Improving the Stability of High-Voltage Lithium Cobalt Oxide with a Multifunctional Electrolyte Additive: Interfacial Analyses
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials, Electrolyte Configuration, and Cell Production
2.2. Electrochemical Properties
3. Results and Discussion
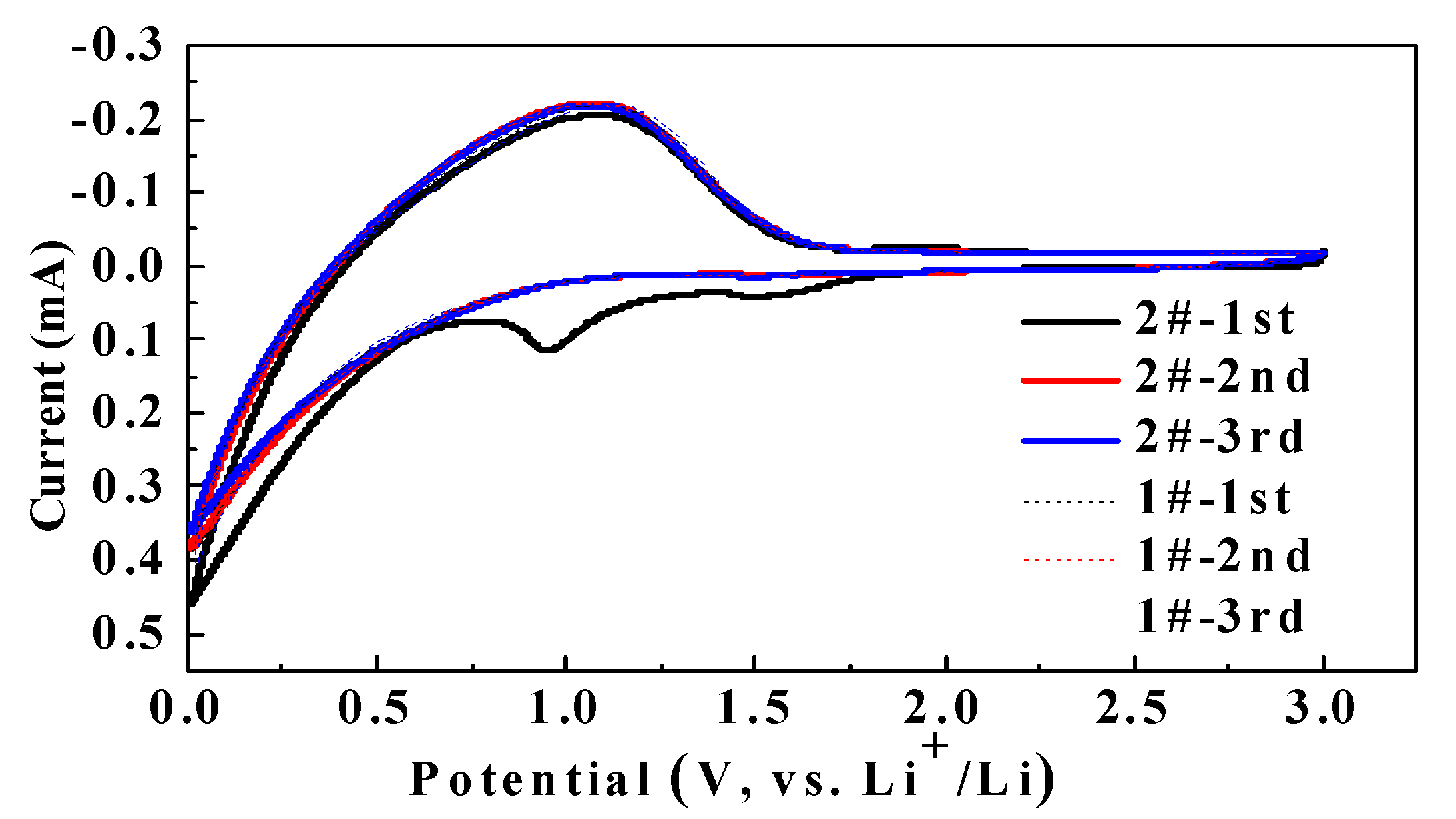
3.1. Influence of DDDT on the Electrochemical Window
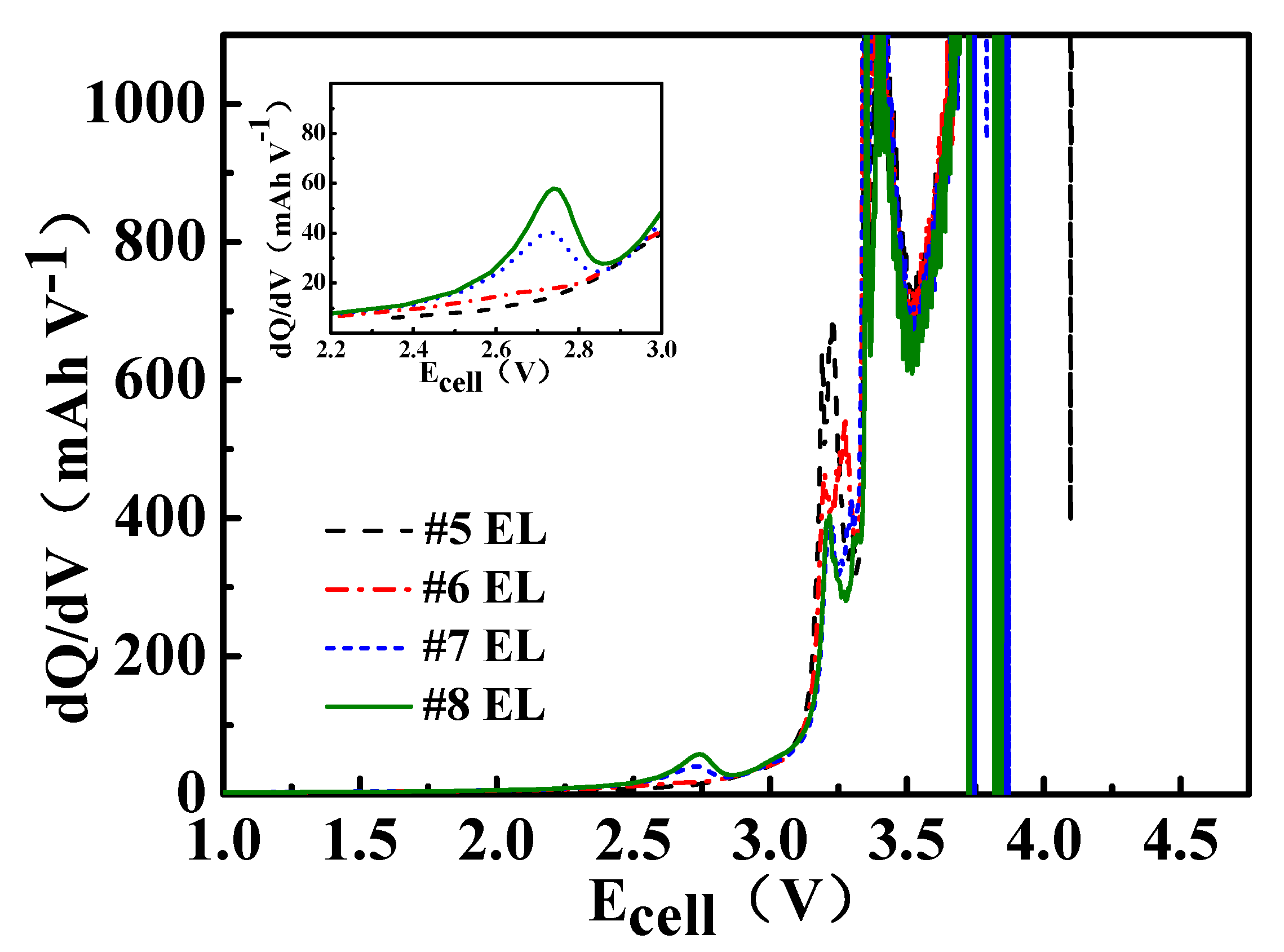
3.2. Electrical Performance of DDDT in Batteries Fully Charged to 4.5 V
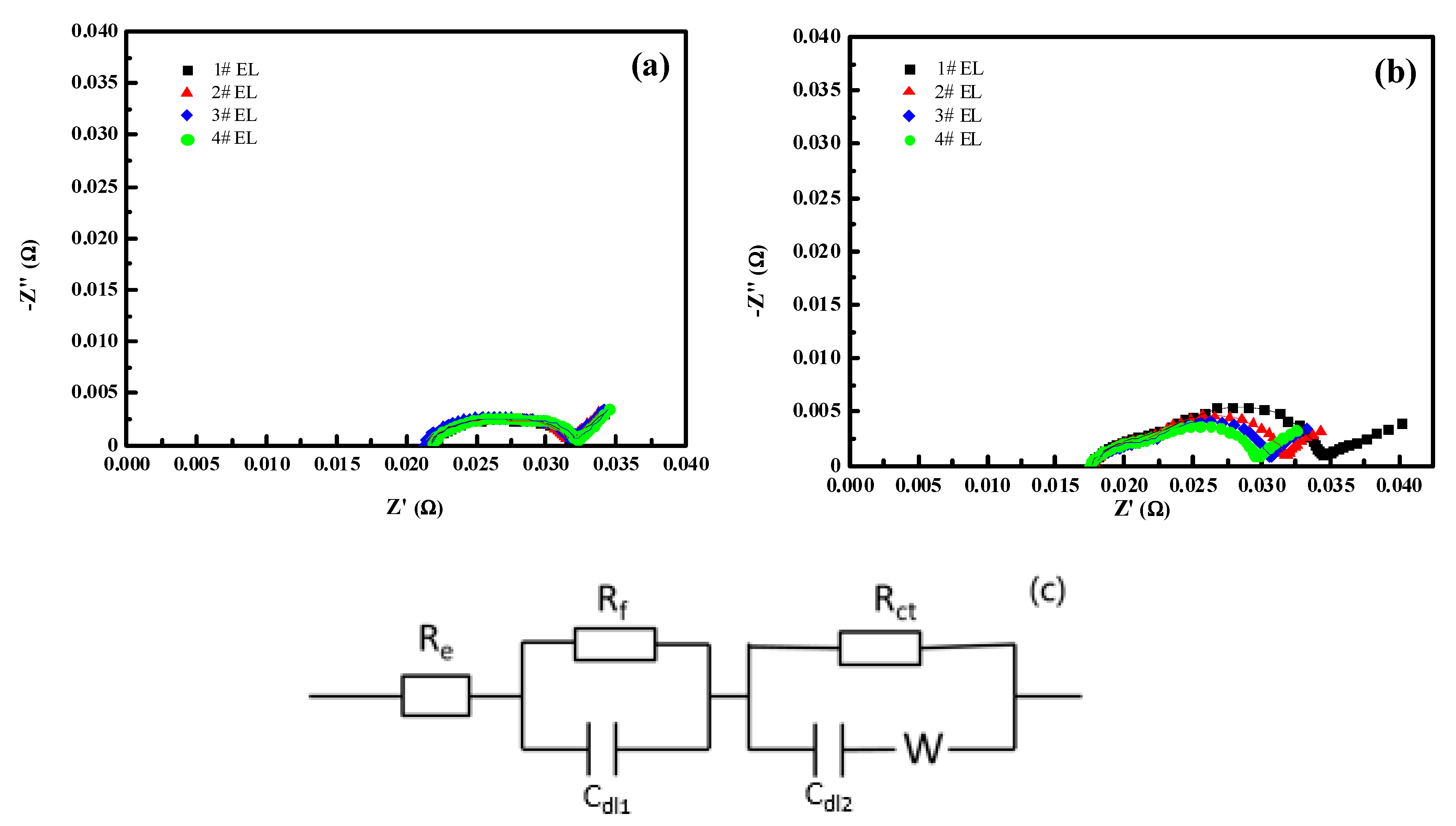
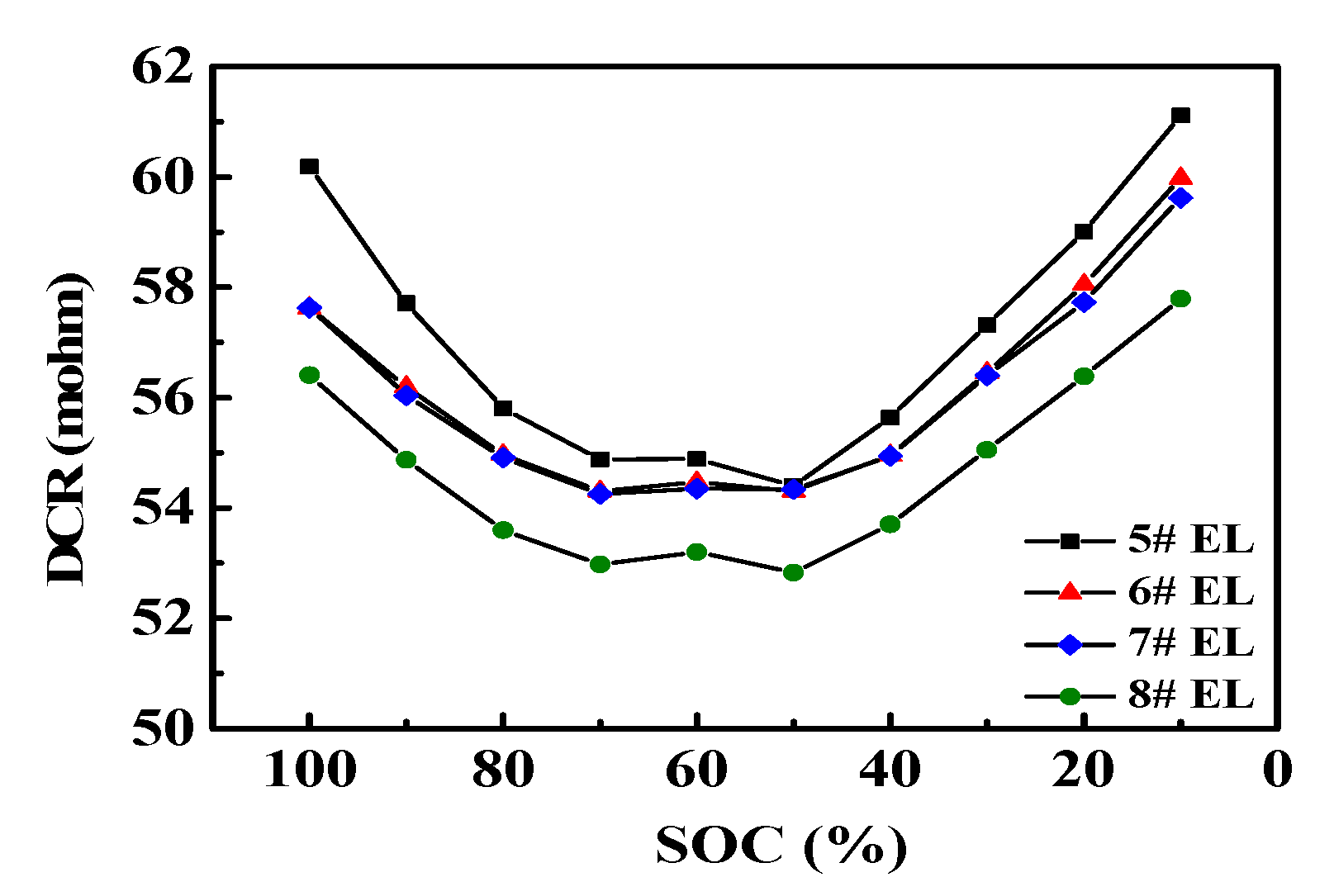
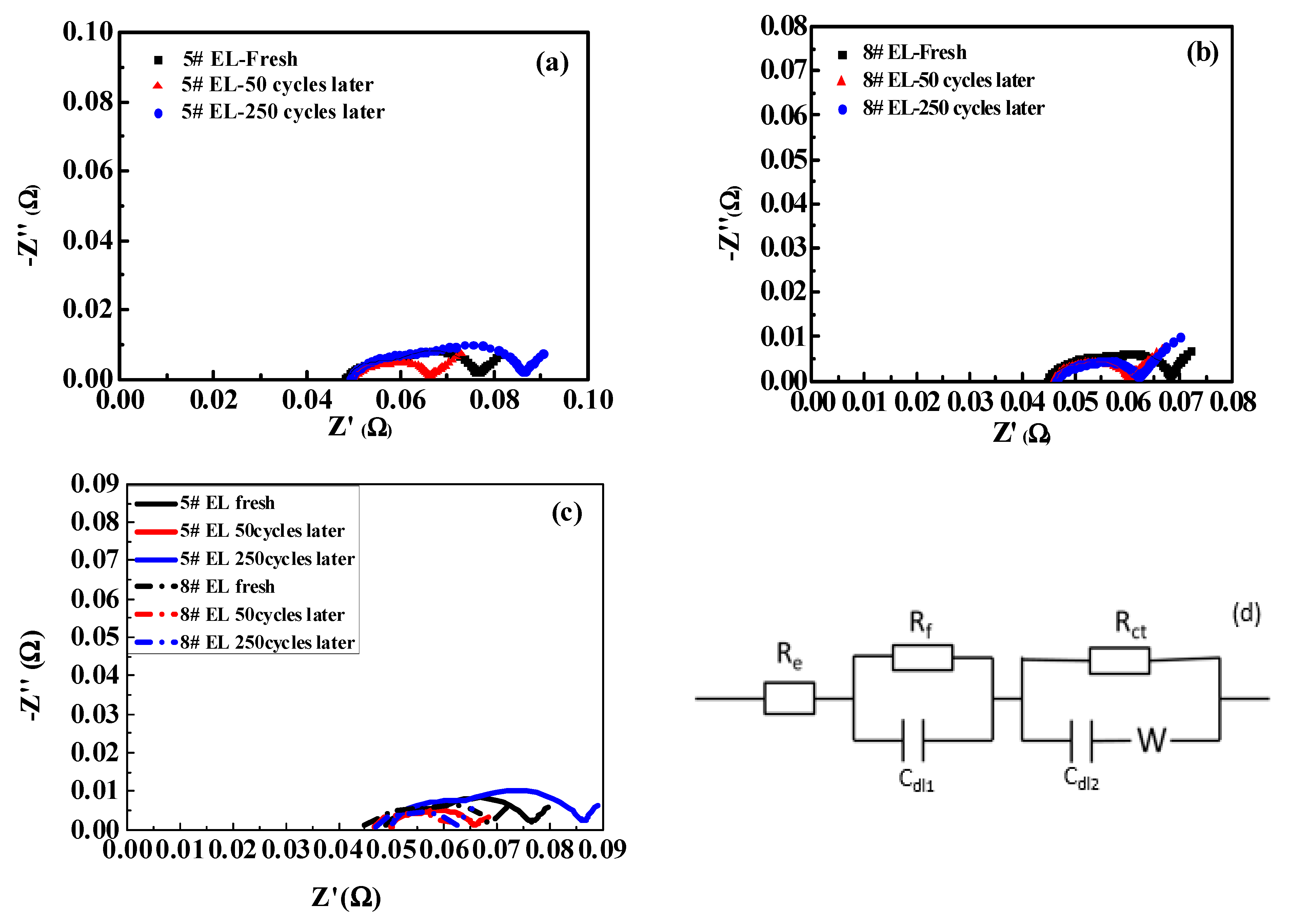
3.2.1. Effect of DDDT on DC Impedance
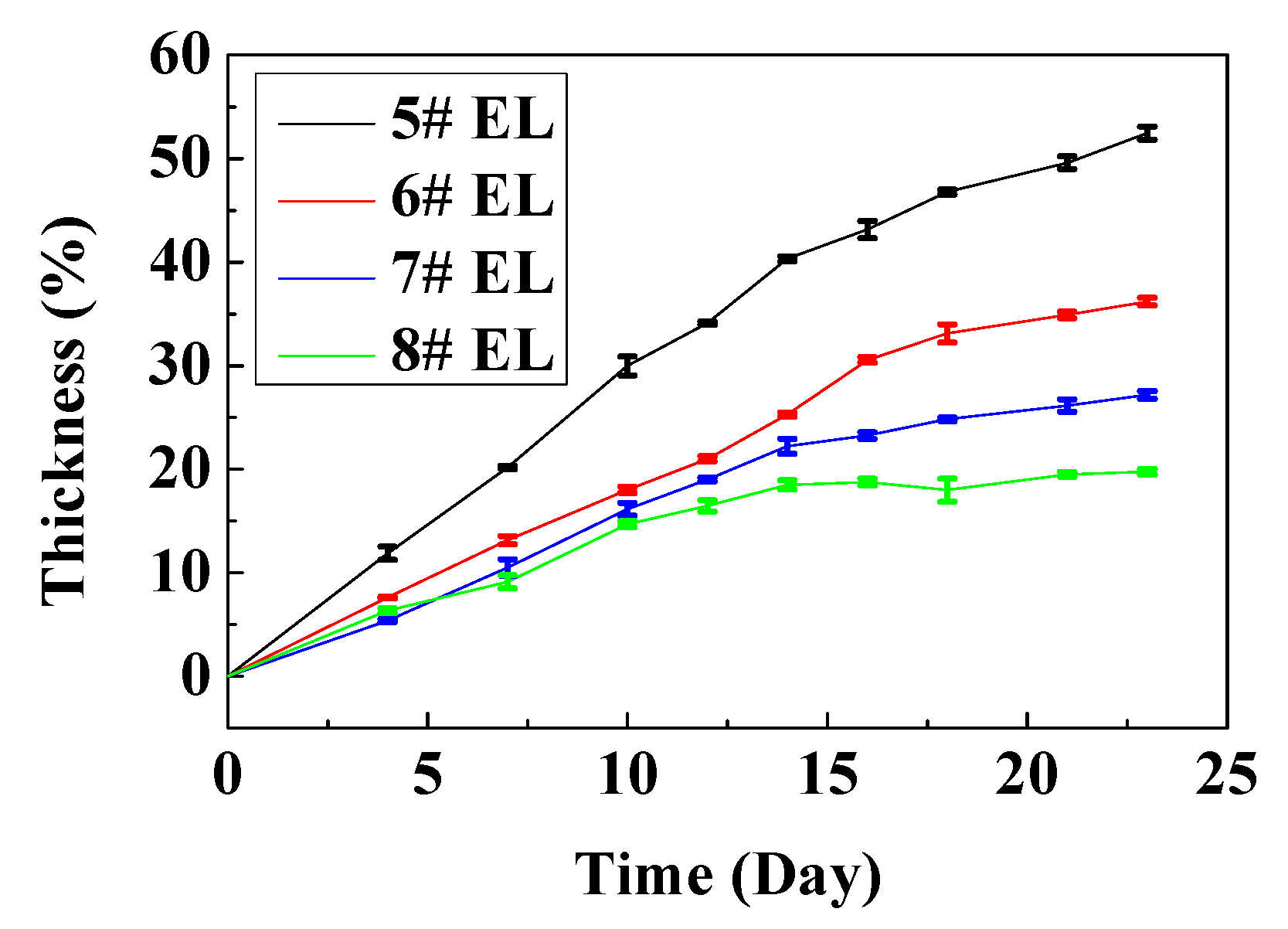
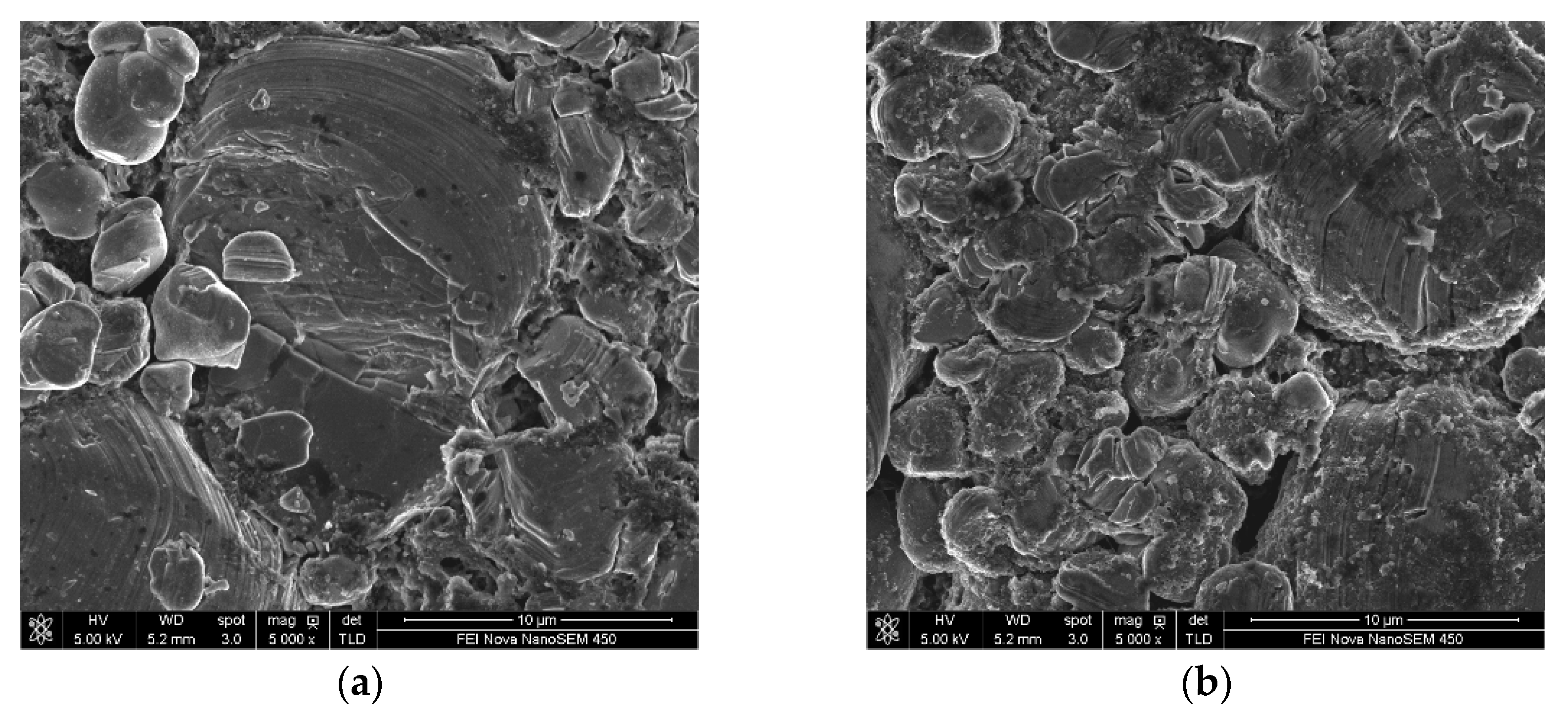
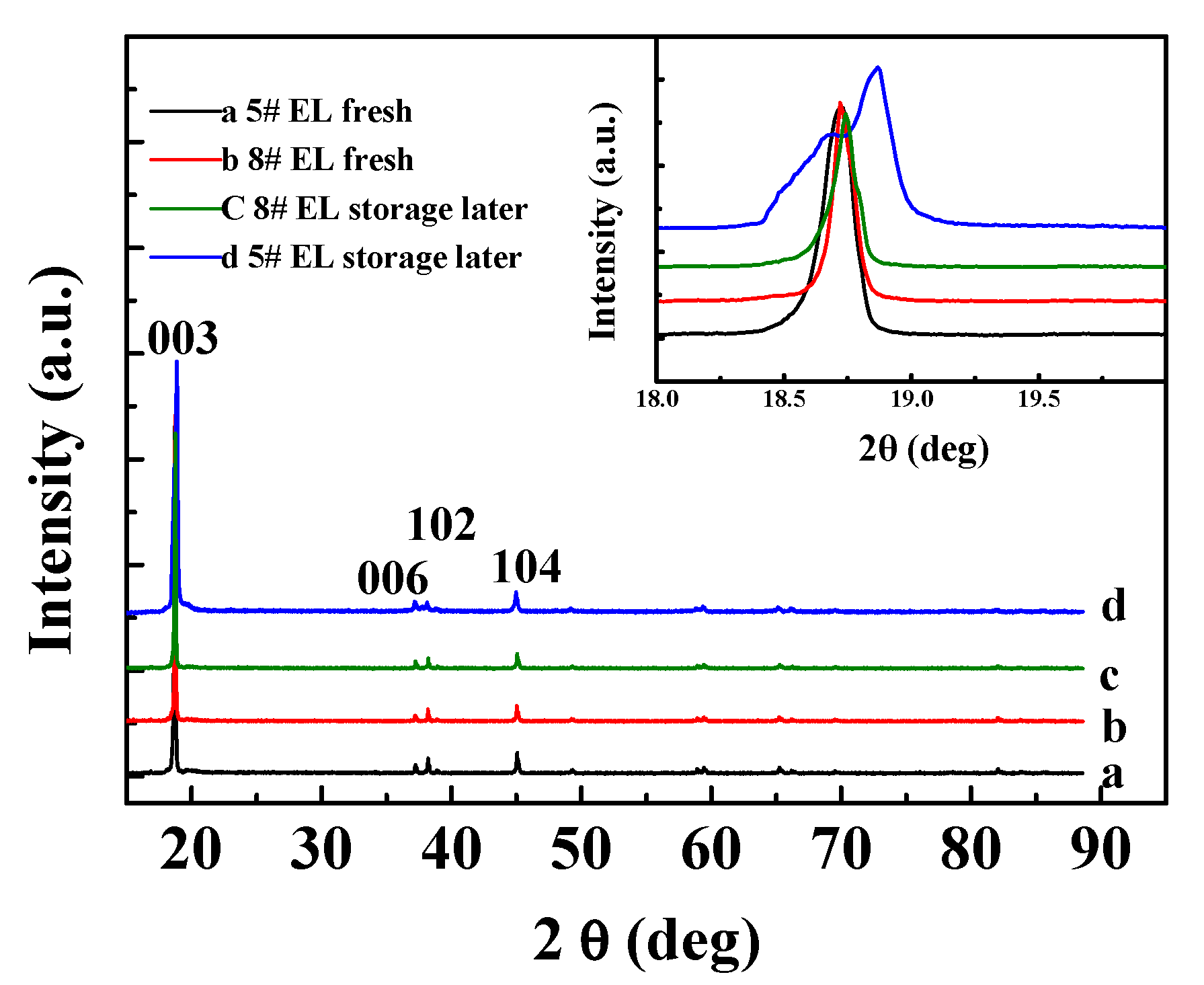
3.2.2. Effect of DDDT Additive on High-Temperature Storage
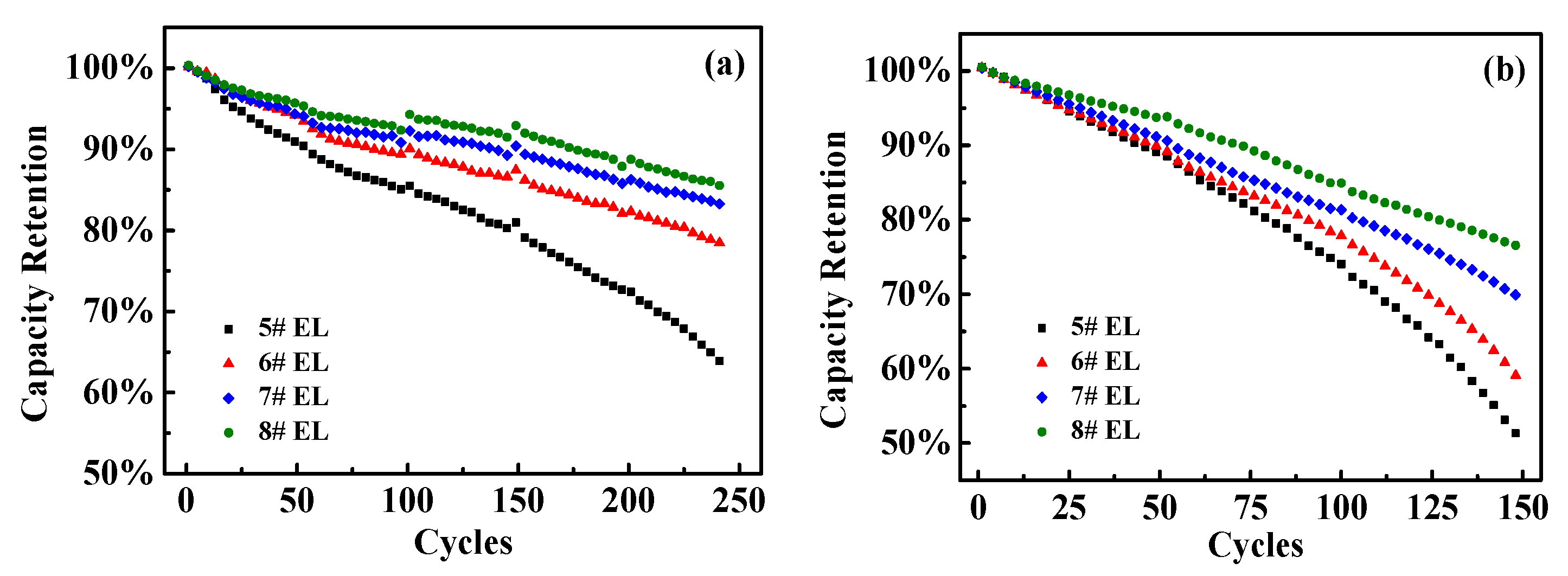
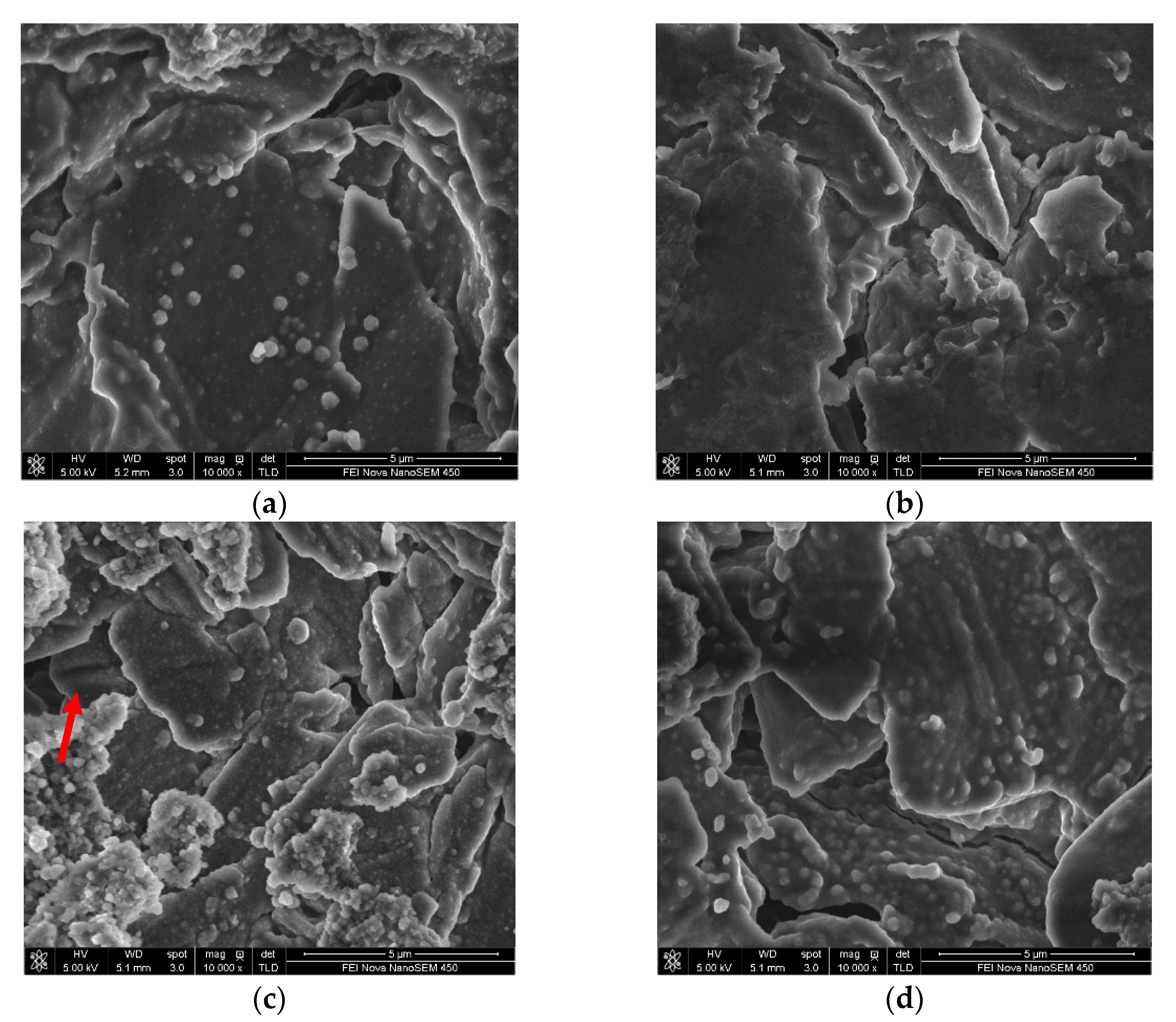
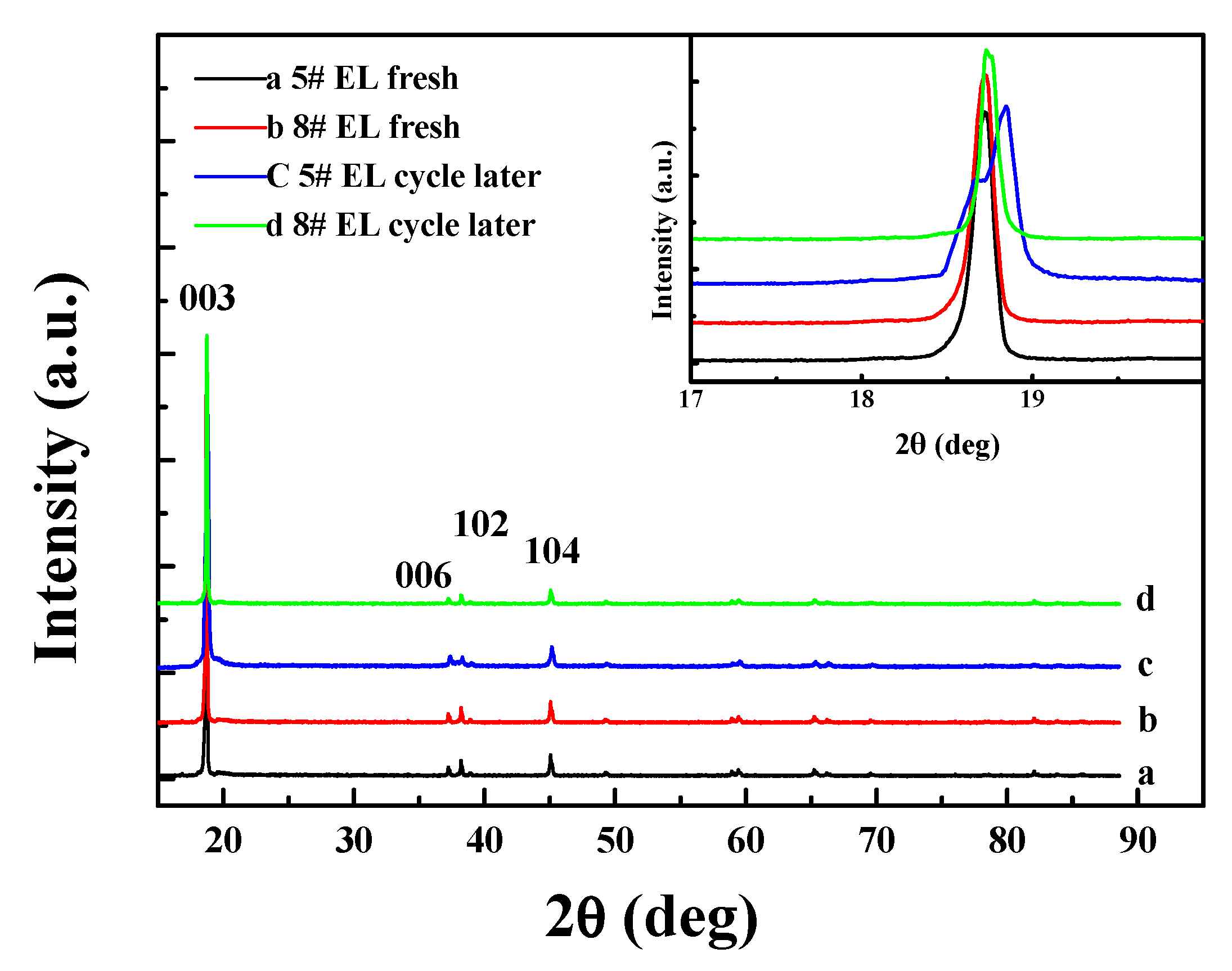
3.2.3. Effect of DDDT Additive on Cycle Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huo, H.; Xing, Y.; Pecht, M.; Züger, B.J.; Khare, N.; Vezzini, A. Safety Requirements for Transportation of Lithium Batteries. Energies 2017, 10, 793. [Google Scholar] [CrossRef]
- Williard, N.; Hendricks, C.; Sood, B.; Chung, J.S.; Pecht, M. Evaluation of Batteries for Safe Air Transport. Energies 2016, 9, 340. [Google Scholar] [CrossRef]
- Song, Y.; Liu, D.; Hou, Y.; Yu, J.; Peng, Y. Satellite lithium-ion battery remaining useful life estimation with an iterative up-dated RVM fused with the KF algorithm. Chin. J. Aeronaut. 2018, 31, 31. [Google Scholar] [CrossRef]
- Levchenko, I.; Xu, S.; Mazouffre, S.; Lev, D.; Pedrini, D.; Goebel, D.; Garrigues, L.; Taccogna, F.; Bazaka, K. Perspectives, frontiers, and new horizons for plasma-based space electric propulsion. Phys. Plasmas 2020, 27, 020601. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, I.; Xu, S.; Wu, Y.-L.; Bazaka, K. Hopes and concerns for astronomy of satellite constellations. Nat. Astron. 2020, 4, 1012–1014. [Google Scholar] [CrossRef]
- Iclodean, C.; Varga, B.; Burnete, N.; Cimerdean, D.; Jurchiş, B. Comparison of different battery types for electric vehicles. IOP Conf. Ser. Mater. Sci. Eng. 2017, 252, 012058. [Google Scholar] [CrossRef] [Green Version]
- Han, G.B.; Ryou, M.H.; Cho, K.Y.; Lee, Y.M.; Park, Y.K. Effect of succinic anhydride as an electrolyte additive on electro-chemical characteristics of silicon thin-film electrode. J. Power Sources 2020, 195, 3709. [Google Scholar] [CrossRef]
- Kang, S.-H.; Kempgens, P.; Greenbaum, S.; Kropf, A.J.; Amine, K.; Thackeray, M.M. Interpreting the structural and electrochemical complexity of 0.5Li2MnO3·0.5LiMO2 electrodes for lithium batteries (M = Mn0.5−xNi0.5−xCo2x, 0 ≤ x ≤ 0.5). J. Mater. Chem. 2007, 17, 2069–2077. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, X.M.; Bommel, A.V.; Xia, X.; Dahn, J.R. Comparison of Li [Li1/9Ni1/3Mn5/9] O2, Li [Li1/5Ni1/5Mn3/5] O2, LiNi0.5Mn1.5O4, and LiNi2/3Mn1/3O2 as high voltage positive electrode materials. J. Electrochem. Soc. 2011, 158, A187. [Google Scholar] [CrossRef]
- Meng, X.; Dou, S.; Wang, W.-L. High power and high capacity cathode material LiNi0.5Mn0.5O2 for advanced lithium-ion batteries. J. Power Sources 2008, 184, 489–493. [Google Scholar] [CrossRef]
- Yazami, R.; Ozawa, Y.; Gabrisch, H.; Fultz, B. Mechanism of electrochemical performance decay in LiCoO2 aged at high voltage. Electrochim. Acta 2004, 50, 385–390. [Google Scholar] [CrossRef]
- Kim, Y.; Veith, G.M.; Nanda, J.; Unocic, R.R.; Chi, M.; Dudney, N.J. High voltage stability of LiCoO2 particles with a nano-scale Lipon coating. Electrochim. Acta 2011, 56, 6573–6580. [Google Scholar] [CrossRef]
- Yang, L.; Markmaitree, T.; Lucht, B.L. Inorganic additives for passivation of high voltage cathode materials. J. Power Sources 2011, 196, 2251–2254. [Google Scholar] [CrossRef]
- Xu, K.; Ding, S.P.; Jow, T.R. Toward Reliable Values of Electrochemical Stability Limits for Electrolytes. J. Electrochem. Soc. 1999, 146, 4172–4178. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]
- Jiao, S.; Ren, X.; Cao, R.; Engelhard, M.H.; Liu, Y.; Hu, D.; Mei, D.; Zheng, J.; Zhao, W.; Li, Q.; et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 2018, 3, 739–746. [Google Scholar] [CrossRef]
- Yoshida, K.; Nakamura, M.; Kazue, Y.; Tachikawa, N.; Tsuzuki, S.; Seki, S.; Dokko, K.; Watanabe, M. Oxidative-stability enhancement and charge transport mechanism in glymeàlithium salt equimolar complexes. J. Am. Chem. Soc. 2011, 133, 13121. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Yu, Z.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z. A new class of ionically conducting fluorinated ether electro-lytes with high electrochemical stability. J. Am. Chem. Soc. 2020, 142, 7393. [Google Scholar] [CrossRef]
- Xu, K.; Angell, C.A. Sulfone-based electrolytes for lithium-ion batteries. J. Electrochem. Soc. 2002, 149, A920. [Google Scholar] [CrossRef]
- Sun, X.G.; Angell, C.A. New sulfone electrolytes for rechargeable lithium batteries: Part I. Oligoether-containing sulfones. Electrochem. Commun. 2005, 7, 261. [Google Scholar] [CrossRef]
- Lewandowski, A.; Świderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Guerfi, A.; Dontigny, M.; Charest, P.; Petitclerc, M.; Lagacé, M.; Vijh, A.; Zaghib, K. Improved electrolytes for Li-ion batteries: Mixtures of ionic liquid and organic electrolyte with enhanced safety and electrochemical performance. J. Power Sources 2010, 195, 845. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Li, B.; Li, W.; Li, X.; Hu, S. Tris (pentafluorophenyl) phosphine: An electrolyte additive for high voltage Li-ion batteries. Electrochem. Commun. 2012, 18, 123–126. [Google Scholar] [CrossRef]
- von Cresce, A.; Xu, K. Electrolyte additive in support of 5 V Li ion chemistry. J. Electrochem. Soc. 2011, 158, 337. [Google Scholar] [CrossRef]
- Lee, J.-N.; Han, G.-B.; Ryou, M.-H.; Lee, D.J.; Song, J.; Choi, J.W.; Park, J.-K. N-(triphenylphosphoranylidene) aniline as a novel electrolyte additive for high voltage LiCoO2 operations in lithium ion batteries. Electrochim. Acta 2011, 56, 5195–5200. [Google Scholar] [CrossRef]
- Zuo, X.; Fan, C.; Xiao, X.; Liu, J.; Nan, J. High-voltage performance of LiCoO2/graphite batteries with methylene me-thanedisulfonate as electrolyte additive. J. Power Sources 2012, 219, 94. [Google Scholar] [CrossRef]
- Wang, Z.; Dupré, N.; Lajaunie, L.; Moreau, P.; Martin, J.-F.; Boutafa, L.; Patoux, S.; Guyomard, D. Effect of glutaric anhydride additive on the LiNi0.4Mn1.6O4 electrode/electrolyte interface evolution: A MAS NMR and TEM/EELS study. J. Power Sources 2012, 215, 170–178. [Google Scholar] [CrossRef]
- Tarnopolskiy, V.; Kalhoff, J.; Nádherná, M.; Bresser, D.; Picard, L.; Fabre, F.; Rey, M.; Passerini, S. Beneficial influence of suc-cinic anhydride as electrolyte additive on the self-discharge of 5 V LiNi0.4Mn1.6O4 cathodes. J. Power Sources 2013, 236, 39. [Google Scholar] [CrossRef]
- Kubota, T.; Ihara, M.; Katayama, S.; Nakai, H.; Ichikawa, J. 1,1-Difluoro-1-alkenes as new electrolyte additives for lithium ion batteries. J. Power Sources 2012, 207, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Dong, S.; Chen, X.; Cui, G. Recent Advances in Non-Aqueous Electrolyte for Rechargeable Li-O2 Batteries. Adv. Energy Mater. 2016, 6, 1600751. [Google Scholar] [CrossRef]
- Aravindan, V.; Cheah, Y.L.; Ling, W.C.; Madhavi, S. Effect of LiBOB Additive on the Electrochemical Performance of LiCoPO4. J. Electrochem. Soc. 2012, 159, A1435–A1439. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.Y.; Park, S.J.; Park, G.T.; Sun, Y.K. Adiponitrile (C6H8N2): A new Bi-functional additive for high-performance Li-metal batteries. Adv. Funct. Mater. 2019, 29, 1902496. [Google Scholar] [CrossRef]
- Yang, T.; Wang, W.; Li, S.; Lu, J.; Fan, W.; Zuo, X.; Nan, J. Sulfur-containing C2H2O8S2 molecules as an overall-functional electrolyte additive for high-voltage LiNi0.5Co0.2Mn0.3O2/graphite batteries with enhanced performance. J. Power Sources 2020, 470, 228462. [Google Scholar] [CrossRef]
- Zuo, X.; Deng, X.; Ma, X.; Wu, J.; Liang, H.; Nan, J. 3-(Phenylsulfonyl) propionitrile as a higher voltage bifunctional electro-lyte additive to improve the performance of lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14725. [Google Scholar] [CrossRef]
- Li, S.-C.; Yu, J.-G. A well-designed CoTiO3 coating for uncovering and manipulating interfacial compatibility between LiCoO2 and Li1.3Al0.3Ti1.7(PO4)3 in high temperature zone. Appl. Surf. Sci. 2020, 526, 146601. [Google Scholar] [CrossRef]
- Madec, L.; Xia, J.; Petibon, R.; Nelson, K.J.; Sun, J.P.; Hill, I.G.; Dahn, J.R. Effect of sulfate electrolyte additives on LiNi1/3Mn1/3Co1/3O2/graphite pouch cell lifetime: Correlation between XPS surface studies and electrochemical test results. J. Phys. Chem. C 2014, 118, 29608. [Google Scholar] [CrossRef]
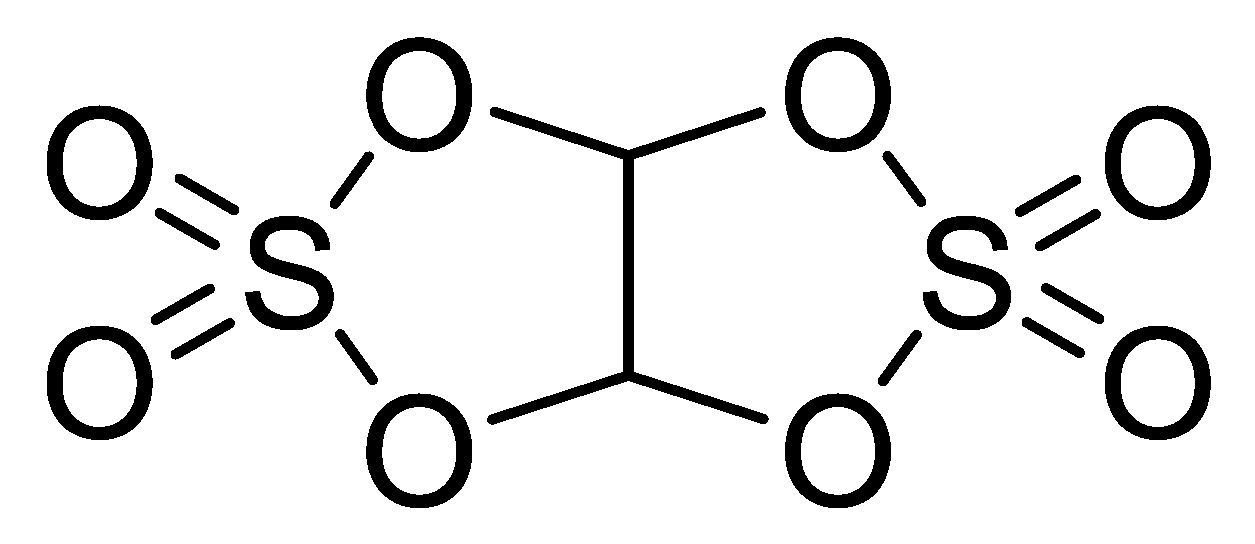



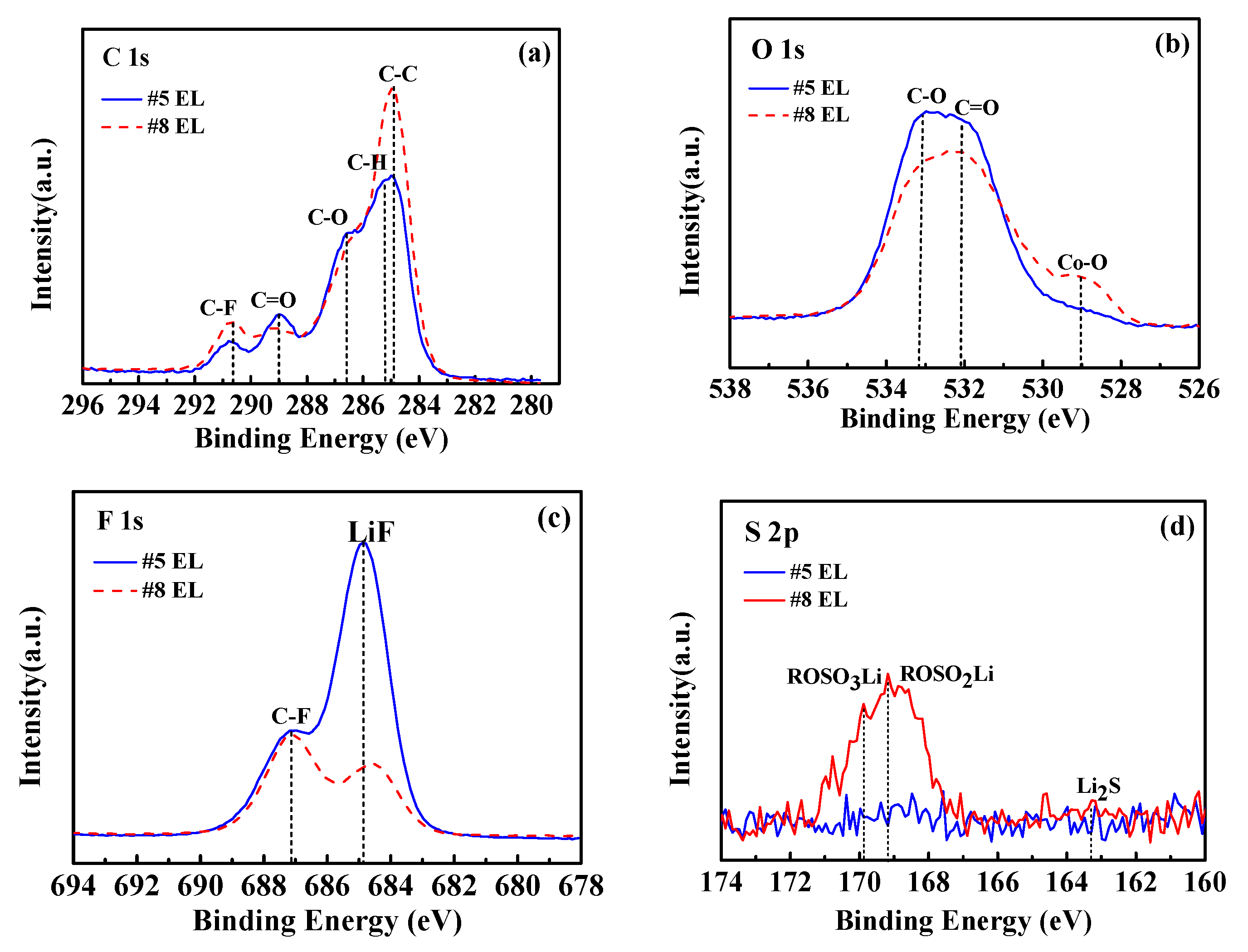
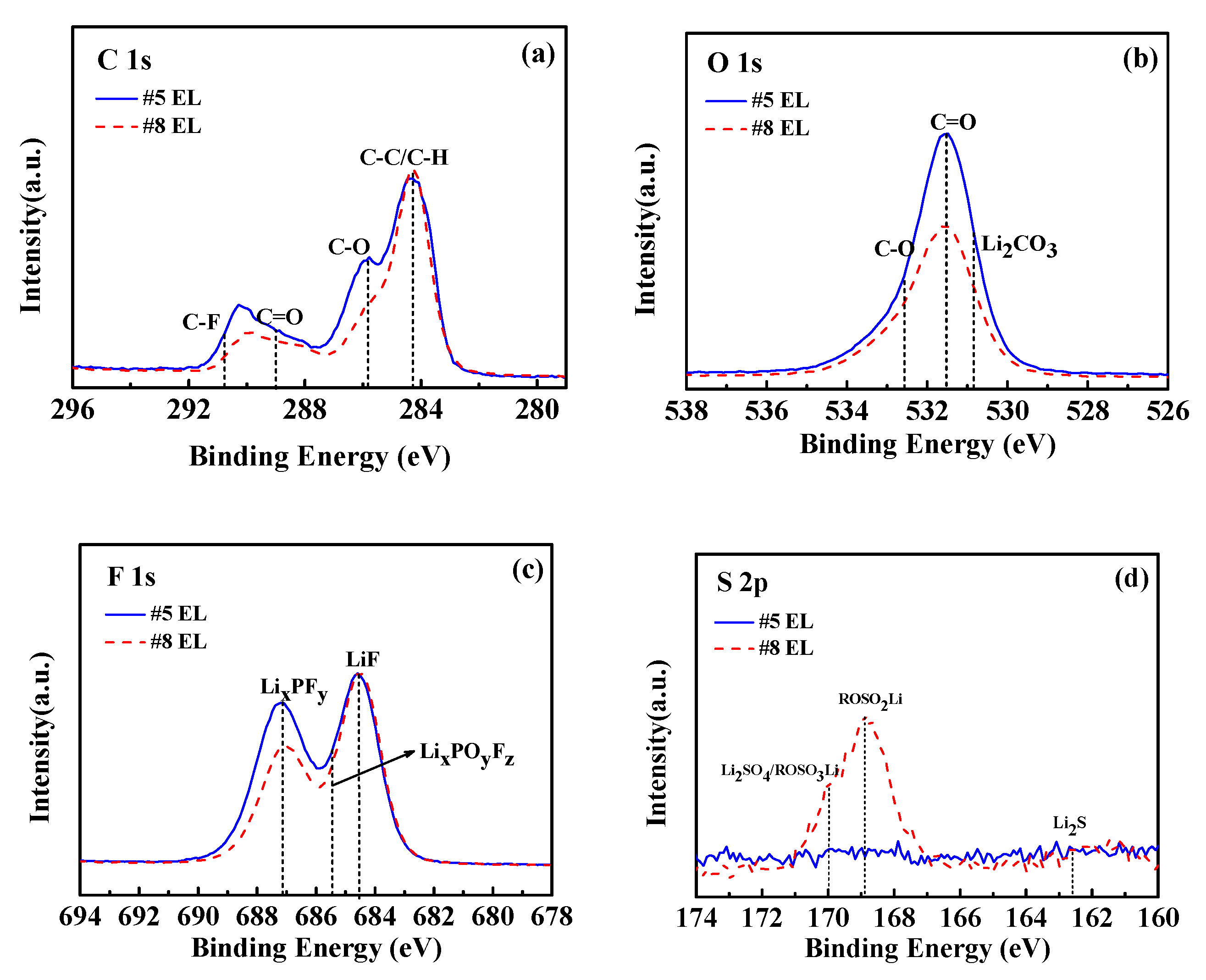
| Code | Solvent | Additive (wt.%) | Lithium Salt (mol/L) | |
|---|---|---|---|---|
| EC | EMC | DDDT | LiPF6 | |
| #1 | 30 | 70 | 0 | 1.15 |
| #2 | 30 | 70 | 0.5 | 1.15 |
| #3 | 30 | 70 | 1 | 1.15 |
| #4 | 30 | 70 | 2 | 1.15 |
| Code | Solvent | Additive (wt.%) | Lithium Salt (mol/L) | |||
|---|---|---|---|---|---|---|
| EC | PC | DEC | PP | DDDT | LiPF6 | |
| #5 | 20 | 10 | 30 | 40 | 0 | 1.15 |
| #6 | 20 | 10 | 30 | 40 | 0.5 | 1.15 |
| #7 | 20 | 10 | 30 | 40 | 1 | 1.15 |
| #8 | 20 | 10 | 30 | 40 | 2 | 1.15 |
| Electrolyte Model | Graphite/Li | LiCoO2/Li | ||
|---|---|---|---|---|
| Rf (mΩ) | Rct (mΩ) | Rf (mΩ) | Rct (mΩ) | |
| #1 EL | 4.07 | 5.62 | 4.4 | 11.95 |
| #2 EL | 4.11 | 5.53 | 4.09 | 9.52 |
| #3 EL | 4.21 | 5.69 | 4.07 | 8.39 |
| #4 EL | 3.99 | 5.63 | 3.76 | 7.15 |
| Elements | #5 EL | #8 EL | |||
|---|---|---|---|---|---|
| Before Storage (ppm) | After Storage (ppm) | Before Storage (ppm) | After Storage (ppm) | ||
| Cathode | C | 18.9 | 16 | 17.4 | 15.6 |
| O | 26.3 | 27.74 | 25.46 | 26.1 | |
| Co | 51.82 | 50 | 52.72 | 52.95 | |
| P | 0.34 | 0.56 | 0.44 | 0.25 | |
| Al | 0.42 | 0.36 | 0.39 | 0.44 | |
| S | / | / | 0.12 | 0.57 | |
| F | 2.22 | 5.34 | 3.47 | 4.09 | |
| Anode | C | 74.89 | 30.07 | 76.18 | 48.59 |
| O | 10.2 | 5.94 | 12.41 | 13.9 | |
| P | 0.59 | 0.76 | 0.54 | 0.69 | |
| Cu | 0.9 | 0.81 | 0.92 | 0.89 | |
| S | / | / | 0.14 | 0.31 | |
| F | 13.42 | 37.32 | 9.81 | 23.52 | |
| Co | / | 25.1 | / | 12.1 | |
| Electrolyte Code | Newly Prepared Cell | After 50 Cycles | After 250 Cycles | |||
|---|---|---|---|---|---|---|
| Rf (mΩ) | Rct (mΩ) | Rf (mΩ) | Rct (mΩ) | Rf (mΩ) | Rct (mΩ) | |
| #5 | 10.2 | 17 | 4.09 | 11.34 | 14.05 | 22.46 |
| #8 | 9.02 | 13.59 | 3.43 | 9.81 | 3.49 | 11.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.-Q.; Li, F.; Zhang, C.-M.; Yin, Z.-L.; Liu, G.-C.; Yu, J.-G. Improving the Stability of High-Voltage Lithium Cobalt Oxide with a Multifunctional Electrolyte Additive: Interfacial Analyses. Nanomaterials 2021, 11, 609. https://doi.org/10.3390/nano11030609
Liao X-Q, Li F, Zhang C-M, Yin Z-L, Liu G-C, Yu J-G. Improving the Stability of High-Voltage Lithium Cobalt Oxide with a Multifunctional Electrolyte Additive: Interfacial Analyses. Nanomaterials. 2021; 11(3):609. https://doi.org/10.3390/nano11030609
Chicago/Turabian StyleLiao, Xing-Qun, Feng Li, Chang-Ming Zhang, Zhou-Lan Yin, Guo-Cong Liu, and Jin-Gang Yu. 2021. "Improving the Stability of High-Voltage Lithium Cobalt Oxide with a Multifunctional Electrolyte Additive: Interfacial Analyses" Nanomaterials 11, no. 3: 609. https://doi.org/10.3390/nano11030609







