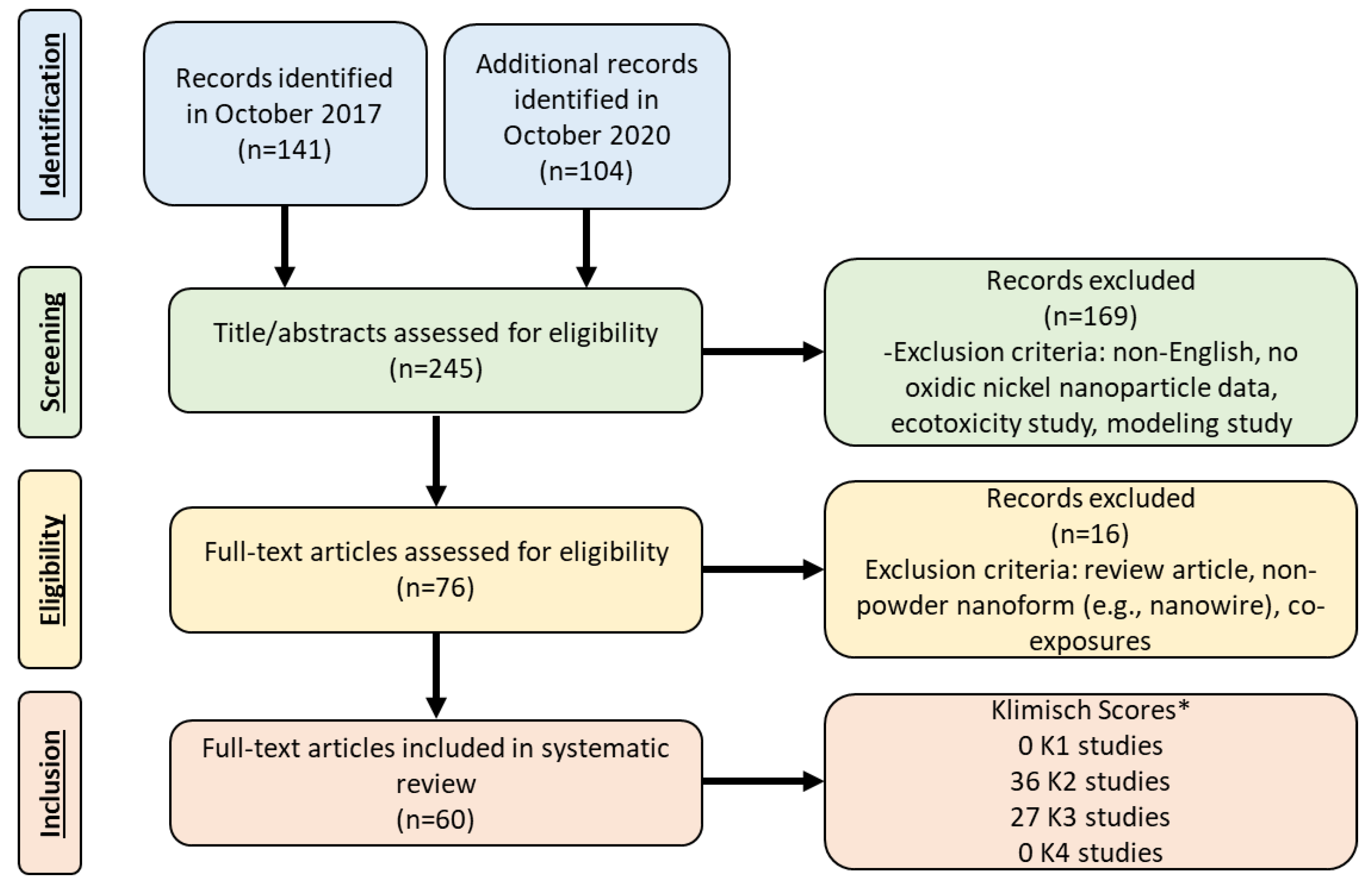
Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles
Abstract
1. Introduction
2. Literature Review
3. Study Quality Assessment
4. Nanomaterial Physicochemical Characteristics
| Study | Nanoparticle Type | Oxidic Nickel Nanoparticle Primary Particle Size (Average ± SD) | Oxidic Nickel Nanoparticle Size in Media (Average ± SD) |
|---|---|---|---|
| Abudayyak et al. [24] | NiO | 15.0 ± 7.54 nm | 135.81 nm |
| Ada et al. [25] | NiO | 20 nm | NR |
| Åkerlund et al. [26] | NiO | <50 nm | 200 nm |
| Ali [27] | NiO | <50 nm | 91.54 nm |
| Bai et al. [28] | NiO | 20 nm | 685.7 nm |
| Cao et al. [29] | NiO | 18.6 ± 5.5 nm | 313 ± 12.6 nm |
| Capasso et al. [30] | NiO | 50 nm | 80 and 450 nm |
| Cho et al. [13] | NiO | 10–20 nm | 92 nm |
| Cho et al. [12] | NiO | 5 nm | 92 nm |
| Cho et al. [14] | NiO | 10–20 nm | 92 nm |
| Cuevas et al. [31] | Ni(OH)2 | 5 nm | 40 ± 1.5 nm |
| Di Bucchianico et al. [32] | NiO | <50 nm | 750 nm |
| Duan et al. [33] | NiO | <50 nm | 306 ± 2 nm |
| Dumala et al. [34] | NiO | 15.6 ± 2.59 nm | 169 ± 17.1 nm |
| Dumala et al. [35] | NiO | 13 ± 3.0 nm | 111 ± 25.9 nm |
| Dumala et al. [36] | NiO | 12.9 ± 3.4 nm | 111 ± 25.9 nm |
| Dumala et al. [37] | NiO | 17.94 ± 3.48 nm | 285.9 ± 19.6 nm |
| Fujita et al. [38] | NiO | 10–20 nm | 59 nm |
| Gillespie et al. 2010 [22] | Ni(OH)2 | 5 nm | 40 ± 1.5 nm |
| Gutierrez et al. [17] | NiO | <20 nm | variable from <100 nm to >1 µm |
| Horie et al. [39] | NiO | 15–35 nm | 20–100 nm |
| Horie et al. [40] | NiO | <100 nm | NR |
| Horie et al. [41] | NiO | 100 nm | 74–108 nm |
| Horie et al. [42] | NiO | 20 nm | 27–39 nm |
| Horie et al. [43] | NiO | 10–20 nm | NR |
| Horie et al. [19] | NiO (Green) (Black) | Green: 100 nm Black: 20 nm | Green: (NR) Black: (38–180 nm) |
| Jeong et al. [44] | NiO | 5.3 ± 1.9 nm | 210 ± 3.7 nm |
| Kadoya et al. [45] | NiO | 26 nm | 54 nm |
| Kang et al. [46] | Ni(OH)2 | 5 nm | 40 ± 1.5 nm |
| Kang et al. [47] | Ni(OH)2 | 38 nm | 38 ± 1.4 nm based on SMPS |
| Katsnelson et al. [48] | NiO | 16.7 ± 8.2 nm | NR |
| Latvala et al. [21] | NiO | <50 nm | 0.7–2.2 nm |
| Lee et al. [49] | NiO | 5.3 ± 0.4 nm | 224 ± 11 nm |
| Liberda et al. [50] | Ni(OH)2 | 5 nm | 40 nm |
| Liberda et al. [51] | Ni(OH)2 | 5 nm | 40 ± 1.5 nm |
| Liberda [52] | Ni(OH)2 | 5 nm | 40 ± 1.5 nm |
| Lu et al. [53] | NiO | 10–20 nm | NR |
| Lu et al. [54] | NiO | 10–20 nm | NR |
| Marzban et al. [55] | NiO | 28–32 nm | NR |
| Minigalieva et al. [56] | NiO | 16.7 ± 8.2 nm | NR |
| Minigalieva et al. [57] | NiO | 16.7 ± 8.2 nm | NR |
| Morimoto et al. [58] | NiO | 20 nm | 139 ± 12 nm |
| Morimoto et al. [59] | NiO | 19 nm | 20–100 nm |
| Morimoto et al. [60] | NiO | 8.41 nm | 0.48–8.69 µm |
| Morimoto et al. [61] | NiO | 20 nm | 26 nm |
| Morimoto et al. [62] | NiO | 8.41 nm | 1.34 µm |
| Nishi et al. [63] | NiO | 20 nm | 26 nm |
| Nishi et al. [23] | NiO | 10–20 nm | 26 nm |
| Ogami et al. [64] | NiO | 20 nm | Instillation: 26 nm Inhalation: 59 ± 3 nm |
| Ogami et al. [65] | NiO | 27 nm | 800 nm |
| Oyabu et al. [66] | NiO | 20 nm | 139 ± 12 nm |
| Oyabu et al. [67] | NiO | 19 nm | 59.7 nm |
| Pietruska et al. [68] | NiO | <100 nm | >100 nm |
| Sager et al. [15] | NiO | Not Specified | 486 ± 5.8 nm 694 ± 3.7 nm 221 ± 6.6 nm 102 ± 2.9 nm 3060 ± 13.5 nm 1313 ± 8.4 nm 4460 ± 85.4 nm 490 ± 8.9 nm |
| Saquib et al. [69] | NiO | 25.1 ± 2.1 nm | 43.3 ± 2.6 and 226 ± 1.5 nm |
| Senoh et al. [70] | NiO | 20 nm | 37–68 nm |
| Shinohara et al. [20] | NiO | Spherical: 20 ± 8 nm Irregular Spherical: 140 ± 67 nm | Spherical: 49 nm Irregular Spherical: 1600 nm |
| Siddiqui et al. [71] | NiO | 22 nm | 151 nm |
| Sutunkova et al. [72] | NiO | 23 ± 5 nm | NR |
| Yu et al. [73] | NiO | 20 nm | NR |
5. Overview of Toxicological Endpoints and Considerations for Potential Human Health Effects
5.1. Estimation of Human Exposures
Dosimetric Adjustment
5.2. Considerations for In Vitro and In Vivo Studies When Evaluating Human Health Effects
5.3. Dose, Deposition, and Clearance
6. Overview of Lung Inflammation
7. Overview of Systemic Toxicological Endpoints
7.1. Mortality
7.2. Cardiovascular
7.3. Other Systemic Effects
8. Overview of Carcinogenicity and Genotoxicity Endpoints
9. Potential Toxic Mechanisms and Comparison to Micron Data
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magaye, R.; Zhao, J. Recent progress in studies of metallic nickel and nickel-based nanoparticles’ genotoxicity and carcinogenicity. Environ. Toxicol. Pharm. 2012, 34, 644–650. [Google Scholar] [CrossRef]
- Polyzois, I.; Nikolopoulos, D.; Michos, I.; Patsouris, E.; Theocharis, S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J. Appl. Toxicol. 2012, 32, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Future Markets Inc. The Global Market for Metal and Metal Oxide Nanoparticles 2010–2027; Future Markets Inc.: Edinburgh, UK, 2017. [Google Scholar]
- Biskos, G.; Schmidt-Ott, A. Airborne engineered nanoparticles: Potential risks and monitoring challenges for assessing their impacts on children. Paediatr. Respir. Rev. 2012, 13, 79–83. [Google Scholar] [CrossRef]
- Fay, M.; Wilbur, S.; Abadin, H.; Ingerman, L.; Swarts, S.G. Toxicological Profile for Nickel. August 2005; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- IARC. Nickel and nickel compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Chromium, Nickel, and Welding; WHO-IARC: Lyon, France, 1990; Volume 49, pp. 257–445. [Google Scholar]
- IARC. Nickel and nickel compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Arsenic, Metals, Fibres and Dusts. Vol. 100C—A Review of Human Carcinogens; WHO-IARC: Lyon, France, 2012; Volume 100C, pp. 169–218. [Google Scholar]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Klimisch, H.J.; Andreae, M.; Tillmann, U. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul. Toxicol. Pharm. 1997, 25, 1–5. [Google Scholar] [CrossRef]
- Card, J.W.; Magnuson, B.A. A method to assess the quality of studies that examine the toxicity of engineered nanomaterials. Int. J. Toxicol. 2010, 29, 402–410. [Google Scholar] [CrossRef]
- Sayes, C.M.; Warheit, D.B. Characterization of nanomaterials for toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 660–670. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Bradley, M.; Megson, I.L.; Macnee, W.; Howie, S.E.; Donaldson, K. NiO and Co3O4 nanoparticles induce lung DTH-like responses and alveolar lipoproteinosis. Eur. Respir. J. 2012, 39, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Poland, C.A.; Howie, S.E.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Metal oxide nanoparticles induce unique inflammatory footprints in the lung: Important implications for nanoparticle testing. Environ. Health Perspect. 2010, 118, 1699–1706. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Poland, C.A.; Duschl, A.; Oostingh, G.J.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 2012, 6, 22–35. [Google Scholar] [CrossRef]
- Sager, T.; Wolfarth, M.; Keane, M.; Porter, D.; Castranova, V.; Holian, A. Effects of nickel-oxide nanoparticle pre-exposure dispersion status on bioactivity in the mouse lung. Nanotoxicology 2016, 10, 151–161. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef]
- Gutierrez, E.R.; Kamens, R.M.; Tolocka, M.; Sexton, K.; Jaspers, I. A comparison of three dispersion media on the physicochemical and toxicological behavior of TiO2 and NiO nanoparticles. Chem. Biol. Interact. 2015, 236, 74–81. [Google Scholar] [CrossRef]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 1–35. [Google Scholar] [CrossRef]
- Horie, M.; Nishio, K.; Fujita, K.; Kato, H.; Nakamura, A.; Kinugasa, S.; Endoh, S.; Miyauchi, A.; Yamamoto, K.; Murayama, H.; et al. Ultrafine NiO particles induce cytotoxicity in vitro by cellular uptake and subsequent Ni(II) release. Chem. Res. Toxicol. 2009, 22, 1415–1426. [Google Scholar] [CrossRef]
- Shinohara, N.; Zhang, G.; Oshima, Y.; Kobayashi, T.; Imatanaka, N.; Nakai, M.; Sasaki, T.; Kawaguchi, K.; Gamo, M. Kinetics and dissolution of intratracheally administered nickel oxide nanomaterials in rats. Part. Fibre Toxicol. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Latvala, S.; Hedberg, J.; Di Bucchianico, S.; Moller, L.; Odnevall Wallinder, I.; Elihn, K.; Karlsson, H.L. Nickel Release, ROS Generation and Toxicity of Ni and NiO Micro- and Nanoparticles. PLoS ONE 2016, 11, e0159684. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.A.; Kang, G.S.; Elder, A.; Gelein, R.; Chen, L.; Moreira, A.L.; Koberstein, J.; Tchou-Wong, K.M.; Gordon, T.; Chen, L.C. Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: Short and long-term studies in mice. Nanotoxicology 2010, 4, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.i.; Kadoya, C.; Ogami, A.; Oyabu, T.; Morimoto, Y.; Ueno, S.; Myojo, T. Changes over time in pulmonary inflammatory response in rat lungs after intratracheal instillation of nickel oxide nanoparticles. J. Occup. Health 2020, 62, e12162. [Google Scholar] [CrossRef] [PubMed]
- Abudayyak, M.; Guzel, E.; Ozhan, G. Nickel oxide nanoparticles are highly toxic to SH-SY5Y neuronal cells. Neurochem. Int. 2017, 108, 7–14. [Google Scholar] [CrossRef]
- Ada, K.; Turk, M.; Oguztuzun, S.; Kilic, M.; Demirel, M.; Tandogan, N.; Ersayar, E.; Latif, O. Cytotoxicity and apoptotic effects of nickel oxide nanoparticles in cultured HeLa cells. Folia Histochem. Cytobiol. 2010, 48, 524–529. [Google Scholar] [CrossRef]
- Åkerlund, E.; Cappellini, F.; Di Bucchianico, S.; Islam, S.; Skoglund, S.; Derr, R.; Odnevall Wallinder, I.; Hendriks, G.; Karlsson, H.L. Genotoxic and mutagenic properties of Ni and NiO nanoparticles investigated by comet assay, γ-H2AX staining, Hprt mutation assay and ToxTracker reporter cell lines. Environ. Mol. Mutagenesis 2018, 59, 211–222. [Google Scholar] [CrossRef]
- Ali, A.A.-M. Evaluation of some biological, biochemical, and hematological aspects in male albino rats after acute exposure to the nano-structured oxides of nickel and cobalt. Environ. Sci. Pollut. Res. 2019, 26, 17407–17417. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.-J.; Chuang, K.-J.; Chen, J.-K.; Hua, H.-E.; Shen, Y.-L.; Liao, W.-N.; Lee, C.-H.; Chen, K.-Y.; Lee, K.-Y.; Hsiao, T.-C. Investigation into the pulmonary inflammopathology of exposure to nickel oxide nanoparticles in mice. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Fang, Y.; Lu, Y.; Qian, F.; Ma, Q.; He, M.; Pi, H.; Yu, Z.; Zhou, Z. Exposure to nickel oxide nanoparticles induces pulmonary inflammation through NLRP3 inflammasome activation in rats. Int. J. Nanomed. 2016, 11, 3331–3346. [Google Scholar] [CrossRef]
- Capasso, L.; Camatini, M.; Gualtieri, M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol Lett. 2014, 226, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.K.; Liberda, E.N.; Gillespie, P.A.; Allina, J.; Chen, L.C. Inhaled nickel nanoparticles alter vascular reactivity in C57BL/6 mice. Inhal. Toxicol. 2010, 22 (Suppl. S2), 100–106. [Google Scholar] [CrossRef][Green Version]
- Di Bucchianico, S.; Gliga, A.R.; Åkerlund, E.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Calcium-dependent cyto-and genotoxicity of nickel metal and nickel oxide nanoparticles in human lung cells. Part. Fibre Toxicol. 2018, 15, 1–14. [Google Scholar] [CrossRef]
- Duan, W.X.; He, M.D.; Mao, L.; Qian, F.H.; Li, Y.M.; Pi, H.F.; Liu, C.; Chen, C.H.; Lu, Y.H.; Cao, Z.W.; et al. NiO nanoparticles induce apoptosis through repressing SIRT1 in human bronchial epithelial cells. Toxicol. Appl. Pharm. 2015, 286, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Dumala, N.; Mangalampalli, B.; Chinde, S.; Kumari, S.I.; Mahoob, M.; Rahman, M.F.; Grover, P. Genotoxicity study of nickel oxide nanoparticles in female Wistar rats after acute oral exposure. Mutagenesis 2017, 32, 417–427. [Google Scholar] [CrossRef]
- Dumala, N.; Mangalampalli, B.; Kalyan Kamal, S.S.; Grover, P. Biochemical alterations induced by nickel oxide nanoparticles in female Wistar albino rats after acute oral exposure. Biomarkers 2018, 23, 1366–5804. [Google Scholar] [CrossRef]
- Dumala, N.; Mangalampalli, B.; Kalyan Kamal, S.S.; Grover, P. Repeated oral dose toxicity study of nickel oxide nanoparticles in Wistar rats: A histological and biochemical perspective. J. Appl. Toxicol. 2019, 39, 1012–1029. [Google Scholar] [CrossRef]
- Dumala, N.; Mangalampalli, B.; Grover, P. In vitro genotoxicity assessment of nickel (II) oxide nanoparticles on lymphocytes of human peripheral blood. J. Appl. Toxicol. 2019, 39, 955–965. [Google Scholar] [CrossRef]
- Fujita, K.; Morimoto, Y.; Ogami, A.; Myojyo, T.; Tanaka, I.; Shimada, M.; Wang, W.N.; Endoh, S.; Uchida, K.; Nakazato, T.; et al. Gene expression profiles in rat lung after inhalation exposure to C60 fullerene particles. Toxicology 2009, 258, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Yoshiura, Y.; Izumi, H.; Oyabu, T.; Tomonaga, T.; Okada, T.; Lee, B.-W.; Myojo, T.; Kubo, M.; Shimada, M.; et al. Comparison of the pulmonary oxidative stress caused by intratracheal instillation and inhalation of NiO nanoparticles when equivalent amounts of NiO are retained in the lung. Antioxidants 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Stowe, M.; Tabei, M.; Kuroda, E. Metal ion release of manufactured metal oxide nanoparticles is involved in the allergic response to inhaled ovalbumin in mice. Occ. Dis. Environ. Med. 2016, 4, 17–26. [Google Scholar] [CrossRef]
- Horie, M.; Fukui, H.; Endoh, S.; Maru, J.; Miyauchi, A.; Shichiri, M.; Fujita, K.; Niki, E.; Hagihara, Y.; Yoshida, Y.; et al. Comparison of acute oxidative stress on rat lung induced by nano and fine-scale, soluble and insoluble metal oxide particles: NiO and TiO2. Inhal. Toxicol. 2012, 24, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Fukui, H.; Nishio, K.; Endoh, S.; Kato, H.; Fujita, K.; Miyauchi, A.; Nakamura, A.; Shichiri, M.; Ishida, N.; et al. Evaluation of acute oxidative stress induced by NiO nanoparticles in vivo and in vitro. J. Occup. Health 2011, 53, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Nishio, K.; Fujita, K.; Endoh, S.; Miyauchi, A.; Saito, Y.; Iwahashi, H.; Yamamoto, K.; Murayama, H.; Nakano, H.; et al. Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells. Chem. Res. Toxicol. 2009, 22, 543–553. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.; Seok, S.H.; Cho, W.S. Indium oxide (In2O3) nanoparticles induce progressive lung injury distinct from lung injuries by copper oxide (CuO) and nickel oxide (NiO) nanoparticles. Arch. Toxicol. 2016, 90, 817–828. [Google Scholar] [CrossRef]
- Kadoya, C.; Lee, B.W.; Ogami, A.; Oyabu, T.; Nishi, K.; Yamamoto, M.; Todoroki, M.; Morimoto, Y.; Tanaka, I.; Myojo, T. Analysis of pulmonary surfactant in rat lungs after inhalation of nanomaterials: Fullerenes, nickel oxide and multi-walled carbon nanotubes. Nanotoxicology 2016, 10, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.S.; Gillespie, P.A.; Gunnison, A.; Moreira, A.L.; Tchou-Wong, K.M.; Chen, L.C. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. EnvIRON. Health Perspect. 2011, 119, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.S.; Gillespie, P.A.; Gunnison, A.; Rengifo, H.; Koberstein, J.; Chen, L.C. Comparative pulmonary toxicity of inhaled nickel nanoparticles; role of deposited dose and solubility. Inhal. Toxicol. 2011, 23, 95–103. [Google Scholar] [CrossRef]
- Katsnelson, B.A.; Minigaliyeva, I.A.; Panov, V.G.; Privalova, L.I.; Varaksin, A.N.; Gurvich, V.B.; Sutunkova, M.P.; Shur, V.Y.; Shishkina, E.V.; Valamina, I.E.; et al. Some patterns of metallic nanoparticles’ combined subchronic toxicity as exemplified by a combination of nickel and manganese oxide nanoparticles. Food Chem. Toxicol. 2015, 86, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hwang, S.-H.; Jeong, J.; Han, Y.; Kim, S.-H.; Lee, D.-K.; Lee, H.-S.; Chung, S.-T.; Jeong, J.; Roh, C.; et al. Nickel oxide nanoparticles can recruit eosinophils in the lungs of rats by the direct release of intracellular eotaxin. Part. Fibre Toxicol. 2016, 13. [Google Scholar] [CrossRef]
- Liberda, E.N.; Cuevas, A.K.; Qu, Q.; Chen, L.C. The acute exposure effects of inhaled nickel nanoparticles on murine endothelial progenitor cells. Inhal. Toxicol. 2014, 26, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Liberda, E.N.; Cuevas, A.K.; Gillespie, P.A.; Grunig, G.; Qu, Q.; Chen, L.C. Exposure to inhaled nickel nanoparticles causes a reduction in number and function of bone marrow endothelial progenitor cells. Inhal. Toxicol. 2010, 22 (Suppl. 2), 95–99. [Google Scholar] [CrossRef][Green Version]
- Liberda, E.N. The Effects of Inhaled Nickel Nanoparticles on Murine Edothelial Progenitor Cells; UMI Dissertations Publishing: An Arbor, MI, USA, 2011. [Google Scholar]
- Lu, S.; Duffin, R.; Poland, C.; Daly, P.; Murphy, F.; Drost, E.; Macnee, W.; Stone, V.; Donaldson, K. Efficacy of simple short-term in vitro assays for predicting the potential of metal oxide nanoparticles to cause pulmonary inflammation. Environ. Health Perspect. 2009, 117, 241–247. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, W.; Zhang, R.; Liu, P.; Wang, Q.; Shang, Y.; Wu, M.; Donaldson, K.; Wang, Q. Comparison of cellular toxicity caused by ambient ultrafine particles and engineered metal oxide nanoparticles. Part Fibre Toxicol. 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Marzban, A.; Seyedalipour, B.; Mianabady, M.; Taravati, A.; Hoseini, S.M. Biochemical, Toxicological, and Histopathological outcome in rat brain following treatment with NiO and NiO nanoparticles. Biol. Trace Elem. Res. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Minigalieva, I.A.; Katsnelson, B.A.; Privalova, L.I.; Sutunkova, M.P.; Gurvich, V.B.; Shur, V.Y.; Shishkina, E.V.; Valamina, I.E.; Makeyev, O.H.; Panov, V.G.; et al. Attenuation of Combined Nickel(II) Oxide and Manganese(II, III) Oxide Nanoparticles’ Adverse Effects with a Complex of Bioprotectors. Int. J. Mol. Sci. 2015, 16, 22555–22583. [Google Scholar] [CrossRef] [PubMed]
- Minigalieva, I.; Bushueva, T.; Frohlich, E.; Meindl, C.; Ohlinger, K.; Panov, V.; Varaksin, A.; Shur, V.; Shishkina, E.; Gurvich, V.; et al. Are in vivo and in vitro assessments of comparative and combined toxicity of the same metallic nanoparticles compatible, or contradictory, or both? A juxtaposition of data obtained in respective experiments with NiO and Mn3O4 nanoparticles. Food Chem. Toxicol. 2017, 109, 393–404. [Google Scholar] [CrossRef]
- Morimoto, Y.; Oyabu, T.; Ogami, A.; Myojo, T.; Kuroda, E.; Hirohashi, M.; Shimada, M.; Lenggoro, W.; Okuyama, K.; Tanaka, I. Investigation of gene expression of MMP-2 and TIMP-2 mRNA in rat lung in inhaled nickel oxide and titanium dioxide nanoparticles. Ind. Health 2011, 49, 344–352. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Lee, B.W.; Okada, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicology 2016, 10, 607–618. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Hashiba, M.; Mizuguchi, Y.; Kambara, T.; Lee, B.W.; Kuroda, E.; et al. Pulmonary toxicity following an intratracheal instillation of nickel oxide nanoparticle agglomerates. J. Occup. Health 2011, 53, 293–295. [Google Scholar] [CrossRef]
- Morimoto, Y.; Ogami, A.; Todoroki, M.; Yamamoto, M.; Murakami, M.; Hirohashi, M.; Oyabu, T.; Myojo, T.; Nishi, K.; Kadoya, C.; et al. Expression of inflammation-related cytokines following intratracheal instillation of nickel oxide nanoparticles. Nanotoxicology 2010, 4, 161–176. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Hashiba, M.; Mizuguchi, Y.; Kambara, T.; Lee, B.W.; Kuroda, E.; et al. Expression of cytokine-induced neutrophil chemoattractant in rat lungs following an intratracheal instillation of micron-sized nickel oxide nanoparticle agglomerates. Toxicol. Ind. Health 2014, 30, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Morimoto, Y.; Ogami, A.; Murakami, M.; Myojo, T.; Oyabu, T.; Kadoya, C.; Yamamoto, M.; Todoroki, M.; Hirohashi, M.; et al. Expression of cytokine-induced neutrophil chemoattractant in rat lungs by intratracheal instillation of nickel oxide nanoparticles. Inhal. Toxicol. 2009, 21, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Ogami, A.; Yamamoto, K.; Morimoto, Y.; Fujita, K.; Hirohashi, M.; Oyabu, T.; Myojo, T.; Nishi, K.; Kadoya, C.; Todoroki, M.; et al. Pathological features of rat lung following inhalation and intratracheal instillation of C(60) fullerene. Inhal. Toxicol. 2011, 23, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Ogami, A.; Morimoto, Y.; Myojo, T.; Oyabu, T.; Murakami, M.; Todoroki, M.; Nishi, K.; Kadoya, C.; Yamamoto, M.; Tanaka, I. Pathological features of different sizes of nickel oxide following intratracheal instillation in rats. Inhal. Toxicol. 2009, 21, 812–818. [Google Scholar] [CrossRef]
- Oyabu, T.; Ogami, A.; Morimoto, Y.; Shimada, M.; Lenggoro, W.; Okuyama, K.; Tanaka, I. Biopersistence of inhaled nickel oxide nanoparticles in rat lung. Inhal. Toxicol. 2007, 19 (Suppl. 1), 55–58. [Google Scholar] [CrossRef]
- Oyabu, T.; Myojo, T.; Lee, B.-W.; Okada, T.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Li, Y.-S.; Kawai, K.; Shimada, M. Biopersistence of NiO and TiO2 nanoparticles following intratracheal instillation and inhalation. Int. J. Mol. Sci. 2017, 18, 2757. [Google Scholar] [CrossRef] [PubMed]
- Pietruska, J.R.; Liu, X.; Smith, A.; McNeil, K.; Weston, P.; Zhitkovich, A.; Hurt, R.; Kane, A.B. Bioavailability, intracellular mobilization of nickel, and HIF-1alpha activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticles. Toxicol. Sci. 2011, 124, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Saquib, Q.; Attia, S.M.; Ansari, S.M.; Al-Salim, A.; Faisal, M.; Alatar, A.A.; Musarrat, J.; Zhang, X.; Al-Khedhairy, A.A. p53, MAPKAPK-2 and caspases regulate nickel oxide nanoparticles induce cell death and cytogenetic anomalies in rats. Int. J. Biol. Macromol. 2017, 105, 228–237. [Google Scholar] [CrossRef]
- Senoh, H.; Kano, H.; Suzuki, M.; Ohnishi, M.; Kondo, H.; Takanobu, K.; Umeda, Y.; Aiso, S.; Fukushima, S. Comparison of single or multiple intratracheal administration for pulmonary toxic responses of nickel oxide nanoparticles in rats. J. Occup. Health 2017, 59, 112–121. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Ahamed, M.; Ahmad, J.; Majeed Khan, M.A.; Musarrat, J.; Al-Khedhairy, A.A.; Alrokayan, S.A. Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem. Toxicol. 2012, 50, 641–647. [Google Scholar] [CrossRef]
- Sutunkova, M.P.; Solovyeva, S.N.; Minigalieva, I.A.; Gurvich, V.B.; Valamina, I.E.; Makeyev, O.H.; Shur, V.Y.; Shishkina, E.V.; Zubarev, I.V.; Saatkhudinova, R.R. Toxic effects of low-level long-term inhalation exposures of rats to nickel oxide nanoparticles. Int. J. Mol. Sci. 2019, 20, 1778. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, F.; Wang, C.; Zhang, J.; Zhu, A.; Zou, L.; Han, A.; Li, J.; Chang, X.; Sun, Y. Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol. Med. Rep. 2018, 17, 3133–3139. [Google Scholar] [CrossRef]
- Ali, A.; Suhail, M.; Mathew, S.; Shah, M.A.; Harakeh, S.M.; Ahmad, S.; Kazmi, Z.; Alhamdan, M.A.; Chaudhary, A.; Damanhouri, G.A.; et al. Nanomaterial Induced Immune Responses and Cytotoxicity. J. Nanosci. Nanotechnol. 2016, 16, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, X.; Castranova, V.; Ding, M. Occupational toxicology of nickel and nickel compounds. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 177–208. [Google Scholar] [CrossRef]
- Karakoti, A.; Hench, L.; Seal, S. The potential toxicity of nanomaterials—the role of surfaces. JOM 2006, 58, 77–82. [Google Scholar] [CrossRef]
- Cena, L.; Chisholm, W.; Keane, M.; Chen, B. A field study on the respiratory deposition of the nano-sized fraction of mild and stainless steel welding fume metals. J. Occup. Environ. Hyg. 2015, 12, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Cena, L.; Keane, M.; Chisholm, W.; Stone, S.; Harper, M.; Chen, B. A novel method for assessing respiratory deposition of welding fume nanoparticles. J. Occup. Environ. Hyg. 2014, 11, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Magrini, A. Engineered nanoparticles at the workplace: Current knowledge about workers’ risk. Occup. Med. 2014, 64, 319–330, Erratum in 2015, 65, 171–173. [Google Scholar] [CrossRef]
- Schulte, P.; Murashov, V.; Zumwalde, R.; Kuempel, E.; Geraci, C. Occupational exposure limits for nanomaterials: State of the art. J. Nanoparticle Res. 2010, 12, 1971–1987. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Sweeney, L.M.; Morris, J.B.; Jarabek, A.M. Advances in inhalation dosimetry models and methods for occupational risk assessment and exposure limit derivation. J. Occup. Environ. Hyg. 2015, 12, S18–S40. [Google Scholar] [CrossRef]
- Applied Research Associates Inc. Multiple-Path Particle Deposition (MPPD 2.1, Beta Version): A Model for Human and Rat Airway Particle Dosimetry; Applied Resarch Associates Inc.: Raleigh, NC, USA.
- Slikker, W., Jr.; Andersen, M.E.; Bogdanffy, M.S.; Bus, J.S.; Cohen, S.D.; Conolly, R.B.; David, R.M.; Doerrer, N.G.; Dorman, D.C.; Gaylor, D.W.; et al. Dose-dependent transitions in mechanisms of toxicity. Toxicol. Appl. Pharm. 2004, 201, 203–225. [Google Scholar] [CrossRef]
- Ahmad Khanbeigi, R.; Kumar, A.; Sadouki, F.; Lorenz, C.; Forbes, B.; Dailey, L.A.; Collins, H. The delivered dose: Applying particokinetics to in vitro investigations of nanoparticle internalization by macrophages. J. Control. Release 2012, 162, 259–266. [Google Scholar] [CrossRef]
- Cohen, J.; Deloid, G.; Pyrgiotakis, G.; Demokritou, P. Interactions of engineered nanomaterials in physiological media and implications for in vitro dosimetry. Nanotoxicology 2013, 7, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Li, W.X.; Xu, J.Y.; Cai, X.Q.; Liu, R.L.; Li, Y.J.; Zhao, Q.F.; Li, Q.N. Comparative study of pathological lesions induced by multiwalled carbon nanotubes in lungs of mice by intratracheal instillation and inhalation. Environ. Toxicol 2007, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Brock, W.J.; Lee, K.P.; Webb, T.R.; Reed, K.L. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: Impact of surface treatments on particle toxicity. Toxicol. Sci. 2005, 88, 514–524. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; Frerichs, S.; Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 2007, 230, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, Y.; Myojo, T.; Oyabu, T.; Hashiba, M.; Lee, B.W.; Yamamoto, M.; Todoroki, M.; Nishi, K.; Kadoya, C.; Ogami, A.; et al. Comparison of dose-response relations between 4-week inhalation and intratracheal instillation of NiO nanoparticles using polimorphonuclear neutrophils in bronchoalveolar lavage fluid as a biomarker of pulmonary inflammation. Inhal. Toxicol. 2013, 25, 29–36. [Google Scholar] [CrossRef]
- NRC. Risk Assessment in the Federal Government Managing the Process; National Research Council of the National Academies: Washington, DC, USA, 1983; pp. 1–191.
- Oller, A.R.; Oberdörster, G. Incorporation of dosimetry in the derivation of reference concentrations for ambient or workplace air: A conceptual approach. J. Aerosol Sci. 2016, 99, 40–45. [Google Scholar] [CrossRef]
- Lippmann, M.; Ito, K.; Hwang, J.-S.; Maciejczyk, P.; Chen, L.-C. Cardiovascular effects of nickel in ambient air. Environ. Health Perspect. 2006, 114, 1662–1669. [Google Scholar] [CrossRef]
- Doll, R. Report of the International Committee on Nickel Carcinogenesis in Man. Final Report; Program Resources, Inc.: Research Triangle Park, NC, USA, 1990. [Google Scholar]
- Kirkland, D.J. Chromosomal aberration tests in vitro: Problems with protocol design and interpretation of results. Mutagenesis 1992, 7, 95–106. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for the Testing of Chemicals: In Vitro Mammalian Chromosomal Aberration Test (473); OECD Publishing: Paris, France, 2016. [Google Scholar]
- OECD. Genetic Toxicology Guidance Document; OECD Publishing: Paris, France, 2015. [Google Scholar]
- Donaldson, K.; Poland, C.A. Nanotoxicity: Challenging the myth of nano-specific toxicity. Curr. Opin. Biotechnol. 2013, 24, 724–734. [Google Scholar] [CrossRef]
- Gebel, T. Small difference in carcinogenic potency between GBP nanomaterials and GBP micromaterials. Arch. Toxicol. 2012, 86, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Kargacin, B.; Klein, C.B.; Costa, M. Mutagenic responses of nickel oxides and nickel sulfides in Chinese hamster V79 cell lines at the xanthine-guanidine phosphoribosyl transferase locus. Mutat. Res. Genet. Toxicol. 1993, 300, 63–72. [Google Scholar] [CrossRef]
- Fletcher, G.G.; Rossetto, F.E.; Turnbull, J.D.; Nieboer, E. Toxicity, uptake, and mutagenicity of particulate and soluble nickel compounds. Environ. Health Perspect. 1994, 102, 69–79. [Google Scholar] [PubMed]
- Miura, T.; Patierno, S.R.; Sakuramoto, T.; Landolph, J.R. Morphological and neoplastic transformation of C3H/10T1/2 Cl 8 mouse embryo cells by insoluble carcinogenic nickel compounds. Environ. Mol. Mutagenesis 1989, 14, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Kuhlbusch, T.A. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10, 38–60. [Google Scholar] [CrossRef]
- Graham, U.M.; Dozier, A.K.; Oberdörster, G.N.; Yokel, R.A.; Molina, R.; Brain, J.D.; Pinto, J.M.; Weuve, J.; Bennett, D.A. Tissue Specific Fate of Nanomaterials by Advanced Analytical Imaging Techniques-A Review. Chem. Res. Toxicol. 2020, 33, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
| Study | Klimisch Score | Model | Dosing Regimen (Exposure Method) (Dose Range and Unit) (Duration/Frequency) (Follow-Up Time) | Health Endpoint (Assay) |
|---|---|---|---|---|
| NiO Nanoparticles | ||||
| [38] | K3 A,B | Male Wistar rats | Whole body inhalation, 200 µg/m3 or 9.2 × 104 particles/cm3 4 w, 5 d/w, 6 h/d, 3 d and 1 m | Disease profile (lung microarray analysis) |
| [45] | K3 A,B | Male Wistar rats | Whole body inhalation 200 µg/m3 4 w, 5 d/w, 6 h/d 3 d, 1 m, and 3 m | Lung inflammation (BALF pulmonary surfactant components, BALF surface tension, histopathology) |
| [64] A | K3 A,B | Male Wistar rats | Whole body inhalation, 200 µg/m3 or 9.2 × 104 particles/cm3 4 w, 5 d/w, 6 h/d, 3 d, 1 m, and 3 m | Lung inflammation (histopathology) |
| [66] | K3 A,B | Male Wistar rats | Whole body inhalation, 1.0 × 105 particles/cm3 * or 2.8 µg/m3, 4 w, unclear d/w, 6 h/d 4 d, 1 m, and 3 m | Lung inflammation (histopathology) Lung clearance (deposited nickel) |
| [58] | K3 A,B | Male Wistar rats | Whole body inhalation, 1.0 × 105 particles/cm3 * or 2.8 µg/m3, 4 w, 5 d/w, 6h/d, 4 d, 1 m, and 3 m | Lung inflammation and fibrosis (BALF cell counts, gene expression, histopathology) |
| [72] | K3 A,B | Female white rats | Nose-only inhalation 1.0 mg/m3 4 h/d, 5 d 1 d 0.23 mg/m3 4 h/d, 5 d/w, 3, 6, 10 m 1 d | Lung inflammation (BALF cell counts, histopathology) |
| [39] | K2 A | Male F344 rats | Intratracheal instillation 0.2, 1 mg for instillation, One time dose 3 d, 1 m, 3 m, 6 m Whole body inhalation 320, 1650 µg/m3 4 w, 5 d/w, 6 h/d 3 d, 1 m, 3 m, 6 m | Lung ROS (oxidative stress markers, BALF gene expression) |
| [59] | K2 A | Male Fischer 344 rats | Intratracheal instillation 0.2, 1 mg One time dose 3 d, 1 w, 1 m, 3 m, 6 m Whole body inhalation 0.32, 1.65 mg/m3 4 w, 7 d/w, 6 h/d 3 d, 1 m, and 3 m | Lung inflammation (BALF cell count, BALF chemokines, BALF LDH, histopathology, morphological features of alveolar macrophages) |
| [28] | K2 A | Female Balb/c mice | Intratracheal instillation 10, 20, 50, 100 µg One time dose 1, 7, 28, 29 d | Lung inflammation (SPECT analysis, CT analysis) Lung damage (BALF protein levels, BALF LDH, histology) |
| [29] | K3 A,B | Male Sprague Dawley rats | Intratracheal instillation 800 µg or 3300 µg/kg One time dose 3, 7, 28 d | Lung inflammation (BALF cell counts, BALF ALP, protein levels, Histopathology, cytokines) Lung damage (BALF LDH, BALF protein levels) |
| [12] | K3 A,B | Female Wistar rats | Intratracheal instillation, 150 cm2/rat, or 163.5 µg/rat, One time dose 24 h and 4 w | Lung inflammation (BALF cell counts, BALF protein and lipids, histopathology, cytokine profile) Macrophage function (surfactant clearance) |
| [13] | K2 A | Female Wistar rats | Intratracheal instillation, 50 and 150 cm2/rat, or 54.5–163.5 µg/rat, One time dose 24 h and 4 w | Lung inflammation (BALF cell counts, protein, histopathology, cytokine profile) |
| [14] | K3 A,B | Female Wistar rats | Intratracheal instillation, 150 cm2/rat, or 163.5 µg/rat, One time dose 24 h and 4 w | Lung inflammation (BALF cell counts, BALF LDH, protein, cytokine profile) |
| [41] | K3 A,B | Male Wistar rats | Intratracheal instillation, 200 µg/rat, One time dose 1 h, 24 h, 72 h, and 1 w | Lung damage and ROS (BALF LDH and protein levels, oxidative stress markers) |
| [42] | K3 A,B | Male Wistar rats | Intratracheal instillation, 200 µg/rat, One time dose 1 h, 4 h, 24 h, 72 h, and 1 w | Lung damage and ROS (BALF LDH levels, oxidative stress markers) |
| [49] | K2 A | Female Wistar rats | Intratracheal instillation 50, 100, and 200 cm2/rat, or 54.5, 109, 218 µg/rat One time dose 1 d, 2 d, 3 d, and 4 d | Lung inflammation (BALF cell counts, BALF total protein, BALF LDH, cytokine levels, levels of anaphylatoxins) Lung clearance (BALF Ni levels) |
| [53] | K3 A,B | Female Wistar rats | Intratracheal instillation, 250 cm2/rat, or 2700 µg/rat, One time dose 24 h | Lung inflammation (BALF LDH and protein levels, BALF cell counts) |
| [60] | K3 A,B | Male Wistar rats | Intratracheal instillation, 1000 µg/rat (3300 µg/kg) One time dos e 3 d, 1 w, 1 m, 3 m, and 6 m | Lung inflammation (BALF cell counts, chemokine levels, histopathology) |
| [61] | K2 A | Male Wistar rats | Intratracheal instillation, 100 and 200 µg/rat (330 or 660 µg/kg) One time dose 3 d, 1 w, 1 m, 3 m, and 6 m | Lung inflammation, fibrosis, and allergy (BALF macrophage counts, BALF alkaline phosphatase release, lung and BALF cytokine profile, histopathology) |
| [62] | K3 A,B | Male Wistar rats | Intratracheal instillation 1000 mg/rat One time dose 3 d, 1 w, 1 m, 3 m, and 6 m | Lung inflammation (BALF cytokine levels, tissue cytokine levels, histopathology) |
| [63] | K2 A | Male Wistar rats | Intratracheal instillation, 100 and 200 µg/rat (330 or 660 µg/kg) One time dose 3 d, 1 w, 1 m, 3 m, and 6 m | Lung inflammation (BALF cell counts, chemokine levels, histopathology) |
| [23] | K2 A | Male Wistar rats | Intratracheal instillation, 100 and 200 µg/rat (330 or 660 µg/kg) One time dose 3 d, 1 w, 1 m, 3 m, and 6 m | Lung inflammation (BALF total protein concentration, BALF phospholipid concentration, BALF surface tension) |
| [65] | K3 A,B | Male Wistar rats | Intratracheal instillation, 2000 µg/rat, One time dose 3 d, 1 w, 1 m, 3 m, and 6 m | Lung inflammation (BALF cell counts, histopathology, collagen deposition) |
| [67] | K2 A | Male Fisher rats | Intratracheal instillation 0.2, 1 mg One time dose 3 d, 1 w, 1 m, 3 m, 6 m Whole body inhalation 320, 1650 µg/m3 4 w, 5 d/w, 6 h/d 3 d, 1 m, 3 m | Lung inflammation (histopathology) Lung clearance (deposited nickel) |
| [70] | K3 A,B | Male F344 rats | Intratracheal instillation 2 mg/kg One time dose or 2–4 divided doses 3, 28, 91 d | Lung inflammation (BALF cell count, BALF protein, histopathology) Lung damage (BALF protein levels, BALF LDH) |
| [20] | K2 A | Male F344/DuCrlCrlj rats | Intratracheal instillation 0.67, 2.0, or 6.0 mg/kg One time dose 3, 28, 91 d | Lung inflammation (histopathology) Lung clearance (organ nickel burden, modeling) |
| [44] | K3 A,B | Female Wistar rats | Pharyngeal aspiration 90 cm2/rat or 98.1 µg/rat One time dose 1, 28 d | Lung inflammation (BALF cell counts, LDH, protein concentration, BALF cytokine profile, BALF phospholipids) |
| [15] | K2 A | Male C57BL/6J mice | Pharyngeal aspiration 20, 40, 80 µg/mouse One time dose 1, 7 d | Lung inflammation (WLL cell count, WLL LDH, WLL albumin levels) |
| Ni(OH)2 Nanoparticles | ||||
| [22] | K2 A | Male C57BL/6 mice | Whole body inhalation, Short-term study: 103.2, 565.0, 1204 µg/m3 4 h, one time dose 24 h Long-term study: 124, 124,5, 129.3 µg/m3, up to 5 m, 5 d/w, 5 h/d, 24 h | Lung inflammation (BALF cell counts, BALF protein levels, histopathology, cytokine, chemokine RT-PCR) |
| [46] | K3 A,B | Male ApoE-/- mice | Whole body inhalation, 100 µg/m3, 1 w or 5 m, 5 d/w, 5 h/d, 24 h | Lung ROS/inflammation (ROS markers, mitochondrial DNA damage, BALF cell counts/protein, cytokine, chemokine, histopathology) |
| [47] | K3 A,B | Male C57BL/6 mice | Whole body inhalation, Ni(OH)2 570 and 1222 µg/m3, | Lung inflammation and ROS (BALF cell counts, BALF protein levels, QT-PCR for Ho-1 and Ccl-2) |
| Study | Klimisch Score | Model | Dosing Regimen (Exposure Method) (Dose Range and Unit) (Duration/Frequency) (Follow-Up Time) | Health Endpoint (Assay) |
|---|---|---|---|---|
| NiO Nanoparticles | ||||
| [72] | K3 A,B | Female white rats | Nose-only inhalation 1.0 mg/m3 4 h/d, 5 d 1 d 0.23 mg/m3 4 h/d, 5 d/w, 3, 6, 10 m 1 d | Organ damage (histopathology of liver, kidney, brain, various functional and biochemical indices) |
| [70] | K3 A,B | Male F344 rats | Intratracheal instillation 2 mg/kg One time dose or 2–4 divided doses 3, 28, 91 d | Organ damage (organ weight of liver, kidney, lung, spleen and brain; histopathology of liver, kidney, lungs, spleen, brain, and pulmonary-related lymph nodes) Hematological analysis (cell count, blood biochemistry) |
| [73] | K2 A | Male Wistar rats | Intratracheal instillation 0.015, 0.06, or 0.24 mg/kg 2 d/w, 6 w | Liver damage (biomarkers of stress, liver weight, histopathology) |
| [27] | K2 A | Male Wistar rats | Oral gavage 500, 1000 mg/kg One time dose 14 days | Clinical toxicology (food consumption, body weight, organ weight) Organ damage (various functional and biochemical indices, RBC and WBC count) |
| [34] | K2 C,D | Female Wistar rats | Oral gavage 5, 50, 300, 2000 mg/kg One time dose 14 d | Organ damage (histopathology of brain, heart, liver, spleen and kidneys) Organ clearance (Ni content) Mortality |
| [35] | K2 A | Female Wistar rats | Oral gavage 125, 250, 500 mg/kg One time dose 24 h | Organ damage (histopathology of liver, kidney, brain, various functional and biochemical indices) |
| [36] | K2 C,D | Male and female Wistar rats | Oral gavage 50, 100, 200 mg/kg 28 d, 7 d/w 24 h | Clinical toxicology (food consumption, body weight, organ weight) Organ damage (histopathology of liver, kidney, brain, various functional and biochemical indices) |
| [48] | K3 A,E | Female rats | Intraperitoneal injection 250, 500 µg/rat 6 w, 3 d/w 24 h | Organ damage (histopathology of liver, spleen, kidney, brain, various functional and biochemical indices) Organ clearance (Ni content of liver, spleen, kidney, brain) |
| [55] | K3 A,D,E | Male rats | Intraperitoneal injection 10, 25, 50 mg/kg 7 d 12 h | Brain damage (Oxidative stress biomarkers including catalase activity, lipid peroxidation by MDA, Glutathione concentration, total antioxidant capacity; histopathology) |
| [56] | K3 A,B,E | Female rats | Intraperitoneal injection 500 µg/rat 6 w, 3 d/w | Organ damage (histopathology of liver, spleen, kidney, brain, various functional and biochemical indices) Organ clearance (Ni content of liver, spleen, kidney, brain) Genotoxicity (DNA damage) |
| [40] | K3 A,B | Female C57BL/6N mice | Pharyngeal aspiration 50 µg/mouse One time dose 21 d | Allergic response (OVA-specific immunoglobulin, gene expression) |
| Ni(OH)2 Nanoparticles | ||||
| [31] | K2 A | Male C57BL/6 mice | Whole body inhalation, 100, 150, 900 µg/m3, 1, 3, or 5 d, 5 h/d, 24 h | Vascular function (carotid artery constriction and relaxation) |
| [46] | K3 A,B | Male ApoE−/− mice | Whole body inhalation, 100 µg/m3, 1 w or 5 m, 5 d/w, 5 h/d, 24 h | Cardiovascular ROS/inflammation (ROS markers, mitochondrial DNA damage, BALF cell counts/protein, cytokine, chemokine) Systemic inflammation (liver SAP protein levels, cytokines/chemokines) Atherosclerosis (plaque formation in aorta, QT-PCR) |
| [50] | K3 A,B | Male C57BL/6 mice | Whole body inhalation 500 µg/m3 5 h 30 m and 12 h | Hematopoietic damage (bone marrow EPC gene expression, EPC count, EPC chemotaxis, tube formation and proliferation, RT-PCR) |
| [51] | K3 A,B | C57BL/6 mice | Whole body inhalation, ∼1200 μg/m3, 2 d, 5 h/d, ∼700 μg/m3, 3 d, 5 h/d, ∼100 μg/m3, 5 d, 5 h/d, 24 h | Endothelial progenitor cell effects (cell counts, cell function, cellular signaling pathways) |
| [52] | K3 A,B | C57BL/6 mice | Whole body inhalation, ∼500 μg/m3, 5 h, 0.5 and 12 h | Endothelial progenitor cell effects (cell counts, cell function, cellular signaling pathways) Atherosclerosis (cellular signaling pathways) |
| Study | Klimisch Score | Model | Dosing Regimen (Exposure Method) (Dose Range and Unit) (Duration/Frequency) (Follow-Up Time) | Health Endpoint (Assay) |
|---|---|---|---|---|
| NiO Nanoparticles | ||||
| [72] | K3 A,B | Female white rats | Nose-only inhalation 1.0 mg/m3 4 h/d, 5d 1 d 0.23 mg/m3 4 h/d, 5 d/w, 3, 6, 10 m 1 d | Genotoxicity (random amplification of polymorphic DNA (RAPD) test) |
| [34] | K2 C,D | Female Wistar rats | Oral gavage 125, 250, 500 mg/kg One time dose 18, 24 h | Genotoxicity (DNA damage, micronucleus test, chromosomal aberration assay) |
| [69] | K2 A | Male Wistar rats | Oral gavage 1, 2, 4 mg/kg/day 7 or 14 d, 7 d/w Immediately | Genotoxicity (chromosomal aberrations, micronuclei formation, DNA damage) Cytotoxicity (apoptosis, ROS generation, mitochondrial membrane potential, apoptotic proteins) |
| [56] | K3 A B,E | Female rats | Intraperitoneal injection 500 µg/rat 6 w, 3 d/w | Genotoxicity (DNA damage) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
More, S.L.; Kovochich, M.; Lyons-Darden, T.; Taylor, M.; Schulte, A.M.; Madl, A.K. Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles. Nanomaterials 2021, 11, 642. https://doi.org/10.3390/nano11030642
More SL, Kovochich M, Lyons-Darden T, Taylor M, Schulte AM, Madl AK. Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles. Nanomaterials. 2021; 11(3):642. https://doi.org/10.3390/nano11030642
Chicago/Turabian StyleMore, Sharlee L., Michael Kovochich, Tara Lyons-Darden, Michael Taylor, Alexandra M. Schulte, and Amy K. Madl. 2021. "Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles" Nanomaterials 11, no. 3: 642. https://doi.org/10.3390/nano11030642
APA StyleMore, S. L., Kovochich, M., Lyons-Darden, T., Taylor, M., Schulte, A. M., & Madl, A. K. (2021). Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles. Nanomaterials, 11(3), 642. https://doi.org/10.3390/nano11030642






