Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
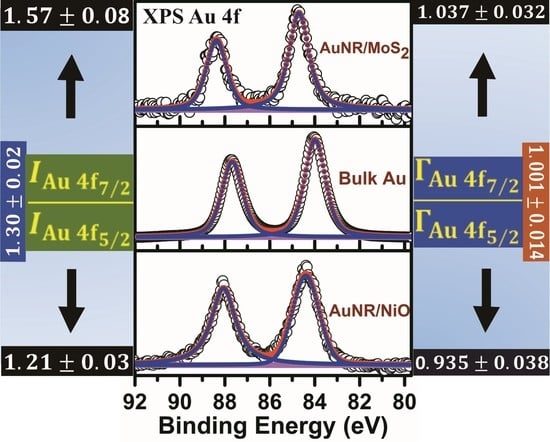
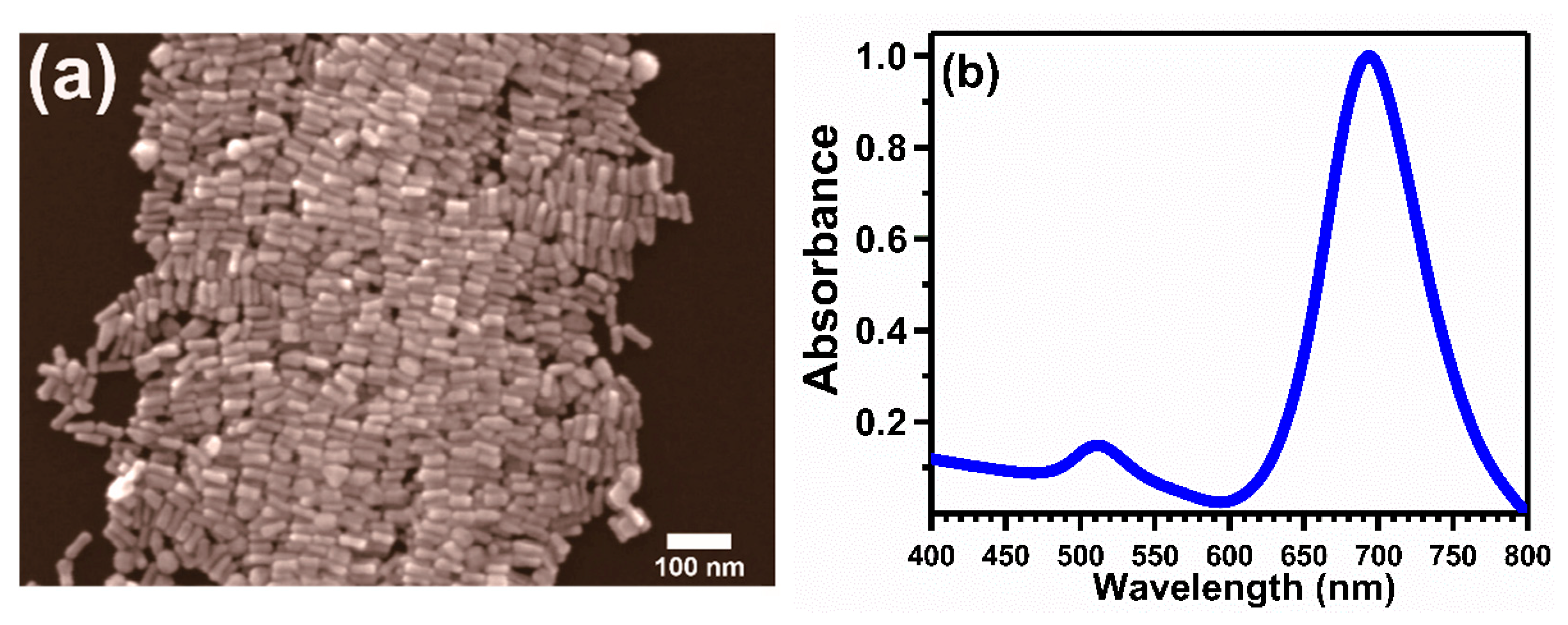
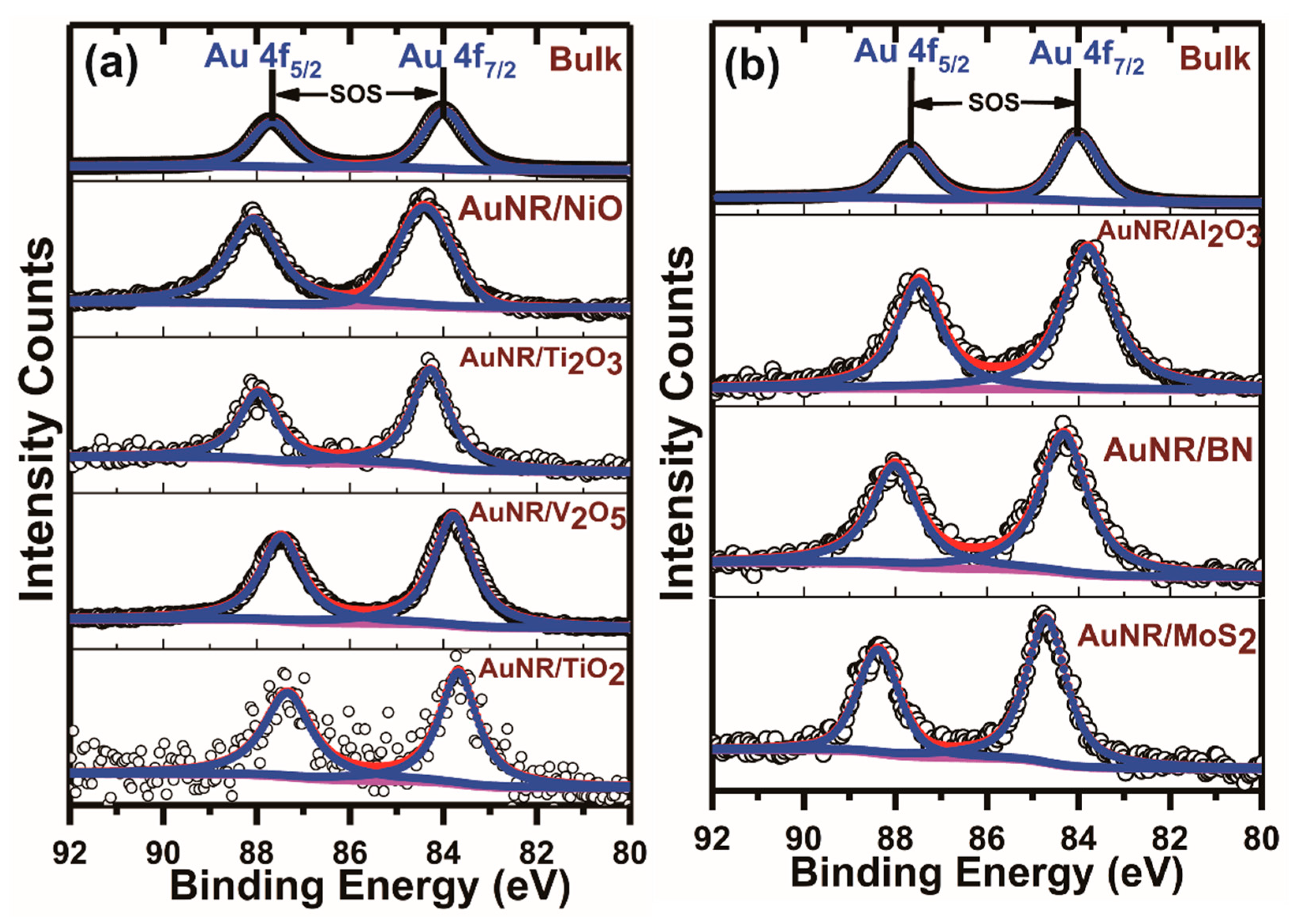
3.1. Au 4f X-ray Photoelectron Spectroscopic Analysis from the Supported Gold Nanoparticles and Nanorods
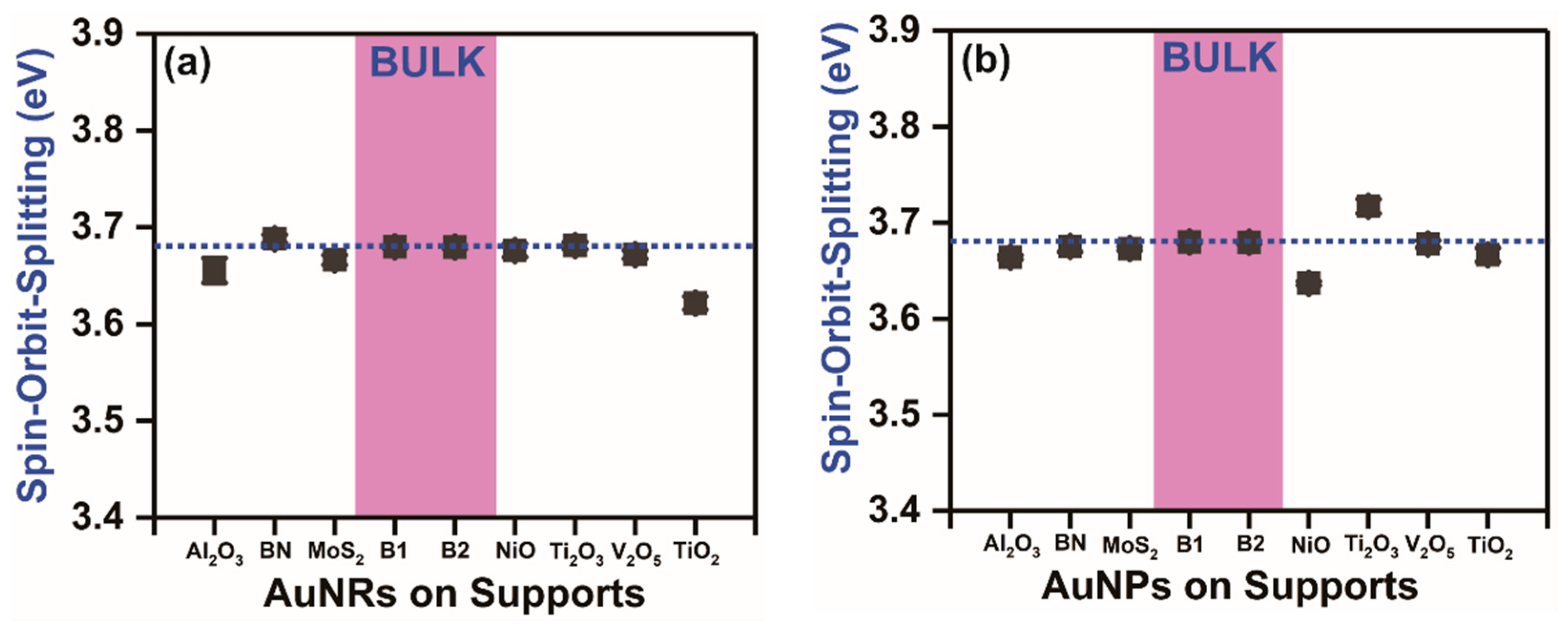
3.2. Au 4f7/2-to-Au 4f5/2 Peak Intensity and Linewidth Ratios in the Supported AuNPs, AuNRs and Bulk Gold
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef]
- Xiao, J.; Qi, L. Surfactant-assisted, shape-controlled synthesis of gold nanocrystals. Nanoscale 2011, 3, 1383–1396. [Google Scholar] [CrossRef]
- Abad, A.; Concepción, P.; Corma, A.; García, H. A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chemie Int. Ed. 2005, 44, 4066–4069. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [Green Version]
- Lohse, S.E.; Murphy, C.J. Applications of colloidal inorganic nanoparticles: From medicine to energy. J. Am. Chem. Soc. 2012, 134, 15607–15620. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, G.J. Catalysis by gold. Catal. Today 2005, 100, 55–61. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.; Zhang, T.; Mou, C.Y. Catalysis by gold: New insights into the support effect. Nano Today 2013, 8, 403–416. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; Van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, J.J. CO oxidation over supported gold catalysts -”Inert” and “active” support materials and their role for the oxygen supply during reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Shekhar, M.; Wang, J.; Lee, W.S.; Williams, W.D.; Kim, S.M.; Stach, E.A.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Size and support effects for the water-gas shift catalysis over gold nanoparticles supported on model Al2O3 and TiO2. J. Am. Chem. Soc. 2012, 134, 4700–4708. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M. Chance and necessity: My encounter with gold catalysts. Angew. Chemie Int. Ed. 2014, 53, 52–56. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.C.; Zanella, R.; Oros-Ruíz, S. Heterogeneous Gold Catalysts and Catalysis; Ma, Z., Dai, S., Eds.; Catalysis Series; Royal Society of Chemistry: London, UK, 2014; ISBN 978-1-84973-917-7. [Google Scholar]
- Hejral, U.; Franz, D.; Volkov, S.; Francoual, S.; Strempfer, J.; Stierle, A. Identification of a Catalytically Highly Active Surface Phase for CO Oxidation over PtRh Nanoparticles under Operando Reaction Conditions. Phys. Rev. Lett. 2018, 120, 126101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascente, P.A.P.; Maluf, S.S.; Afonso, C.R.M.; Landers, R.; Pinheiro, A.N.; Leite, E.R. Au and Pd nanoparticles supported on CeO2, TiO2, and Mn2O3 oxides. Appl. Surf. Sci. 2014, 315, 490–498. [Google Scholar] [CrossRef]
- Elumalai, G.; Noguchi, H.; Dinh, H.C.; Uosaki, K. An efficient electrocatalyst for oxygen reduction to water—Boron nitride nanosheets decorated with small gold nanoparticles (~5 nm) of narrow size distribution on gold substrate. J. Electroanal. Chem. 2018, 819, 107–113. [Google Scholar] [CrossRef]
- Miljevic, M.; Geiseler, B.; Bergfeldt, T.; Bockstaller, P.; Fruk, L. Enhanced photocatalytic activity of Au/TiO2 nanocomposite prepared using bifunctional bridging linker. Adv. Funct. Mater. 2014, 24, 907–915. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, W.; Wang, Y. Effect of size of catalytically active phases in the dehydrogenation of alcohols and the challenging selective oxidation of hydrocarbons. Chem. Commun. 2011, 47, 9275–9292. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-Atom Catalysts Based on the Metal-Oxide Interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Madkour, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Zhang, B.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Li, L.; Ren, Y.; Huang, J.; Haruta, M.; et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, M.S.; Trafela, Š.; Rožman, K.Ž.; Kovač, J.; Djinović, P.; Pintar, A. Determination of Schottky barrier height and enhanced photoelectron generation in novel plasmonic immobilized multisegmented (Au/TiO2) nanorod arrays (NRAs) suitable for solar energy conversion applications. J. Mater. Chem. C 2017, 5, 10509–10516. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Comer, B.M.; Liu, Y.; Dixit, M.B.; Hatzell, K.B.; Crumlin, E.J.; Hatzell, M.C.; Medford, A.J. The Role of Adventitious Carbon on Photocatalytic Nitrogen Fixation by Titania. J. Am. Chem. Soc. 2018, 1–4. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Veith, G.M.; Wang, D.; Manzoli, M.; Prati, L.; Hutchings, G.J. Characterisation of gold catalysts. Chem. Soc. Rev. 2016, 45, 4953–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venezia, A.M. X-ray photoelectron spectroscopy (XPS) for catalysts characterization. Catal. Today 2003, 77, 359–370. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wang, D.; Rosynek, M.P.; Lunsford, J.H. Conversion of methane to benzene over transition metal ion ZSM-5 zeolites: II. Catalyst characterization by X-ray photoelectron spectroscopy. J. Catal. 1998, 175, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Tan, P. Active phase, catalytic activity, and induction period of Fe/zeolite material in nonoxidative aromatization of methane. J. Catal. 2016, 338, 21–29. [Google Scholar] [CrossRef]
- Baer, D.R. Guide to making XPS measurements on nanoparticles. J. Vac. Sci. Technol. A 2020, 38, 031201. [Google Scholar] [CrossRef] [Green Version]
- Chenakin, S.P.; Kruse, N. Au 4f spin-orbit coupling effects in supported gold nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 22778–22782. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Ramacharyulu, P.V.R.K.; Ke, S.-C. Impact of Nonideal Nanoparticles on X-ray Photoelectron Spectroscopic Quantitation: An Investigation Using Simulation and Modeling of Gold Nanoparticles. Anal. Chem. 2018, 90, 1621–1627. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef]
- Chang, H.H.; Murphy, C.J. Mini Gold Nanorods with Tunable Plasmonic Peaks beyond 1000 nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef]
- Nehl, C.L.; Hafner, J.H. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419. [Google Scholar] [CrossRef] [Green Version]
- Nyholm, R.; Berndtsson, A.; Martensson, N. Core level binding energies for the elements Hf to Bi (Z = 72−83). J. Phys. C Solid State Phys. 1980, 13. [Google Scholar] [CrossRef]
- Patanen, M.; Aksela, S.; Urpelainen, S.; Kantia, T.; Heinäsmäki, S.; Aksela, H. Free atom 4f photoelectron spectra of Au, Pb, and Bi. J. Electron Spectros. Relat. Phenom. 2011, 183, 59–63. [Google Scholar] [CrossRef]
- Aksela, S.; Kantia, T.; Patanen, M.; Mäkinen, A.; Urpelainen, S.; Aksela, H. Accurate free atom-solid binding energy shifts for Au and Ag. J. Electron Spectros. Relat. Phenom. 2012, 185, 273–277. [Google Scholar] [CrossRef]
- Pyykkö, P. Theoretical chemistry of gold. II. Inorg. Chim. Acta 2005, 358, 4113–4130. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Cronje, S.; Djordjevic, B.; Schuster, O. Understanding gold chemistry through relativity. Chem. Phys. 2005, 311, 151–161. [Google Scholar] [CrossRef]
- Schmidbaur, H. The aurophilicity phenomenon: A decade of experimental findings, theoretical concepts and emerging applications. Gold Bull. 2000, 33, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Jansen, M. Effects of relativistic motion of electrons on the chemistry of gold and platinum. Solid State Sci. 2005, 7, 1464–1474. [Google Scholar] [CrossRef] [Green Version]
- Mattheiss, L.F.; Dietz, R.E. Relativistic tight-binding calculation of core-valence transitions in Pt and Au. Phys. Rev. B 1980, 22, 1663–1676. [Google Scholar] [CrossRef]
- Nyholm, R.; Martensson, N. N-shell core-level widths for the elements 74W to 83Bi. Phys. Rev. B 1987, 36, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Riffe, D.M.; Wertheim, G.K.; Citrin, P.H.; Buchanan, D.N.E. Core-hole lifetime and screening are different in the surface of W(110). Phys. Scr. 1990, 41, 1009–1013. [Google Scholar] [CrossRef] [Green Version]
- Riffe, D.M.; Wertheim, G.K. Ta(110) surface and subsurface core-level shifts and 4f7/2 line shapes. Phys. Rev. B 1993, 47, 6672–6679. [Google Scholar] [CrossRef] [Green Version]
- Van Der Veen, J.F.; Himpsel, F.J.; Eastman, D.E. Chemisorption-induced 4f-core-electron binding-energy shifts for surface atoms of W(111), W(100), and Ta(111). Phys. Rev. B 1982, 25, 7388–7397. [Google Scholar] [CrossRef]
- Nyholm, R.; Schmidt-May, J. Surface core level shift in polycrystalline hafnium. J. Phys. C Solid State Phys. 1984, 17, L113–L116. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, S.R.; Ke, S.-C. Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles. Nanomaterials 2021, 11, 554. https://doi.org/10.3390/nano11020554
Sahoo SR, Ke S-C. Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles. Nanomaterials. 2021; 11(2):554. https://doi.org/10.3390/nano11020554
Chicago/Turabian StyleSahoo, Smruti R., and Shyue-Chu Ke. 2021. "Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles" Nanomaterials 11, no. 2: 554. https://doi.org/10.3390/nano11020554
APA StyleSahoo, S. R., & Ke, S.-C. (2021). Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles. Nanomaterials, 11(2), 554. https://doi.org/10.3390/nano11020554