Gold Inks for Inkjet Printing on Photo Paper: Complementary Characterisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
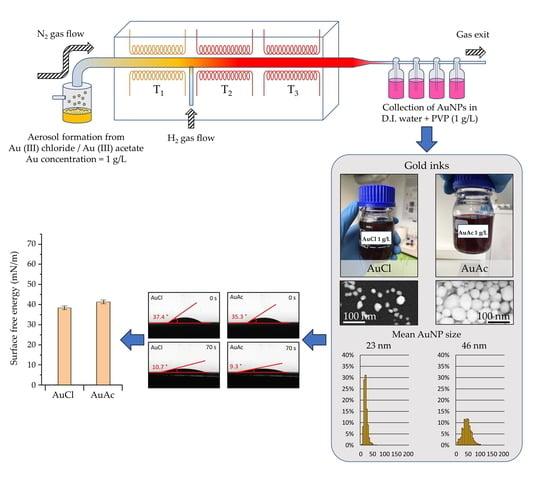
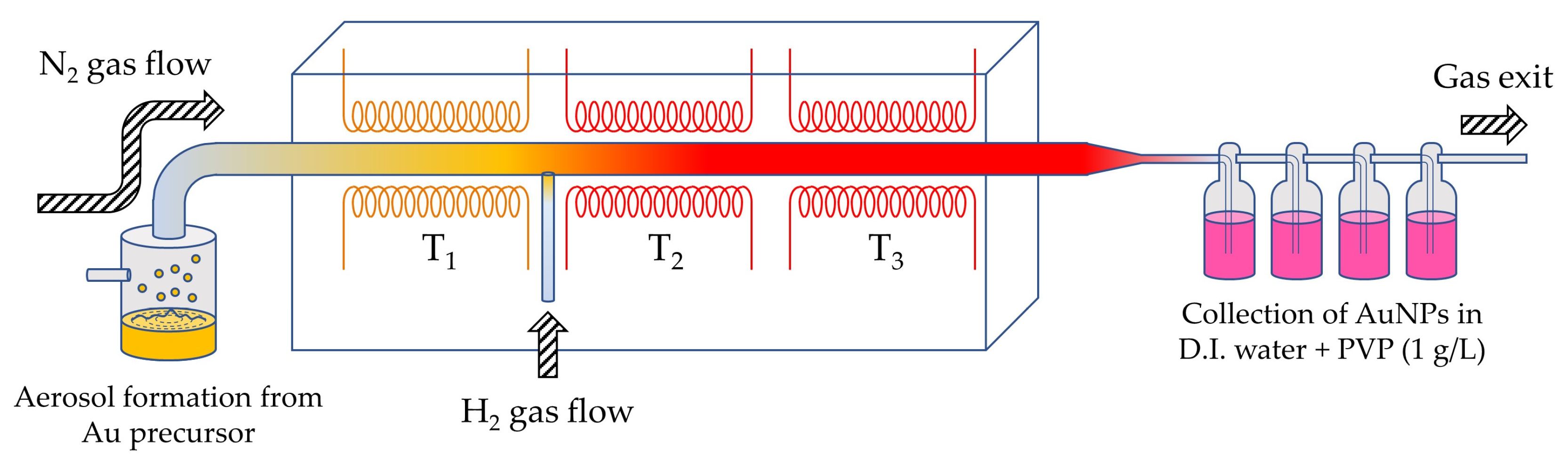
2.2.1. Preparation of AuNPs
2.2.2. Preparation of Gold Inks
2.2.3. Inkjet Printing
2.3. Characterisation
2.3.1. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
2.3.2. Dynamic and Electrophoretic Light Scattering (DLS)
2.3.3. UV–VIS Spectrophotometry
2.3.4. Scanning Electron Microscopy
2.3.5. Optical Goniometry
2.3.6. Statistics
3. Results and Discussion
3.1. ICP-MS
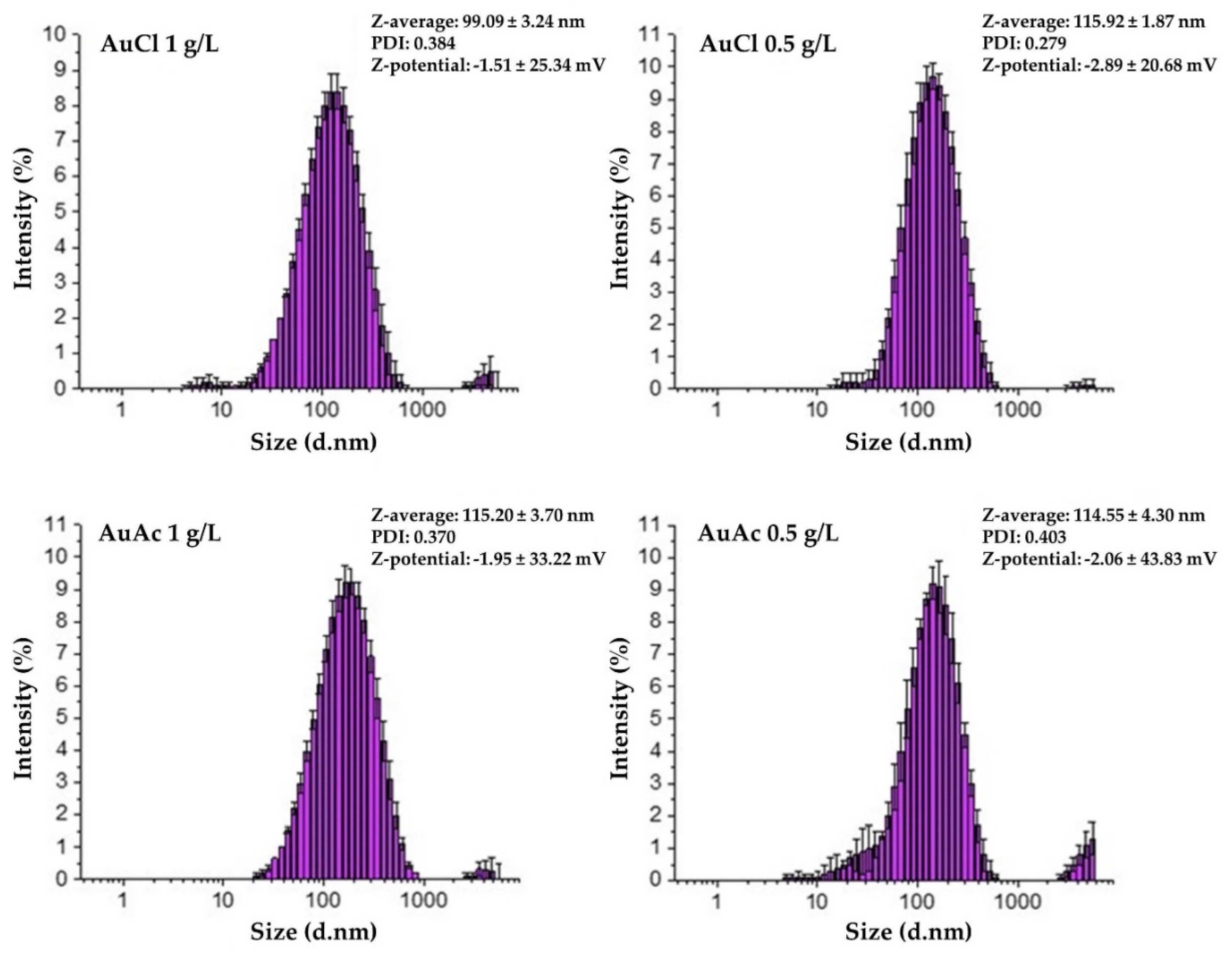
3.2. AuNPs’ Size and ζ-Potential of Gold Inks
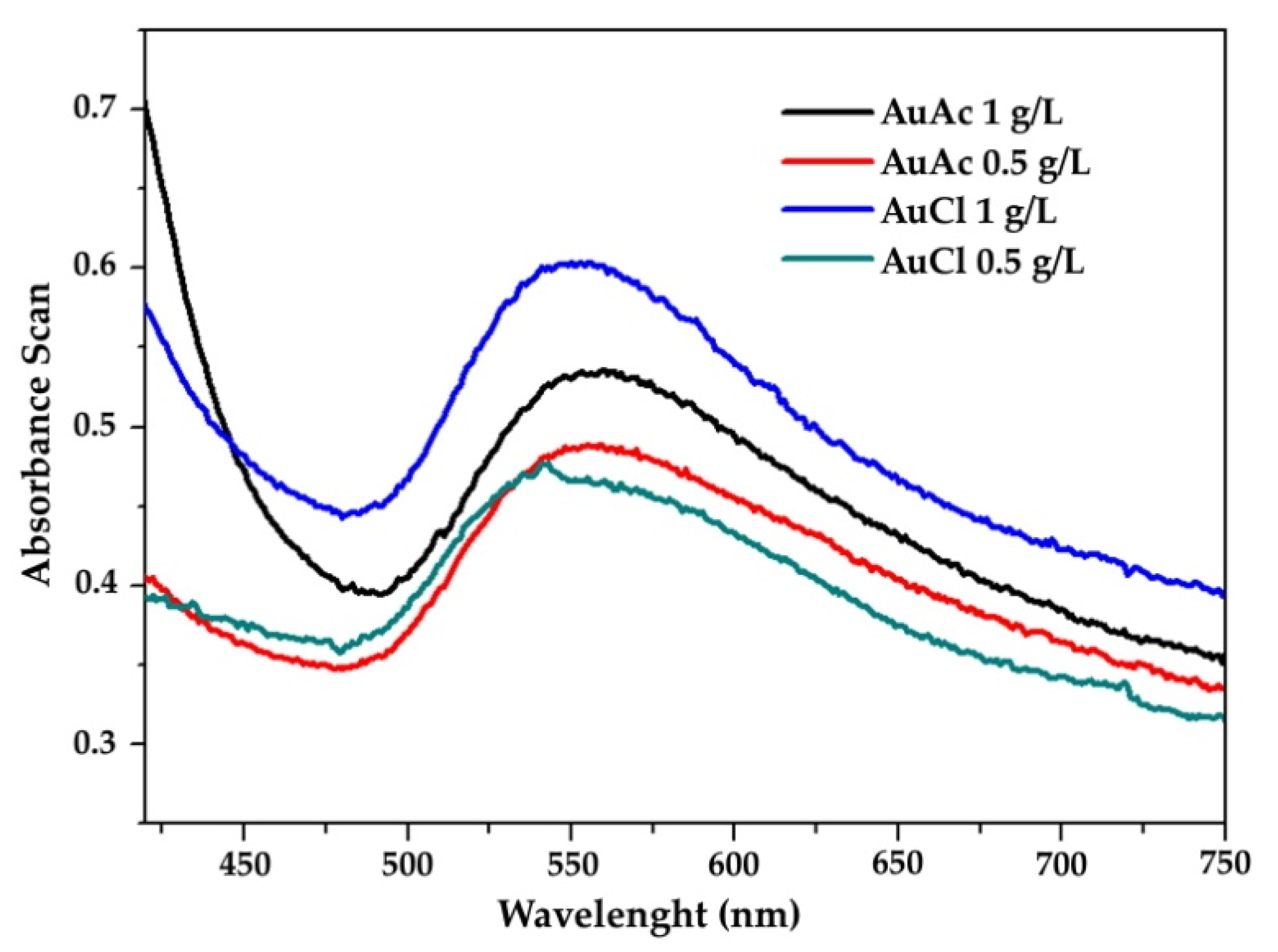
3.3. UV–VIS
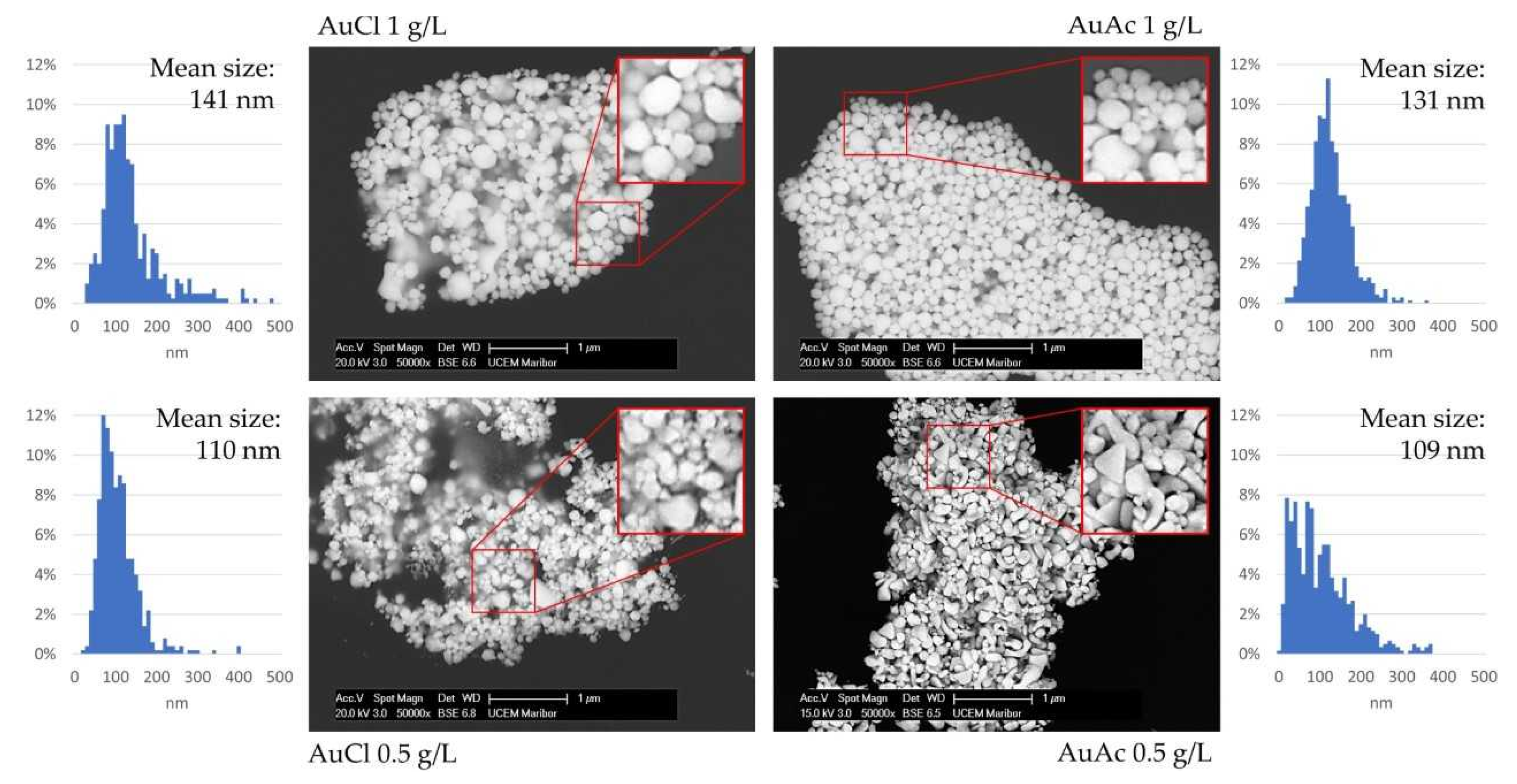
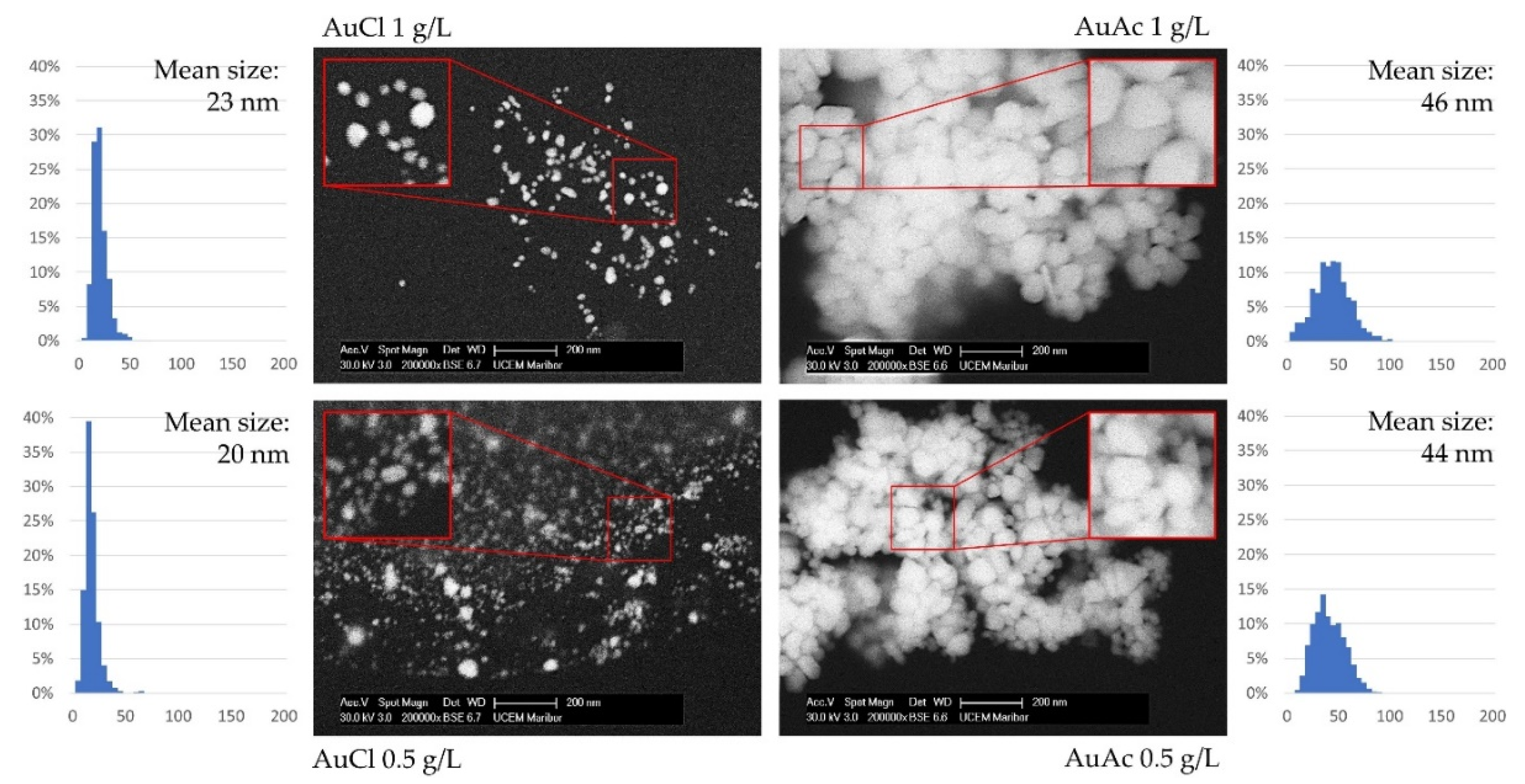
3.4. SEM Analysis of AuNPs
3.5. Contact Angle and Surface Energy Measurements of Gold Inks
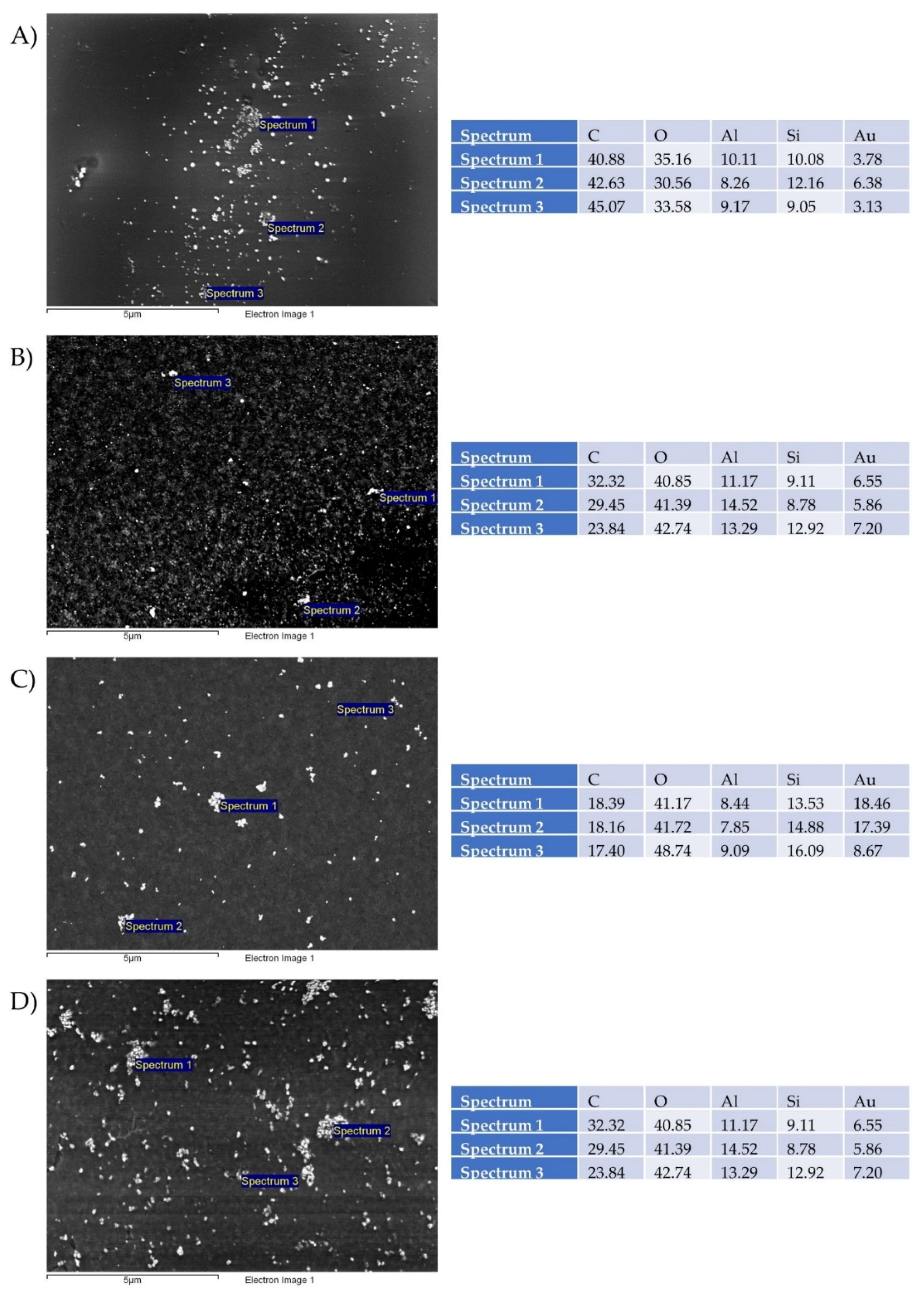
3.6. SEM Analysis of Printing Pattern
4. Conclusions
- The USP synthesis, in combination with the rotavapor, allowed successful preparation of gold inks with AuNPs reaching Au concentrations more than 300 ppm.
- SEM observation revealed that AuNPs in the as prepared inks had mostly spherical shape, with the presence of some irregularly shaped AuNPs.
- AuNPs in the prepared inks exhibited Z-average hydrodynamic diameters, ranging from 99 to 115 nm.
- Due to the fact that some AuNPs were over 1 µm in size, consequently, the filtration of as prepared gold inks was mandatory to avoid problems with the inkjet printing, as AuNPs agglomerated, which can cause nozzle blockage.
- After filtration, the AuNPs in gold inks had an average size between 20 and 46 nm, suitable for inkjet printing. The AuNPs size was directly dependent on the Au concentration in the precursor (lower AuNPs’ size–lower Au concentration).
- The measured UV–VIS spectra of as prepared gold inks were within the range of 510–560 nm.
- The contact angle and surface energy results showed that the printed patterns could be controlled by varying the concentration of AuNPs in the inks.
- The filtered gold inks were printed on photo paper with selected parameters, and it confirmed relative homogeneous distribution of AuNPs on their surfaces. Additional EDS measurements showed the presence of Au.
Author Contributions
Funding
Conflicts of Interest
References
- Lin, Y.; Gritsenko, D.; Liu, Q.; Lu, X.; Xu, J. Recent Advancements in Functionalized Paper-Based Electronics. ACS Appl. Mater. Interfaces 2016, 8, 20501–20515. [Google Scholar] [CrossRef] [PubMed]
- Serpelloni, M.; Cantù, E.; Borghetti, M.; Sardini, E. Printed Smart Devices on Cellulose-Based Materials by means of Aerosol-Jet Printing and Photonic Curing. Sensors 2020, 20, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, F.; Operamolla, A.; Castro-Hermosa, S.; Lucarelli, G.; Manca, V.; Farinola, G.M.; Brown, T.M. Printed Solar Cells and Energy Storage Devices on Paper Substrates. Adv. Funct. Mater. 2019, 29, 1806798. [Google Scholar] [CrossRef] [Green Version]
- Gozutok, Z.; Kinj, O.; Torun, I.; Ozdemir, A.T.; Onses, M.S. One-step deposition of hydrophobic coatings on paper for printed-electronics applications. Cellulose 2019, 26, 3503–3512. [Google Scholar] [CrossRef] [Green Version]
- Alkin, K.; Stockinger, T.; Zirkl, M.; Stadlober, B.; Bauer-Gogonea, S.; Kaltenbrunner, M.; Bauer, S.; Müller, U.; Schwödiauer, R. Paper-based printed impedance sensors for water sorption and humidity analysis. Flex. Print. Electron. 2017, 2, 14005. [Google Scholar] [CrossRef] [Green Version]
- Öhlund, T.; Schuppert, A.K.; Hummelgård, M.; Bäckström, J.; Nilsson, H.-E.; Olin, H. Inkjet Fabrication of Copper Patterns for Flexible Electronics: Using Paper with Active Precoatings. ACS Appl. Mater. Interfaces 2015, 7, 18273–18282. [Google Scholar] [CrossRef]
- Öhlund, T.; Örtegren, J.; Forsberg, S.; Nilsson, H.-E. Paper surfaces for metal nanoparticle inkjet printing. Appl. Surf. Sci. 2012, 259, 731–739. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, C.; Cheng, S.-Y.; Xu, Z.-Q.; Sun, X.; Xu, Y.-H.; Chen, J.-J.; Jiang, Z.; Liang, K.; Feng, Z.-S. Flexible RFID Tag Metal Antenna on Paper-Based Substrate by Inkjet Printing Technology. Adv. Funct. Mater. 2019, 29, 1902579. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Kou, T.; Song, Y.; Liu, T.; Li, Y. Paper-Based Electrodes for Flexible Energy Storage Devices. Adv. Sci. 2017, 4, 1700107. [Google Scholar] [CrossRef]
- Ling, H.; Chen, R.; Huang, Q.; Shen, F.; Wang, Y.; Wang, X. Transparent, flexible and recyclable nanopaper-based touch sensors fabricated via inkjet-printing. Green Chem. 2020, 22, 3208–3215. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Chen, J.; Sowade, E.; Wang, Y.; Liang, K.; Marcus, K.; Baumann, R.R.; Feng, Z. Paper-Based Inkjet-Printed Flexible Electronic Circuits. ACS Appl. Mater. Interfaces 2016, 8, 26112–26118. [Google Scholar] [CrossRef]
- Beedasy, V.; Smith, P.J. Printed Electronics as Prepared by Inkjet Printing. Materials 2020, 13, 704. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Chon, S.; Cho, S.; Lee, Y.; Yoo, M.; Kim, D.; Lee, D.Y.; Lim, J.K. A novel photolithographic method for fabrication of flexible micro-patterned glucose sensors. J. Electroanal. Chem. 2020, 876, 114720. [Google Scholar] [CrossRef]
- Dou, Y.; Chen, L.; Li, H.; Tang, B.; Henzen, A.; Zhou, G. Photolithography Fabricated Spacer Arrays Offering Mechanical Strengthening and Oil Motion Control in Electrowetting Displays. Sensors 2020, 20, 494. [Google Scholar] [CrossRef] [Green Version]
- Sameenoi, Y.; Nongkai, P.N.; Nouanthavong, S.; Henry, C.S.; Nacapricha, D. One-step polymer screen-printing for microfluidic paper-based analytical device (μPAD) fabrication. Analyst 2014, 139, 6580–6588. [Google Scholar] [CrossRef]
- Somalu, M.R.; Muchtar, A.; Daud, W.R.W.; Brandon, N.P. Screen-printing inks for the fabrication of solid oxide fuel cell films: A review. Renew. Sustain. Energy Rev. 2017, 75, 426–439. [Google Scholar] [CrossRef]
- Özkan, M.; Dimic-Misic, K.; Karakoc, A.; Hashmi, S.G.; Lund, P.; Maloney, T.; Paltakari, J. Rheological characterization of liquid electrolytes for drop-on-demand inkjet printing. Org. Electron. 2016, 38, 307–315. [Google Scholar] [CrossRef]
- Ma, S.; Ribeiro, F.; Powell, K.; Lutian, J.; Møller, C.; Large, T.; Holbery, J. Fabrication of Novel Transparent Touch Sensing Device via Drop-on-Demand Inkjet Printing Technique. ACS Appl. Mater. Interfaces 2015, 7, 21628–21633. [Google Scholar] [CrossRef] [PubMed]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef]
- Van der Meulen, M.-J.; Reinten, H.; Wijshoff, H.; Versluis, M.; Lohse, D.; Steen, P. Nonaxisymmetric Effects in Drop-On-Demand Piezoacoustic Inkjet Printing. Phys. Rev. Appl. 2020, 13, 54071. [Google Scholar] [CrossRef]
- Raut, N.C.; Al-Shamery, K. Inkjet printing metals on flexible materials for plastic and paper electronics. J. Mater. Chem. C 2018, 6, 1618–1641. [Google Scholar] [CrossRef]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A review on inkjet printing of nanoparticle inks for flexible electronics. J. Mater. Chem. C 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Mahato, K.; Nagpal, S.; Shah, M.A.; Srivastava, A.; Maurya, P.K.; Roy, S.; Jaiswal, A.; Singh, R.; Chandra, P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech 2019, 9, 57. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Shariq, M.; Chattopadhyaya, S.; Rudolf, R.; Rai Dixit, A. Characterization of AuNPs based ink for inkjet printing of low cost paper based sensors. Mater. Lett. 2020, 264, 1–4. [Google Scholar] [CrossRef]
- Shariq, M.; Marić, N.; Gorše, G.K.; Kargl, R.; Rudolf, R. Synthesis of gold nanoparticles with ultrasonic spray pyrolysis and its feasibility for inkjet printing on paper. Micro Nanosyst. 2018, 10, 102–109. [Google Scholar] [CrossRef]
- Majerič, P.; Rudolf, R. Advances in Ultrasonic Spray Pyrolysis Processing of Noble Metal Nanoparticles—Review. Materials 2020, 13, 3485. [Google Scholar] [CrossRef]
- Shariq, M.; Majerič, P.; Friedrich, B.; Budic, B.; Jenko, D.; Dixit, A.R.; Rudolf, R. Application of Gold(III) Acetate as a New Precursor for the Synthesis of Gold Nanoparticles in PEG Through Ultrasonic Spray Pyrolysis. J. Clust. Sci. 2017, 28, 1647–1665. [Google Scholar] [CrossRef]
- Majerič, P.; Rudolf, R.; Friedrich, B.; Ternik, P. Numerical and experimental analysis of the single droplet evaporation in a ultrasonic spray pyrolysis device. Dry. Technol. 2018, 36, 11–20. [Google Scholar] [CrossRef]
- Rudolf, R.; Majerič, P.; Štager, V.; Albreht, B. Process for the Production of Gold Nanoparticles by Modified Ultrasonic Spray Pyrolysis. Slovenia Patent Application No. P-202000079, 5 May 2020. [Google Scholar]
- Della Volpe, C.; Siboni, S. Acid–base surface free energies of solids and the definition of scales in the Good–van Oss–Chaudhury theory. J. Adhes. Sci. Technol. 2000, 14, 235–272. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Majerič, P.; Anžel, I.; Rudolf, R. Low-cost synthesis of AuNPs through ultrasonic spray pyrolysis. Mater. Res. Express 2020, 1, 1–21. [Google Scholar] [CrossRef]
- Abbas, A.; Bajwa, I. Inkjet Printing of Ag Nanoparticles using Dimatix Inkjet Printer, No 1. Protoc. Rep. 2017. [Google Scholar] [CrossRef]
- Das, T.; Kolli, V.; Karmakar, S.; Sarkar, N. Functionalisation of Polyvinylpyrrolidone on Gold Nanoparticles Enhances Its Anti-Amyloidogenic Propensity towards Hen Egg White Lysozyme. Biomedicines 2017, 5, 19. [Google Scholar]
- Trotsiuk, L.; Antanovich, A.; Lizunova, A.; Kulakovich, O. Direct synthesis of amphiphilic polyvinylpyrrolidone-capped gold nanoparticles in chloroform. Colloid Interface Sci. Commun. 2020, 37, 100289. [Google Scholar] [CrossRef]
- Bhatti, Q.A.; Baloch, M.K.; Schwarz, S.; Petzold, G. Effect of Various Parameters on the Stability of Silica Dispersions. J. Solut. Chem. 2014, 43, 1916–1928. [Google Scholar] [CrossRef]
- Guo, M.; Li, W.; Yang, F.; Liu, H. Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 73–79. [Google Scholar] [CrossRef]
- Van Oss, C.J. Interfacial Forces in Aqueous Media, 2nd ed.; Routledge: London, UK, 2006; ISBN 9781420015768. [Google Scholar]
- Knorr, S.D.; Combe, E.C.; Wolff, L.F.; Hodges, J.S. The surface free energy of dental gold-based materials. Dent. Mater. 2005, 21, 272–277. [Google Scholar] [CrossRef]



| Gold Ink | Au Concentration (ppm) |
|---|---|
| AuCl 0.5 g/L | 300 |
| AuCl 1.0 g/L | 400 |
| AuAc 0.5 g/L | 340 |
| AuAc 1.0 g/L | 450 |
| Author | Model | (mN/m) | ||||
|---|---|---|---|---|---|---|
| PVP by Ma et al. [13] | GvOC | 48.5 | 43.4 | 5.1 | 0.4 | 15.3 |
| PVP by Van Oss et al. [40] | GvOC | 43.4 | 43.4 | 0.0 | 0.0 | 29.7 |
| Gold by Knorr et al. [41] | Least square analysis | 36.5 | 33.5 | 3.01 | 0.17 | 19.3 |
| Photo paper (this study) | GvOC | 42.6 | 33.8 | 0.9 | 21.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiyyagura, H.R.; Majerič, P.; Bračič, M.; Anžel, I.; Rudolf, R. Gold Inks for Inkjet Printing on Photo Paper: Complementary Characterisation. Nanomaterials 2021, 11, 599. https://doi.org/10.3390/nano11030599
Tiyyagura HR, Majerič P, Bračič M, Anžel I, Rudolf R. Gold Inks for Inkjet Printing on Photo Paper: Complementary Characterisation. Nanomaterials. 2021; 11(3):599. https://doi.org/10.3390/nano11030599
Chicago/Turabian StyleTiyyagura, Hanuma Reddy, Peter Majerič, Matej Bračič, Ivan Anžel, and Rebeka Rudolf. 2021. "Gold Inks for Inkjet Printing on Photo Paper: Complementary Characterisation" Nanomaterials 11, no. 3: 599. https://doi.org/10.3390/nano11030599