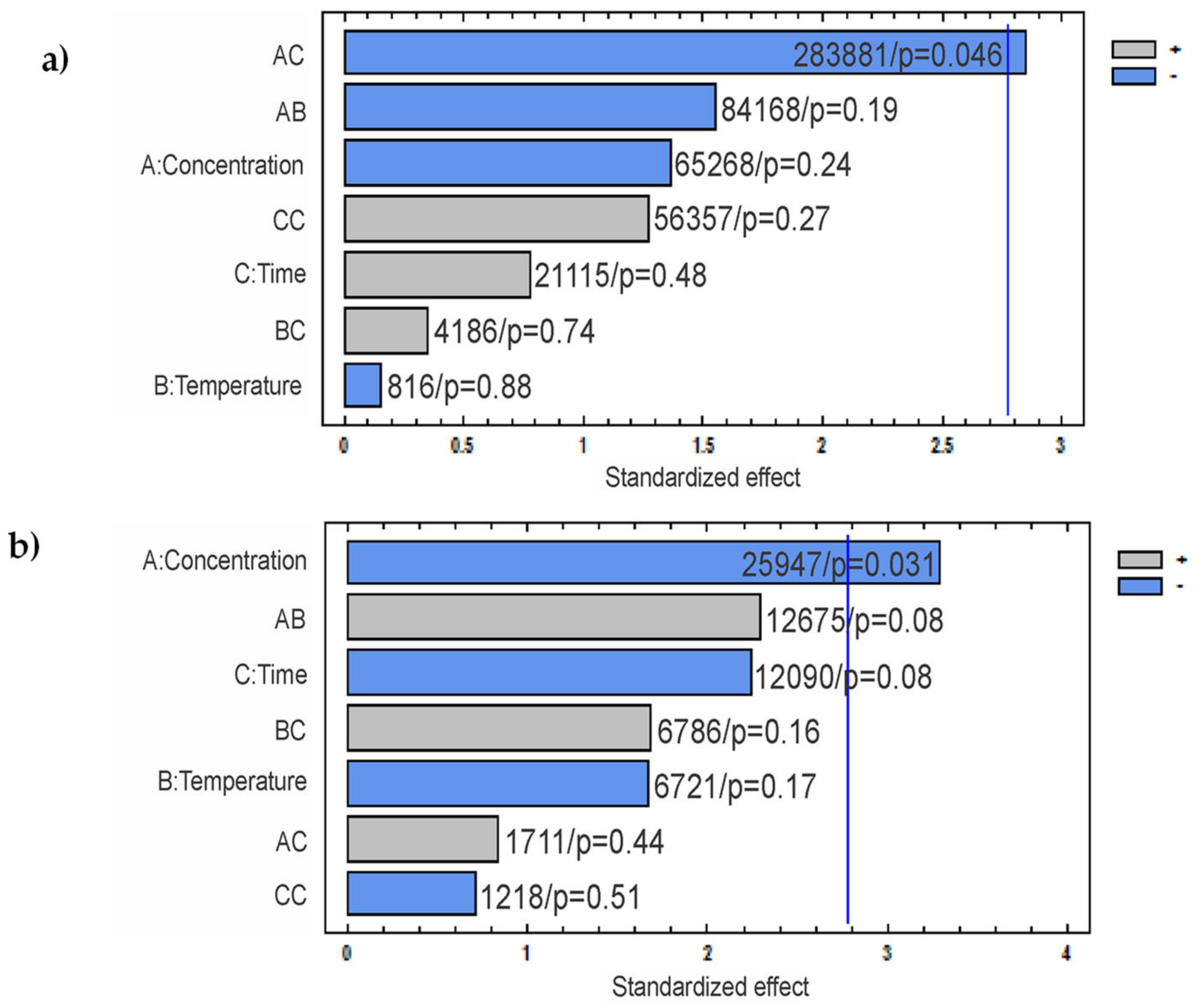
- The Pareto analysis revealed that HCl concentration and time factor interaction (AC) is statistically significant on CNC-H length, whereas H2SO4 concentration is the only statistically significant factor for CNC-S length.
- The CNC lengths obtained by AFM are very similar to the diameter (d) obtained using DLS.
- The CNC-S lengths were shorter and thinner than those of CNC-H, probably attributed to sulfate group insertion and less agglomeration of the nanocrystals in dispersion.
- The smallest CNC-H length was observed with 8N HCl (266–350 nm, except for sample E2H), and the longest CNC-H was obtained with 2N HCl (256–867 nm). The smallest CNC-S length was obtained with 65 wt% H2SO4 (137–244 nm) and the longest CNC-S was obtained with 60 wt.% H2SO4 (185–404 nm).
- As the obtained CNC have an ellipsoidal shape, the studied dimensions were L, H, and W. For both acids (H2SO4 and HCl), CNC height ranged between 8.6–9.3 nm, whereas the width ranged between 20–85 nm, and 47–300 nm, for CNC-S, and CNC-H, respectively. Considering that 2 of 3 dimensions are in the nanometer range, the CNC obtained in this work can be considered as nanomaterials.
- Owing to their exceptional mechanical properties, CNC are excellent candidates to be used as reinforcement materials.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Lam, E.; Chong, J.; Hrapovic, S.; Luong, J.H.T. Reinforced plastics and aerogels by nanocrystalline cellulose. J. Nanoparticle Res. 2013, 15, 1–24. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. Cellulose nanocrystals reinforced poly(oxyethylene). Polymer 2004, 45, 4149–4157. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef]
- Saeed, A.A.; Abbas, M.N.; Singh, B.; Abou-Zeid, R.E.; Kamel, S. Cellulose nanocrystals decorated with gold nanoparticles immobilizing GOx enzyme for non-invasive biosensing of human salivary glucose. Anal. Methods 2019, 11, 6073–6083. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Doosthoseini, K.; Ganjian, E.; Azin, M. Manufacturing of bacterial nano-cellulose reinforced fiber-cement composites. Constr. Build. Mater. 2015, 101, 958–964. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, N.; Bahr, D.; Zavattieri, P.D.; Youngblood, J.; Moon, R.J.; Weiss, J. The influence of cellulose nanocrystals on the microstructure of cement paste. Cem. Concr. Compos. 2016, 74, 164–173. [Google Scholar] [CrossRef]
- Fu, T.; Montes, F.; Suraneni, P.; Youngblood, J.; Weiss, J. The Influence of Cellulose Nanocrystals on the Hydration and Flexural Strength of Portland Cement Pastes. Polymers 2017, 9, 424. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Ferreira, S.; Filho, A.; Fangueiro, R. Ultrasonic dispersion of micro crystalline cellulose for developing cementitious composites with excellent strength and stiffness. Ind. Crops Prod. 2018, 122, 156–165. [Google Scholar] [CrossRef]
- Yu, H.Y.; Qin, Z.Y.; Liu, L.; Yang, X.G.; Zhou, Y.; Yao, J.M. Comparison of the reinforcing effects for cellulose nanocrystals obtained by sulfuric and hydrochloric acid hydrolysis on the mechanical and thermal properties of bacterial polyester. Compos. Sci. Technol. 2013, 87, 22–28. [Google Scholar] [CrossRef]
- Jemaa, N.; Paleologou, M.; Zhang, X.; Repoit, I.S. Fractionation of a Waste Liquor Stream from Nanocrystalline Cellulose Production. U.S. Patent 8,709,203 B2, 29 April 2014. [Google Scholar]
- Espino, E.; Cakir, M.; Domenek, S.; Román-Gutiérrez, A.D.; Belgacem, N.; Bras, J. Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind. Crops Prod. 2014, 62, 552–559. [Google Scholar] [CrossRef]
- Dhar, P.; Bhasney, S.M.; Kumar, A.; Katiyar, V. Acid functionalized cellulose nanocrystals and its effect on mechanical, thermal, crystallization and surfaces properties of poly (lactic acid) bionanocomposites films: A comprehensive study. Polymer 2016, 101, 75–92. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Abdullah, I. Isolation and characterization of cellulose nanocrystals from agave angustifolia fibre. BioResources 2013, 8, 1893–1908. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.-L.; Heux, L.; Dubreuil, F.; Rochas, C. The Shape and Size Distribution of Crystalline Nanoparticles Prepared by Acid Hydrolysis of Native Cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Chen, Y.; Hu, S.; Ren, Z.; Zhu, M. Efficient Extraction of Cellulose Nanocrystals through Hydrochloric Acid Hydrolysis Catalyzed by Inorganic Chlorides under Hydrothermal Conditions. ACS Sustain. Chem. Eng. 2017, 5, 4656–4664. [Google Scholar] [CrossRef]
- Montanari, S.; Roumani, M.; Heux, L.; Vignon, M.R. Topochemistry of Carboxylated Cellulose Nanocrystals Resulting from TEMPO-Mediated Oxidation. Macromolecules 2005, 38, 1665–1671. [Google Scholar] [CrossRef]
- Yu, H.Y.; Qin, Z.Y.; Sun, B.; Yan, C.F.; Yao, J.M. One-pot green fabrication and antibacterial activity of thermally stable corn-like CNC/Ag nanocomposites. J. Nanoparticle Res. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Consejo Regulador del Tequila (CRT), Información Estadística 2020. Available online: https://www.crt.org.mx/EstadisticasCRTweb/ (accessed on 20 October 2020).
- Huitrón, C.; Pérez, R.; Sánchez, A.; Lappe, P.; Zavaleta, L.R. Agricultural waste from the tequila industry as substrate for the production of commercially important enzymes. J. Environ. Biol. 2008, 1, 37–41. [Google Scholar]
- Gallardo-Sánchez, M.A.; Hernández, J.A.; Casillas, R.R.; Vázquez, J.I.E.; Hernández, D.E.; Martínez, J.F.A.S.; Enríquez, S.G.; Balleza, E.R.M. Obtaining Soluble-grade cellulose pulp from Agave tequilana Weber var. Azul Bagasse. BioResources 2019, 14, 9867–9881. [Google Scholar] [CrossRef]
- Ponce-Reyes, C.E.; Chanona-Pérez, J.J.; Garibay-Febles, V.; Palacios-González, E.; Karamath, J.; Terrés-Rojas, E.; Calderón-Domínguez, G. Preparation of cellulose nanoparticles from agave waste and its morphological and structural characterization. Rev. Mex. Ing. Quim. 2014, 13, 897–906. [Google Scholar]
- Pech-Cohuo, S.C.; Canche-Escamilla, G.; Valadez-González, A.; Fernández-Escamilla, V.V.A.; Uribe-Calderon, J. Production and Modification of Cellulose Nanocrystals from Agave tequilana Weber Waste and Its Effect on the Melt Rheology of PLA. Int. J. Polym. Sci. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Zheng, L.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef]
- Ramírez Casillas, R.; del Carmen López López, M.; Becerra Aguilar, B.; Dávalos Olivares, F.; Satyanarayana, K.G. Preparation and Characterization of Cellulose Nanocrystals using Soluble Grade Cellulose from Acid Hydrolysis of Huizache (Acacia farnesiana L. Willd.). BioResources 2019, 14, 3319–3338. [Google Scholar]
- Elhalil, A.; Tounsadi, H.; Elmoubarki, R.; Mahjoubi, F.Z.; Farnane, M.; Sadiq, M.; Abdennouri, M.; Qourzal, S.; Barka, N. Factorial experimental design for the optimization of catalytic degradation of malachite green dye in aqueous solution by Fenton process. Water Resour. Ind. 2016, 15, 41–48. [Google Scholar] [CrossRef]
- Erper, I.; Turkkan, M.; Odabas, M.S. The mathematical approach to the effect of potassium bicarbonate on mycelial growth of Sclerotinia sclerotiorum and Rhizoctonia solani in vitro. Zemdirbyste= Agriculture 2011, 98, 195–204. [Google Scholar]
- Filson, P.B.; Dawson-Andoh, B.E. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009, 100, 2259–2264. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Ramirez Casillas, R.; del Carmen López López, M.; Becerra Aguilar, B.; Dávalos Olivares, F.; Satyanarayana, K.G. Obtaining dissolving grade cellulose from the huizache (Acacia farnesiana L. Willd.) plant. BioResources 2019, 14, 3301–3318. [Google Scholar] [CrossRef]
- Vickerman, J.C.; Gilmore, I.S. Surface Analysis—The Principal Techniques; Vickerman, J.C., Gilmore, I.S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 9780470721582. [Google Scholar]
- Briggs, D.; Seah, M.P. Practical Surface Analysis: Auger and X-ray Photoelectron Spectroscopy, 2nd ed.; Briggs, D., Seah, M.P., Eds.; John Wiley and Sons Ltd.: New York, NY, USA, 1996; ISBN 0471953407. [Google Scholar]
- Snyder, R.L. Analytical profile fitting of X-ray powder diffraction profiles in Rietveld analysis. In The Rietveld Method; Oxford University Press: Oxford, UK, 1993; Volume 1, pp. 111–131. ISBN 0198555776. [Google Scholar]
- Antoy, J. Design of Experiments for Engineers and Scientists, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780080994178. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley and Sons Ltd.: New York, NY, USA, 1992; ISBN 0471935921. [Google Scholar]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Robles, E.; Fernández-Rodríguez, J.; Barbosa, A.M.; Gordobil, O.; Carreño, N.L.V.; Labidi, J. Production of cellulose nanoparticles from blue agave waste treated with environmentally friendly processes. Carbohydr. Polym. 2018, 183, 294–302. [Google Scholar] [CrossRef]
- Anwar, B.; Bundjali, B.; Arcana, I.M. Isolation of Cellulose Nanocrystals from Bacterial Cellulose Produced from Pineapple Peel Waste Juice as Culture Medium. Procedia Chem. 2015, 16, 279–284. [Google Scholar] [CrossRef]
- Contreras, H.J.; Trujillo, H.A.; Arias, G.; Pérez, J.; Delgado, E. Espectroscopia Atr-Ftir De Celulosa: Aspecto Instrumental Y Tratamiento Matemático De Espectros. e-Gnosis 2010, 8, 1–13. [Google Scholar]
- O’Connor, R.T.; DuPré, E.F.; Mitcham, D. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.; Zattera, A. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2018, 25, 37–48. [Google Scholar] [CrossRef]
- Hurtubise, F.G.; KrÄSSIG, H. Classification of Fine Structural Characteristics in Cellulose by Infrared Spectroscopy Use of Potassium Bromide Pellet Technique. Anal. Chem. 1960, 32, 177–181. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Nelson, M.L.; O′Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part, I. Spectra of lattice types I, II, III and of amorphous cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Tronc, E.; Hernández-Escobar, C.A.; Ibarra-Gómez, R.; Estrada-Monje, A.; Navarrete-Bolaños, J.; Zaragoza-Contreras, E.A. Blue agave fiber esterification for the reinforcement of thermoplastic composites. Carbohydr. Polym. 2007, 67, 245–255. [Google Scholar] [CrossRef]
- Popa, N.C.; Balzar, D. Size-broadening anisotropy in whole powder pattern fitting. Application to zinc oxide and interpretation of the apparent crystallites in terms of physical models. J. Appl. Crystallogr. 2008, 41, 615–627. [Google Scholar] [CrossRef]
- De Figueiredo, L.P.; Ferreira, F.F. The Rietveld Method as a Tool to Quantify the Amorphous Amount of Microcrystalline Cellulose. J. Pharm. Sci. 2014, 103, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 2015, 22, 1753–1762. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Valdez-Fausto, E.M.; Rentería-Urquiza, M.; Jiménez-Amezcua, R.M.; Anzaldo Hernández, J.; Torres-Rendon, J.G.; García Enriquez, S. Study of green nanocomposites based on corn starch and cellulose nanofibrils from Agave tequilana Weber. Carbohydr. Polym. 2018, 201, 9–19. [Google Scholar] [CrossRef]
- Naseri, N.; Mathew, A.P.; Girandon, L.; Fröhlich, M.; Oksman, K. Porous electrospun nanocomposite mats based on chitosan–cellulose nanocrystals for wound dressing: Effect of surface characteristics of nanocrystals. Cellulose 2015, 22, 521–534. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; da Silva Perez, D.; Dufresne, A. Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2010, 17, 1147–1158. [Google Scholar] [CrossRef]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Particle Size Analysis-Dynamic Light Scattering (DLS); ISO 22412; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Ramírez Casillas, R.; Báez Rodríguez, K.F.; Cruz-Estrada, R.H.; Dávalos-Olivares, F.; Navarro-Arzate, F.; Satyanarayana, K.G. Isolation and Characterization of Cellulose Nanocrystals Created from Recycled Laser Printed Paper. BioResources 2018, 13, 7404–7429. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, J.R. Obtención y modificación de Nanocristales de celulosa de bagazo de Agave tequilana Weber variedad azul y su uso en matrices poliméricas. Master′s Thesis, Universidad de Guadalajara, Guadalajara, Mexico, 2020. Unpublished. [Google Scholar]
- Gallardo-Sanchez, M.A. Obtención y caracterización de nanocristales de celulosa a partir de desechos agro-industriales de Agave tequilana Weber var. azul y su uso como refuerzo en matrices cementantes. Ph.D. Thesis, Universidad de Guadalajara, Guadalajara, Mexico, 2019. Unpublished. [Google Scholar]





| H2SO4 | HCl | |||||
|---|---|---|---|---|---|---|
| −1 | 0 | +1 | −1 | 0 | +1 | |
| Concentration | 60 wt% | - | 65 wt% | 2 N | - | 8 N |
| Temperature (°C) | 40 | - | 60 | 50 | - | 90 |
| Time (min) | 40 | 55 | 70 | 30 | 115 | 200 |
| H2SO4 | HCl | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Conc. (wt%) | T (°C) | t (min) | Sample | Conc. (N) | T (°C) | t (min) |
| E1S | 60 | 40 | 40 | E1H | 2 | 50 | 30 |
| E2S | 65 | 40 | 40 | E2H | 8 | 50 | 30 |
| E3S | 60 | 60 | 40 | E3H | 2 | 50 | 200 |
| E4S | 65 | 60 | 40 | E4H | 8 | 50 | 200 |
| E5S | 60 | 40 | 70 | E5H | 2 | 90 | 30 |
| E6S | 65 | 40 | 70 | E6H | 8 | 90 | 30 |
| E7S | 60 | 60 | 70 | E7H | 2 | 90 | 200 |
| E8S | 65 | 60 | 70 | E8H | 8 | 90 | 200 |
| E9S | 60 | 40 | 55 | E9H | 2 | 50 | 115 |
| E10S | 65 | 40 | 55 | E10H | 8 | 50 | 115 |
| E11S | 60 | 60 | 55 | E11H | 2 | 90 | 115 |
| E12S | 65 | 60 | 55 | E12H | 8 | 90 | 115 |
| T(ºC) [H2SO4] (wt%) | 40 | 60 | ||||
|---|---|---|---|---|---|---|
| a (mmol/kg) | b (1/min) | R2 | a (mmol/kg) | b (1/min) | R2 | |
| 60 | 1.90 ± 0.84 | 0.04 ± 8 × 10−3 | 0.8976 | 1.46 ± 0.69 | 0.07 ± 8 × 10−3 | 0.9446 |
| 65 | 1.95 ± 0.13 | 0.01 ± 1 × 10−3 | 0.9983 | 5.38 ± 0.015 | 0.05 ± 5 × 10−5 | 1.0 |
| Sample Z | Soluble-Grade Cellulose Pulp | CNC (E1S) | |||||
|---|---|---|---|---|---|---|---|
| C | Atom % | Atom % | C | Atom % | Atom % | ||
| C1s | C-O-C | 4.51 | 10.96 | 57.82 | 5.63 | 7.58 | 56.12 |
| C-O, C-OH | 12.89 | 31.32 | 23.58 | 31.72 | |||
| C-C | 6.40 | 15.54 | 12.50 | 16.82 | |||
| O1s | O-H | 15.44 | 37.49 | 42.18 | 25.74 | 34.63 | 38.96 |
| O-C | 1.93 | 4.69 | 1.90 | 2.56 | |||
| O-S | - | - | 1.31 | 1.77 | |||
| S2p | S-O | 41.17 | 100 | 0 | 3.66 | 4.92 | 4.92 |
| Total | 41.17 | 100 | 100 | 74.33 | 100 | 100 | |
| Source | Conditions | Diameter (nm) | Length (nm) | L/D | Reference |
|---|---|---|---|---|---|
| Agave tequilanaWeber var. azul bagasse | All E1H (2N, 50 °C, 50 min) E8H (8N, 90 °C, 115 min) | 8.6–9.1 8.9 8.6 | 216–829 216 266 | 29.9–95.2 29.9 30.9 | This work |
| MCC | 4N, 80 °C, 225 min | 10–20 | - | - | [63] |
| MCC | 8N, 110 °C, 180 min | 14–16 | 200–250 | 14–15 | [13] |
| MCC | 6N, 110 °C, 180 min | 10–30 | 190–250 | 10–25 | [22] |
| Acacia farnesiana L. Willd | 2–8 N, 50–90 °C, 30–200 min | - | 100–512 | - | [32] |
| Source | Conditions | Diameter (nm) | Length (nm) | L/D | Reference |
|---|---|---|---|---|---|
| Agave tequilanaWeber var. Azul | All E1S (60 wt%, 40 °C, 40 min) E8S (65 wt% N, 60 °C, 70 min) | 8.7–9.3 9.3 9.2 | 137–404 404 149 | 14.7–44.4 43.4 16.2 | This work |
| Agave angustifolia | 60 wt%, 45 °C, 45 min | 8–15 | 170–500 | 10–45 | [17] |
| Agave sisalana | 55 wt%, 45–60 °C, 20–30 min | 5.9–10.5 | 177–433 | 14–15 | [65] |
| Agave tequilana | 65 wt%, 50 °C, 60 min | 11 | 323 ± 112 | 28 | [15] |
| Barley | 65 wt%, 50 °C, 60 min | 10 | 329 ± 123 | 32 | [15] |
| MCC | 64 wt%, 44 °C, 130 min | 16 | 218 ± 56 | 13 | [15] |
| MCC | 50 °C, 60 min | 14–16 | 200–250 | 14–15 | [13] |
| Acacia farnesiana L. Willd | 60–65 wt%, 45–55 °C, 45–65 min | – | 100–260 | – | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Sánchez, M.A.; Diaz-Vidal, T.; Navarro-Hermosillo, A.B.; Figueroa-Ochoa, E.B.; Ramirez Casillas, R.; Anzaldo Hernández, J.; Rosales-Rivera, L.C.; Soltero Martínez, J.F.A.; García Enríquez, S.; Macías-Balleza, E.R. Optimization of the Obtaining of Cellulose Nanocrystals from Agave tequilana Weber Var. Azul Bagasse by Acid Hydrolysis. Nanomaterials 2021, 11, 520. https://doi.org/10.3390/nano11020520
Gallardo-Sánchez MA, Diaz-Vidal T, Navarro-Hermosillo AB, Figueroa-Ochoa EB, Ramirez Casillas R, Anzaldo Hernández J, Rosales-Rivera LC, Soltero Martínez JFA, García Enríquez S, Macías-Balleza ER. Optimization of the Obtaining of Cellulose Nanocrystals from Agave tequilana Weber Var. Azul Bagasse by Acid Hydrolysis. Nanomaterials. 2021; 11(2):520. https://doi.org/10.3390/nano11020520
Chicago/Turabian StyleGallardo-Sánchez, Manuel Alberto, Tania Diaz-Vidal, Alejandra Berenice Navarro-Hermosillo, Edgar Benjamin Figueroa-Ochoa, Rogelio Ramirez Casillas, José Anzaldo Hernández, Luis Carlos Rosales-Rivera, J. Felix Armando Soltero Martínez, Salvador García Enríquez, and Emma Rebeca Macías-Balleza. 2021. "Optimization of the Obtaining of Cellulose Nanocrystals from Agave tequilana Weber Var. Azul Bagasse by Acid Hydrolysis" Nanomaterials 11, no. 2: 520. https://doi.org/10.3390/nano11020520
APA StyleGallardo-Sánchez, M. A., Diaz-Vidal, T., Navarro-Hermosillo, A. B., Figueroa-Ochoa, E. B., Ramirez Casillas, R., Anzaldo Hernández, J., Rosales-Rivera, L. C., Soltero Martínez, J. F. A., García Enríquez, S., & Macías-Balleza, E. R. (2021). Optimization of the Obtaining of Cellulose Nanocrystals from Agave tequilana Weber Var. Azul Bagasse by Acid Hydrolysis. Nanomaterials, 11(2), 520. https://doi.org/10.3390/nano11020520







