Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nickel Molybdate and Nickel Tungstate
2.2. Physical Characterization of NiMoO4 and NiWO4
2.3. Electrochemical Analysis
3. Results and Discussion
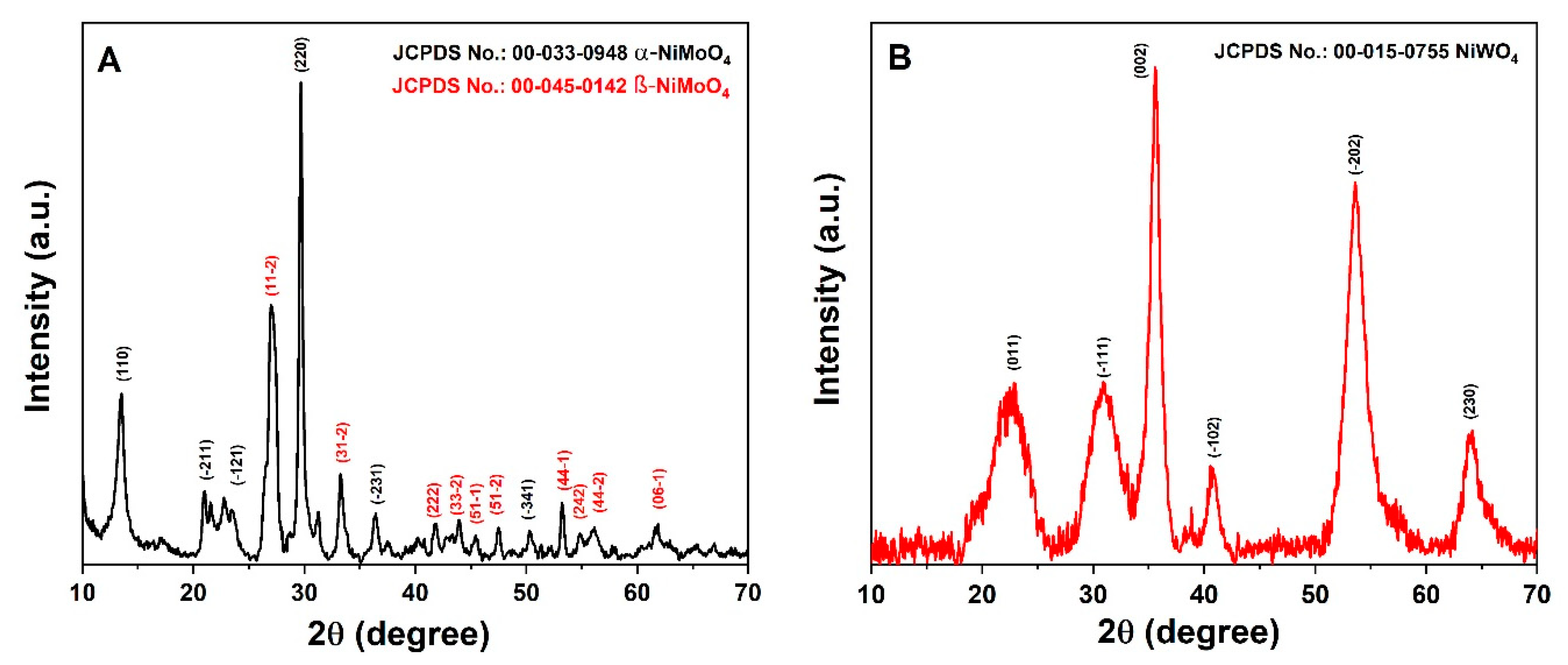
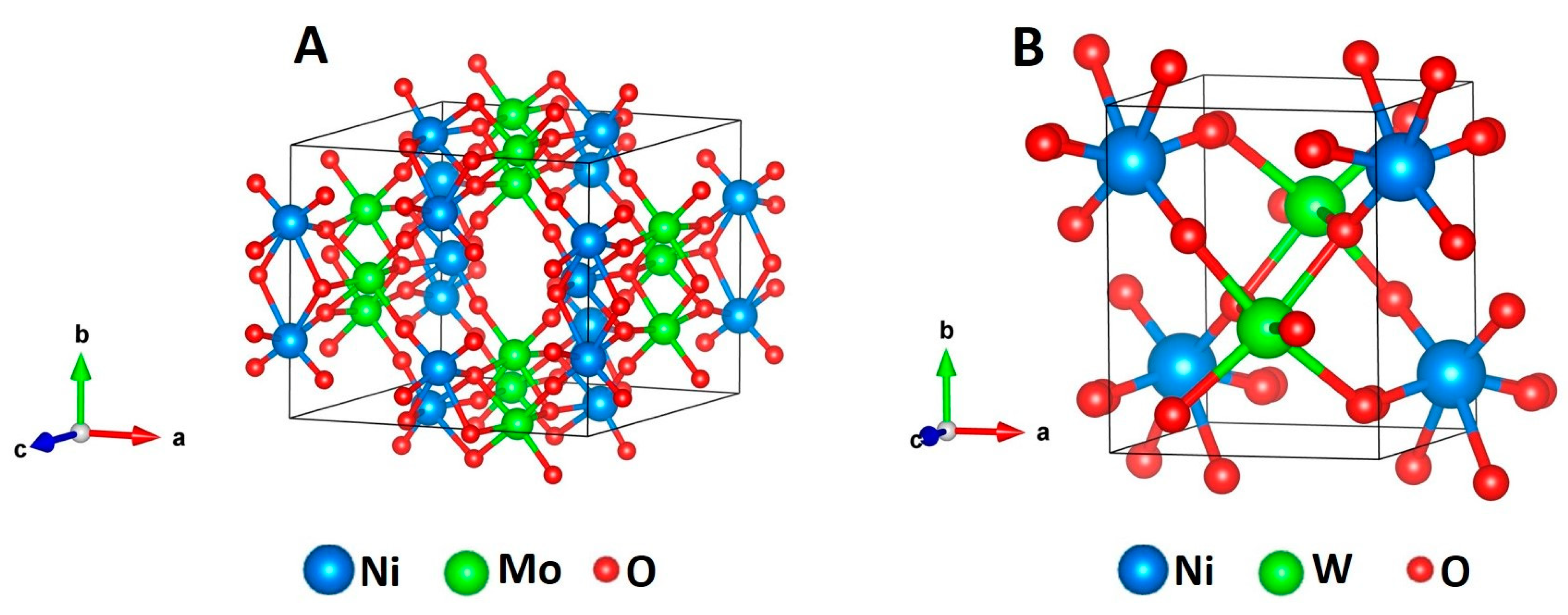
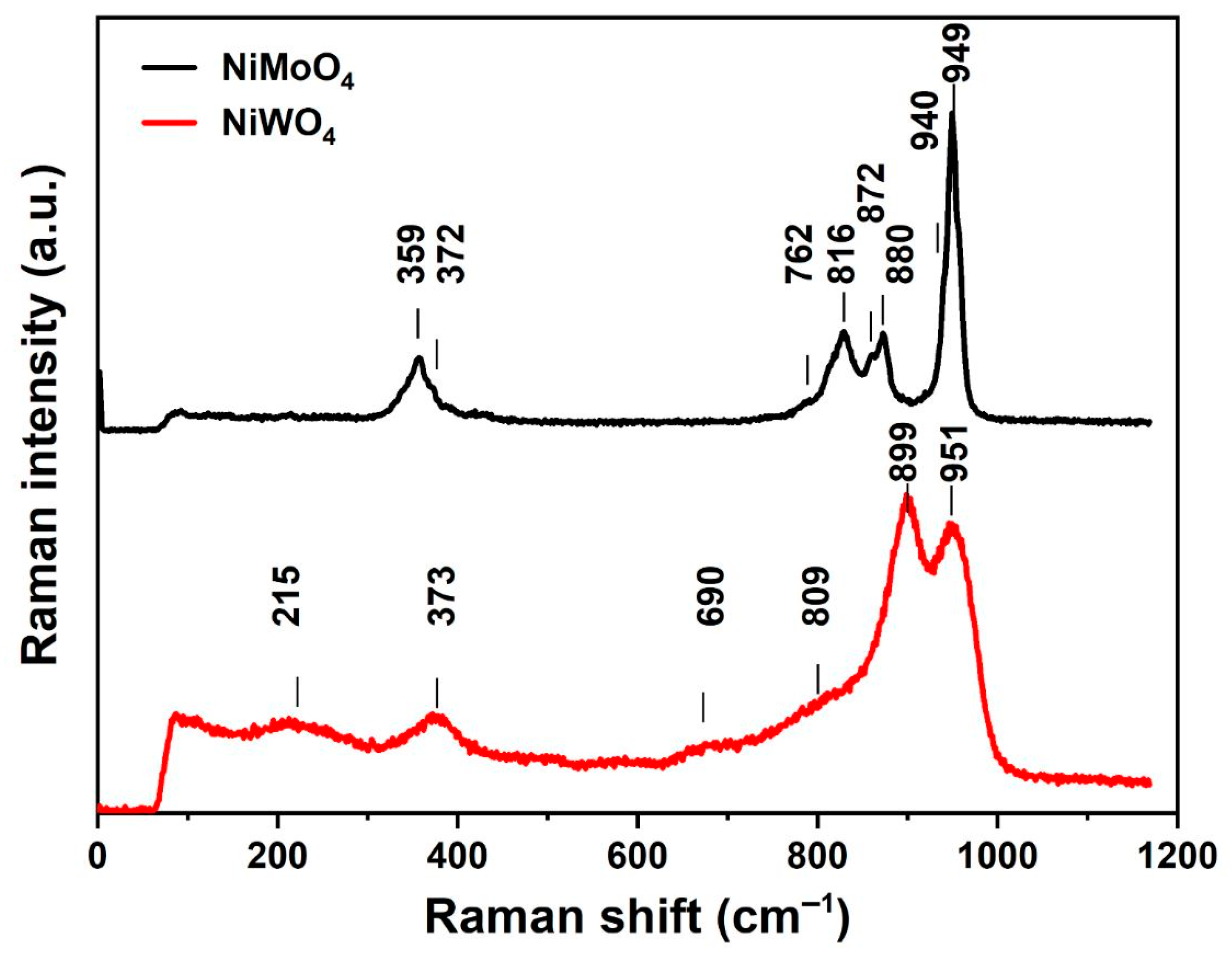
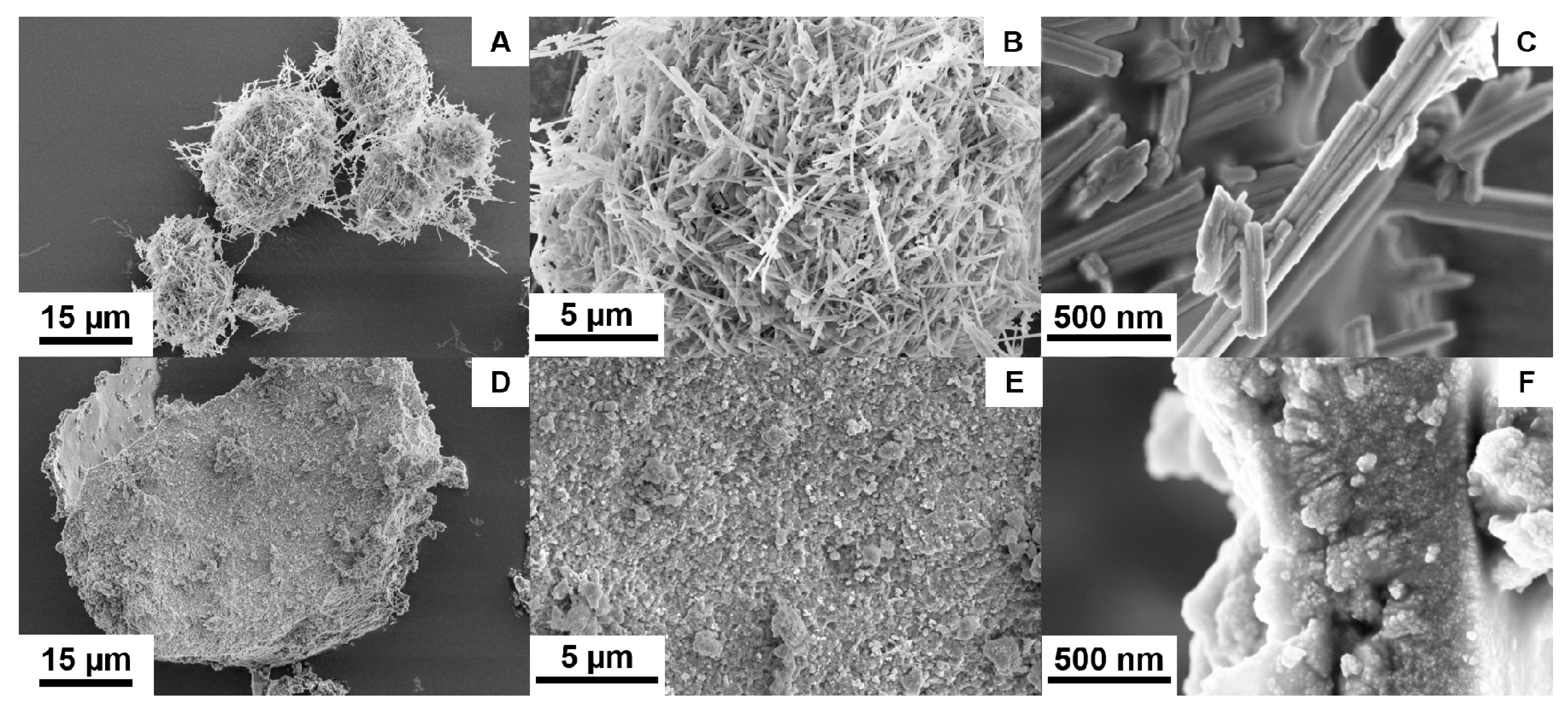
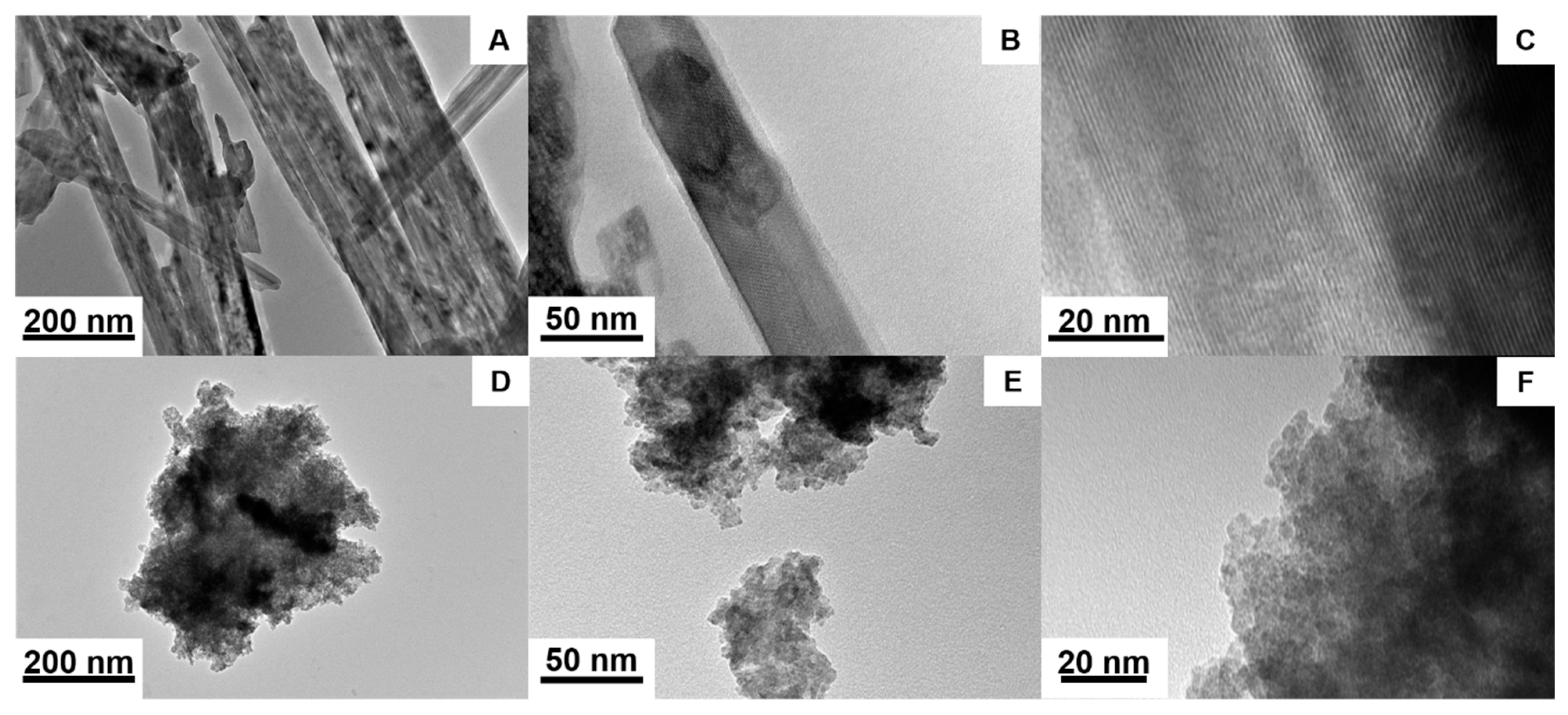
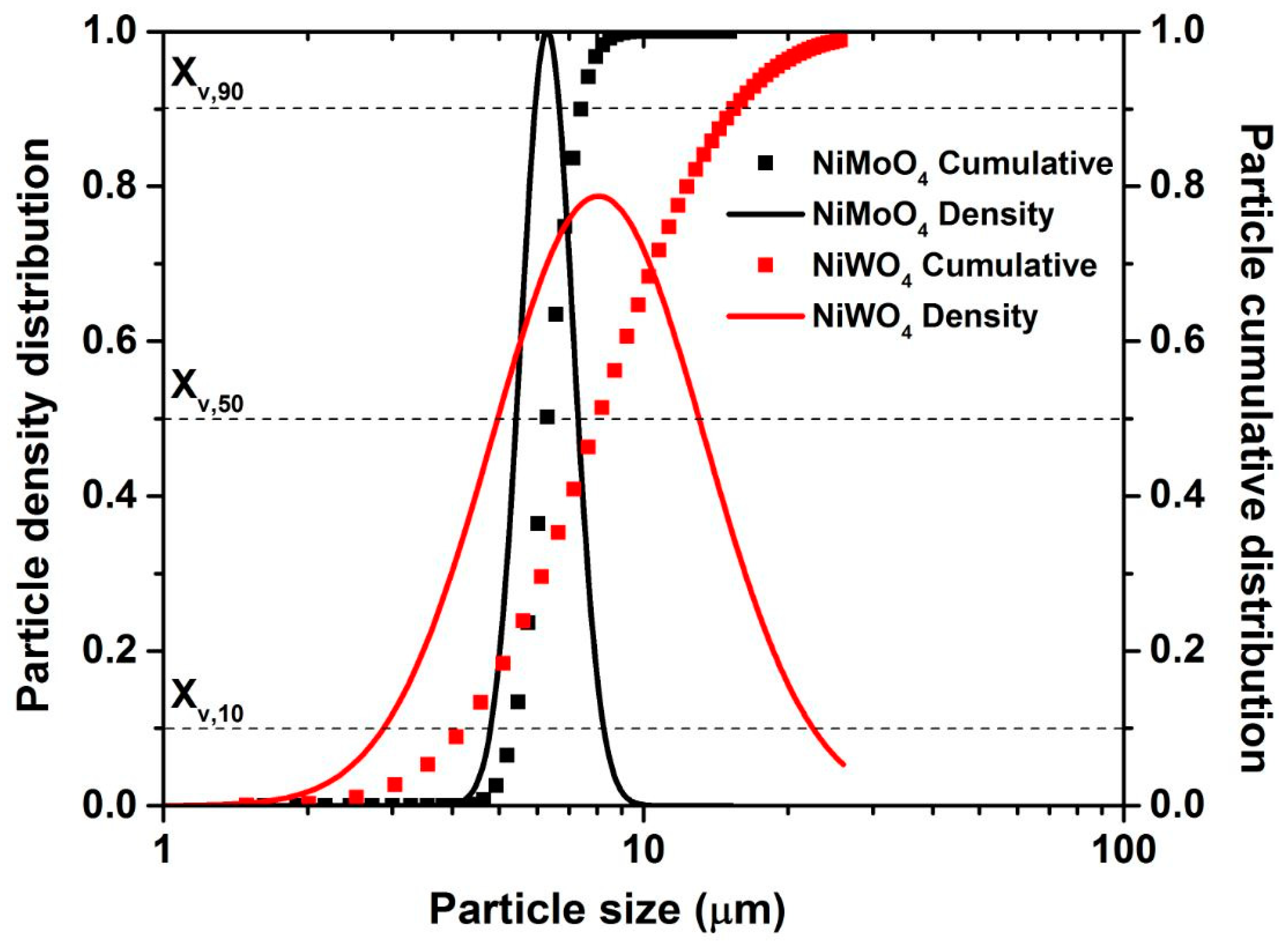
3.1. Chemical and Structural Characterization of NiMoO4 and NiWO4
3.2. Electrochemical Characterization
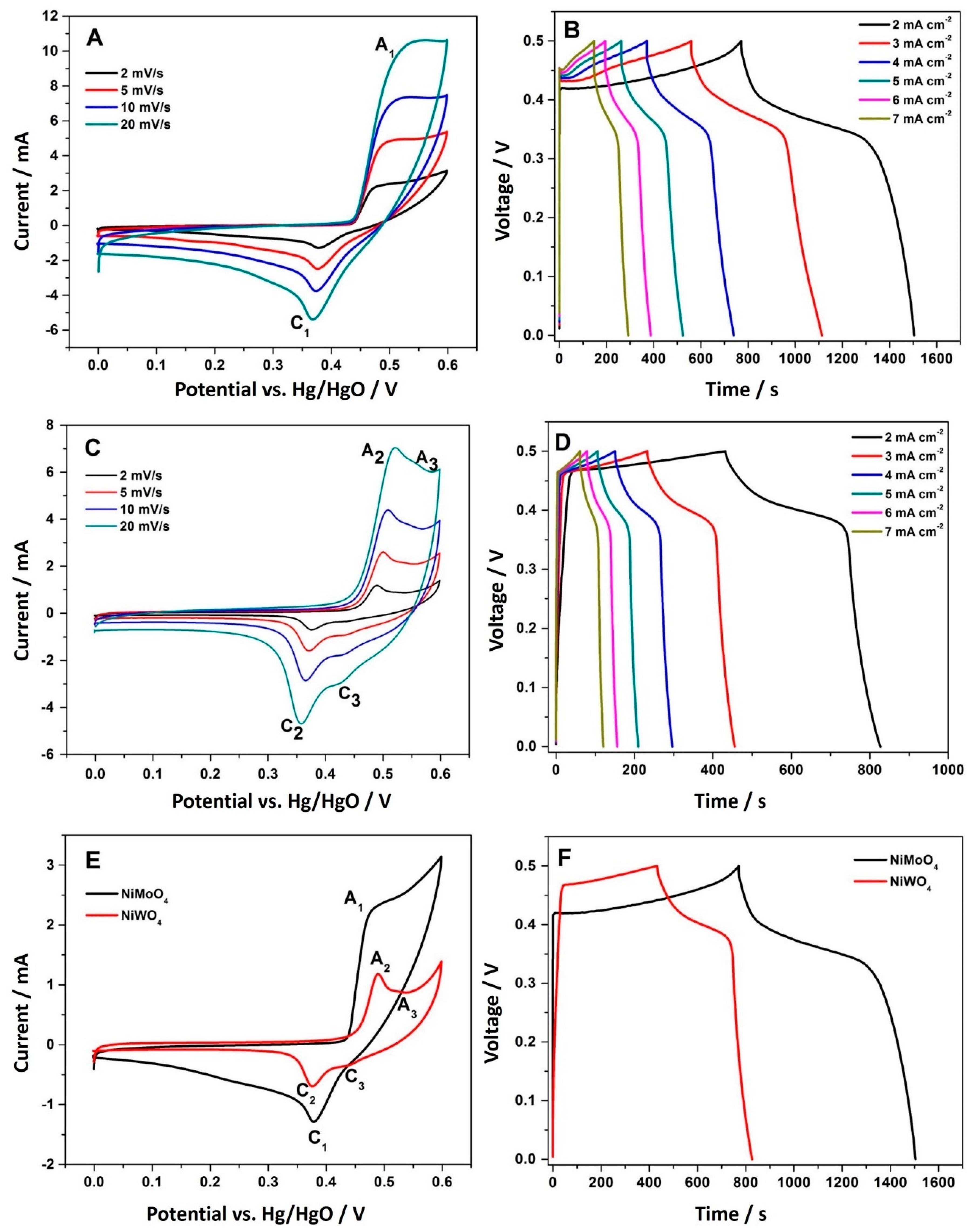
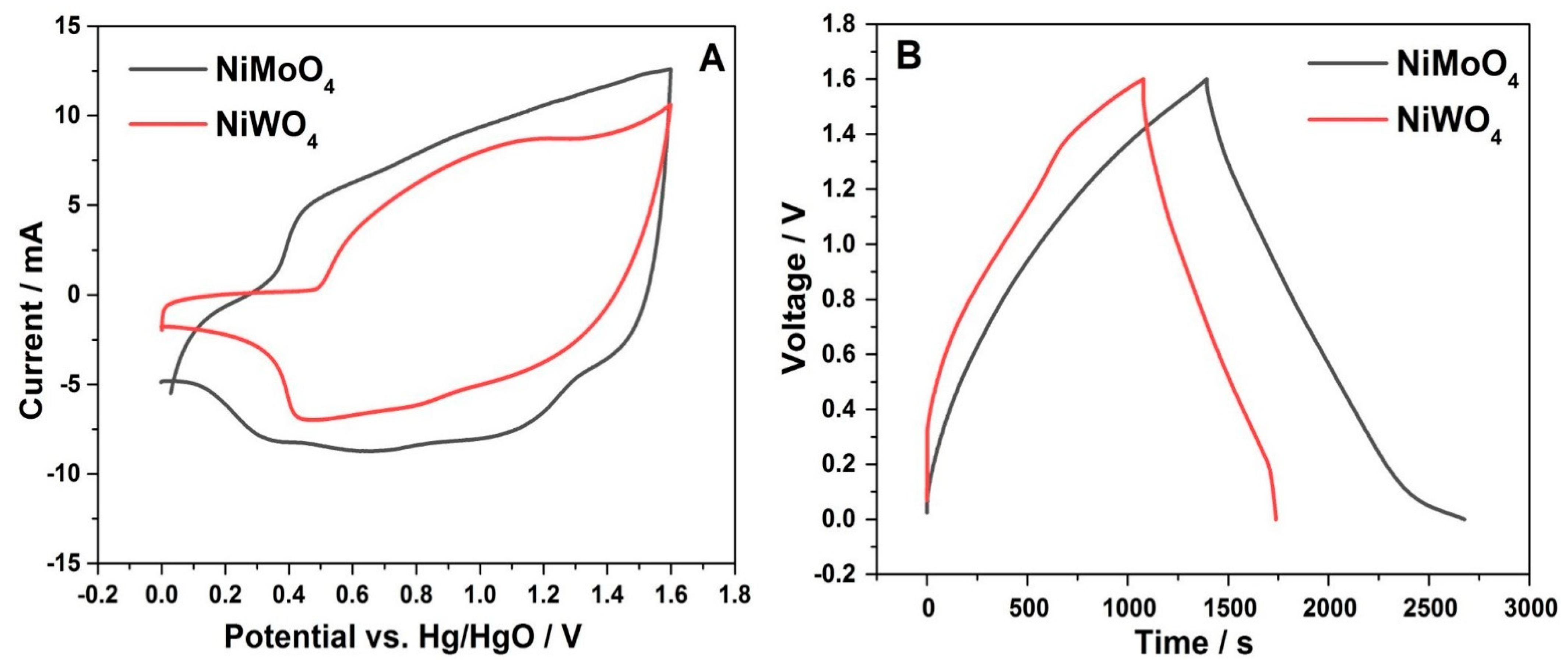
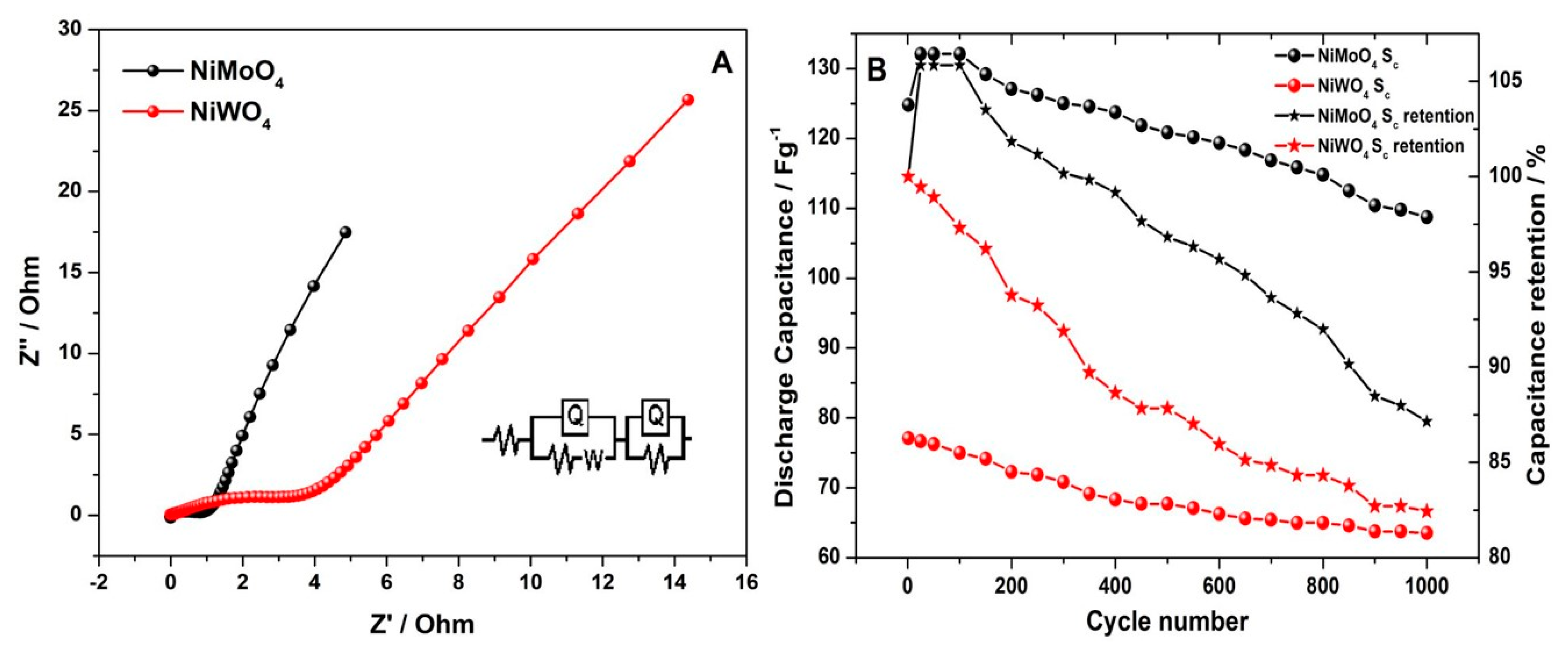
3.2.1. Three-Electrode Configuration
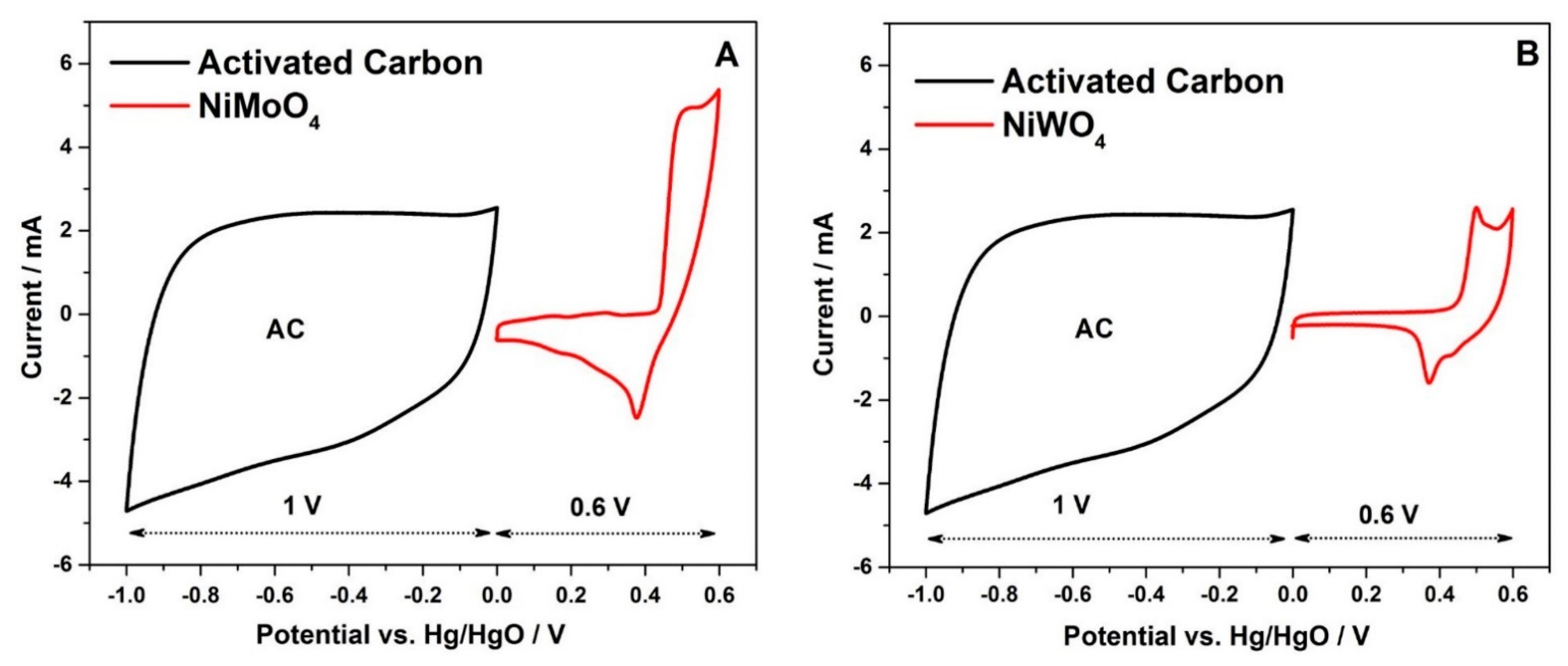
3.2.2. Two-Electrode Configuration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [Green Version]
- Minakshi, M.; Mitchell, D.R.G.; Jones, R.T.; Pramanik, N.C.; Jean-Fulcrand, A.; Garnweitner, G. A Hybrid Electrochemical Energy Storage Device Using Sustainable Electrode Materials. ChemistrySelect 2020, 5, 1597–1606. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Lou, X.W.D. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef]
- Cai, D.; Wang, D.; Liu, B.; Wang, Y.; Liu, Y.; Wang, L.; Li, H.; Huang, H.; Li, Q.; Wang, T. Comparison of the electrochemical performance of NiMoO4 nanorods and hierarchical nanospheres for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 12905–12910. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Lui, Y.H.; Hu, S. Ni-Mn bimetallic oxide nanosheets as high-performance electrode materials for asymmetric supercapacitors. J. Energy Storage 2019, 25, 100897. [Google Scholar] [CrossRef]
- De Oliveira, A.L.M.; Ferreira, J.M.; Silva, M.R.S.; de Souza, S.C.; Vieira, F.T.G.; Longo, E.; Souza, A.G.; Santos, I.M.G. Influence of the thermal treatment in the crystallization of NiWO4 and ZnWO4. J. Therm. Anal. Calorim. 2009, 97, 167–172. [Google Scholar] [CrossRef]
- Baoyi, S.; Aiju, X.; Jiang, W. The impact of preparation methods on the structure and catalytic performance of NiMoO4 for oxidative dehydrogenation of propane. Integr. Ferroelectr. 2016, 171, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Klissurski, D.; Mancheva, M.; Iordanova, R.; Tyuliev, G.; Kunev, B. Mechanochemical synthesis of nanocrystalline nickel molybdates. J. Alloys Compd. 2006, 422, 53–57. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Ahmed, J.; Alzahrani, A.M.; Ahamad, T. Synthesis, characterization, and enhanced photocatalytic properties of NiWO4 nanobricks. New J. Chem. 2017, 41, 8178–8186. [Google Scholar] [CrossRef]
- Wang, B.; Li, S.; Wu, X.; Tian, W.; Liu, J.; Yu, M. Integration of network-like porous NiMoO4 nanoarchitectures assembled with ultrathin mesoporous nanosheets on three-dimensional graphene foam for highly reversible lithium storage. J. Mater. Chem. A 2015, 3, 13691–13698. [Google Scholar] [CrossRef]
- Moreno, B.; Chinarro, E.; Colomer, M.T.; Jurado, J.R. Combustion Synthesis and Electrical Behavior of Nanometric β-NiMoO4. J. Phys. Chem. C 2010, 114, 4251–4257. [Google Scholar] [CrossRef]
- Niu, L.; Li, Z.; Xu, Y.; Sun, J.; Hong, W.; Liu, X.; Wang, J.; Yang, S. Simple synthesis of amorphous NiWO4 nanostructure and its application as a novel cathode material for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 8044–8052. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Jia, Y.; Zheng, H. Three-dimensional NiCo2O4@NiWO4 core–shell nanowire arrays for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 1028–1034. [Google Scholar] [CrossRef]
- Garnweitner, G.; Xue, D. Crystal engineering for electrochemical applications. CrystEngComm 2020, 22, 1498–1499. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Zhang, Y.; Zhang, X.; Tang, T.; Luo, H.; Niu, S.; Dai, Z.-H.; Wan, L.-J.; Hu, J.-S. Self-Templated Fabrication of MoNi4 /MoO3-x Nanorod Arrays with Dual Active Components for Highly Efficient Hydrogen Evolution. Adv. Mater. 2017, 29, 1703311. [Google Scholar] [CrossRef]
- Dury, F.; Gaigneaux, E.M.; Ruiz, P. The active role of CO2 at low temperature in oxidation processes: The case of the oxidative dehydrogenation of propane on NiMoO4 catalysts. Appl. Catal. A 2003, 242, 187–203. [Google Scholar] [CrossRef]
- De Moura, A.P.; de Oliveira, L.H.; Rosa, I.L.V.; Xavier, C.S.; Lisboa-Filho, P.N.; Li, M.S.; La Porta, F.A.; Longo, E.; Varela, J.A. Structural, optical, and magnetic properties of NiMoO4 nanorods prepared by microwave sintering. Sci. World J. 2015, 2015, 315084. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Dayem, H.M. Dynamic Phenomena during Reduction of α-NiMoO4 in Different Atmospheres: In-Situ Thermo-Raman Spectroscopy Study. Ind. Eng. Chem. Res. 2007, 46, 2466–2472. [Google Scholar] [CrossRef]
- Zou, J.Y.; Schrader, G.L. Deposition of multiphase molybdate thin films by reactive sputtering. Thin Solid Films 1997, 324, 52–62. [Google Scholar] [CrossRef]
- Hanuza, J.; Maczka, M.; Van der Maas, J.H. Vibrational Properties of Double Tungstates of the MIMIII(WO4)2 Family (MI = Li, Na, K; MIII = Bi, Cr). J. Solid State Chem. 1995, 117, 177–188. [Google Scholar] [CrossRef]
- Ross-Medgaarden, E.I.; Wachs, I.E. Structural Determination of Bulk and Surface Tungsten Oxides with UV−vis Diffuse Reflectance Spectroscopy and Raman Spectroscopy. J. Phys. Chem. C 2007, 111, 15089–15099. [Google Scholar] [CrossRef]
- Zawawi, S.M.; Yahya, R.; Hassan, A.; Ekramul Mahmud, H.N.M.; Daud, M.N. Structural and optical characterization of metal tungstates (MWO4; M=Ni, Ba, Bi) synthesized by a sucrose-templated method. Chem. Cent. J. 2013, 7, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doudin, N.; Pomp, S.; Blatnik, M.; Resel, R.; Vorokhta, M.; Goniakowski, J.; Noguera, C.; Netzer, F.P.; Surnev, S. Epitaxial NiWO4 films on Ni(110): Experimental and theoretical study of surface stability. Surface Sci. 2017, 659, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Vroulias, D.; Gkoulemani, N.; Papadopoulou, C.; Matralis, H. W–modified Ni/Al2O3 catalysts for the dry reforming of methane: Effect of W loading. Catal. Today 2019, 355, 704–715. [Google Scholar] [CrossRef]
- Lima, N.A.; Alencar, L.D.S.; Siu-Li, M.; Feitosa, C.A.C.; Mesquita, A.; M’peko, J.-C.; Bernardi, M.I.B. NiWO4 powders prepared via polymeric precursor method for application as ceramic luminescent pigments. J. Adv. Ceram. 2020, 9, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Harshan, H.; Priyanka, K.P.; Sreedevi, A.; Jose, A.; Varghese, T. Structural, optical and magnetic properties of nanophase NiWO4 for potential applications. Eur. Phys. J. B 2018, 91, 10356. [Google Scholar] [CrossRef]
- Anspoks, A.; Kalinko, A.; Timoshenko, J.; Kuzmin, A. Local structure relaxation in nanosized tungstates. Solid State Commun. 2014, 183, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn Metal Atom Doping on the Surface Plane of One-Dimesional NiMoO4 Nanorods with Improved Redox Chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Green, S.V.; Kuzmin, A.; Purans, J.; Granqvist, C.G.; Niklasson, G.A. Structure and composition of sputter-deposited nickel-tungsten oxide films. Thin Solid Films 2011, 519, 2062–2066. [Google Scholar] [CrossRef] [Green Version]
- Heumann, T.H.; Stolica, N. The electrochemical behaviour of tungsten-II. The dissolution of tungsten in NaOH solutions. Electrochim. Acta 1971, 16, 1635–1646. [Google Scholar] [CrossRef]
- Tuvić, T.; Pašti, I.; Mentus, S. Tungsten electrochemistry in alkaline solutions—Anodic dissolution and oxygen reduction reaction. Russ. J. Phys. Chem. 2011, 85, 2399–2405. [Google Scholar] [CrossRef]
- Ortiz, P.I.; Giordano, M.C.; Lopez Teijelo, M. Electrochemical behaviour of tungsten in alkaline media Part II. Sodium carbonate solutions. J. Electroanal. Chem. Interfacial Electrochem. 1988, 251, 393–401. [Google Scholar] [CrossRef]
- Krtil, P.; Fattakhova, D.; Yoshimura, M. Mechanism of soft solution processing formation of alkaline earth metal tungstates: An electrochemical and in situ AFM study. J. Solid State Electrochem. 2002, 6, 367–373. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Minakshi, M.; Whale, J.; Jean-Fulcrand, A.; Garnweitner, G. Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications. Nanomaterials 2021, 11, 580. https://doi.org/10.3390/nano11030580
Sharma P, Minakshi M, Whale J, Jean-Fulcrand A, Garnweitner G. Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications. Nanomaterials. 2021; 11(3):580. https://doi.org/10.3390/nano11030580
Chicago/Turabian StyleSharma, Pratigya, Manickam Minakshi, Jonathan Whale, Annelise Jean-Fulcrand, and Georg Garnweitner. 2021. "Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications" Nanomaterials 11, no. 3: 580. https://doi.org/10.3390/nano11030580
APA StyleSharma, P., Minakshi, M., Whale, J., Jean-Fulcrand, A., & Garnweitner, G. (2021). Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications. Nanomaterials, 11(3), 580. https://doi.org/10.3390/nano11030580






