6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
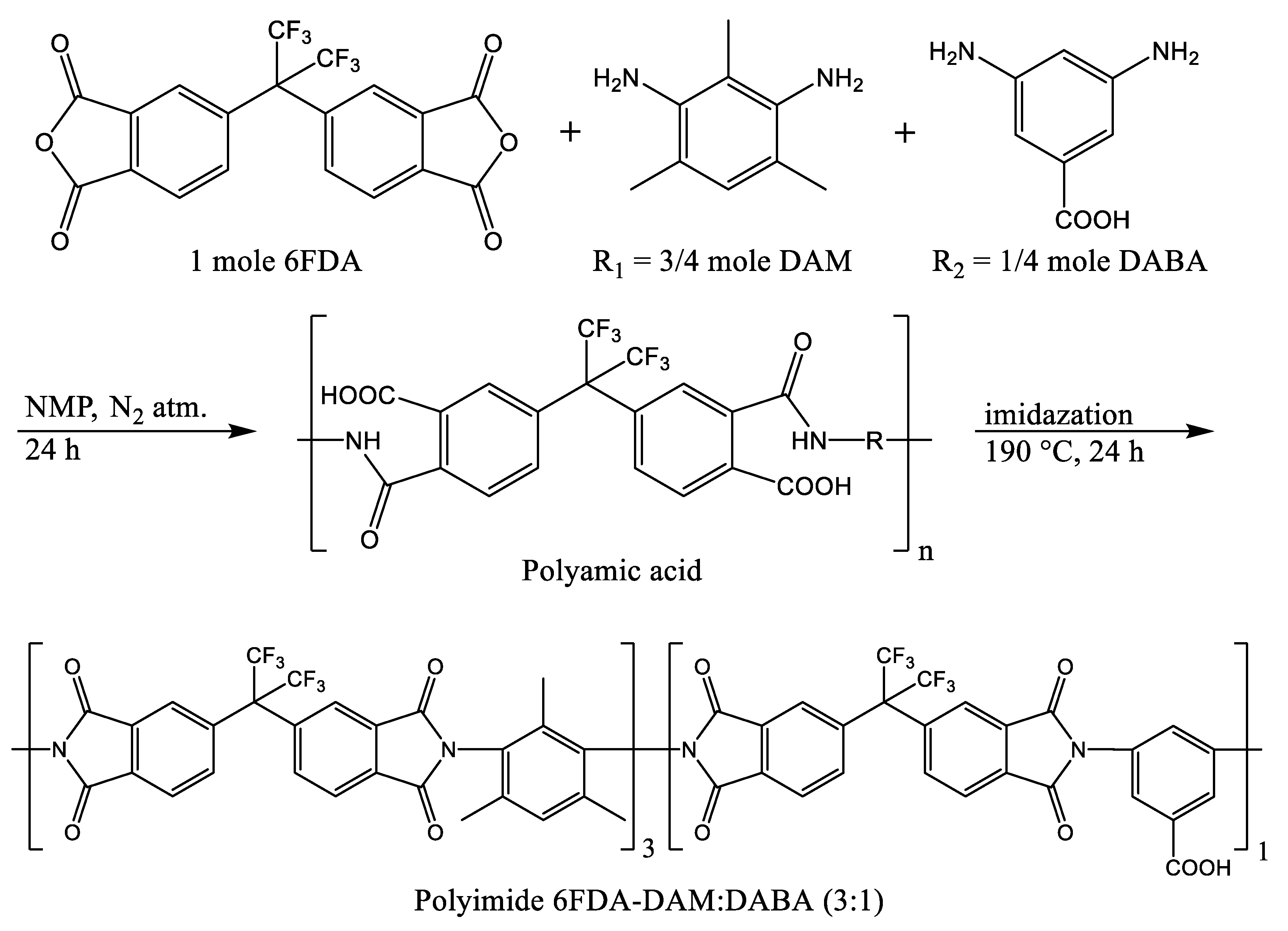
2.2. Co-Polyimide and Nanomaterials Syntheses
- Dry method: The slurry solution was centrifuged at 20,000 rpm (20 min) and the supernatant methanol was removed and replaced with fresh methanol (30 mL), followed by an ultrasound sonication (Kraintek K-10LE, ultrasonic power 300 W at the frequency 38 kHz for 15 min) to re-disperse the nanoparticles in the fresh solvent. This procedure was repeated for 3 cycles and the final supernatant methanol was discarded. The obtained nanoparticles were dried at 90 °C overnight.
- Wet method: At the third cycle of the dry method, the methanol was exchanged by 30 mL NMP and centrifuged at 20,000 rpm (20 min). The supernatant NMP was discarded and replaced by fresh NMP and the cycle was repeated 5 times, producing a solution of ZIF-8 nanoparticles in NMP. To determine the ZIF-8 powder concentration, 1 g of ZIF-8/NMP solution was spread onto a glass plate and kept dry in a vacuum oven at 90 °C for 24 h. The final dried weight was used to calculate the ZIF-8 concentration and determined at 0.074 g·mL−1. The solution was tightly sealed and kept stirred at room temperature before the MMM preparation.
2.3. Membrane Fabrication
2.4. Characterization
2.5. Gas Separation Measurement
3. Results
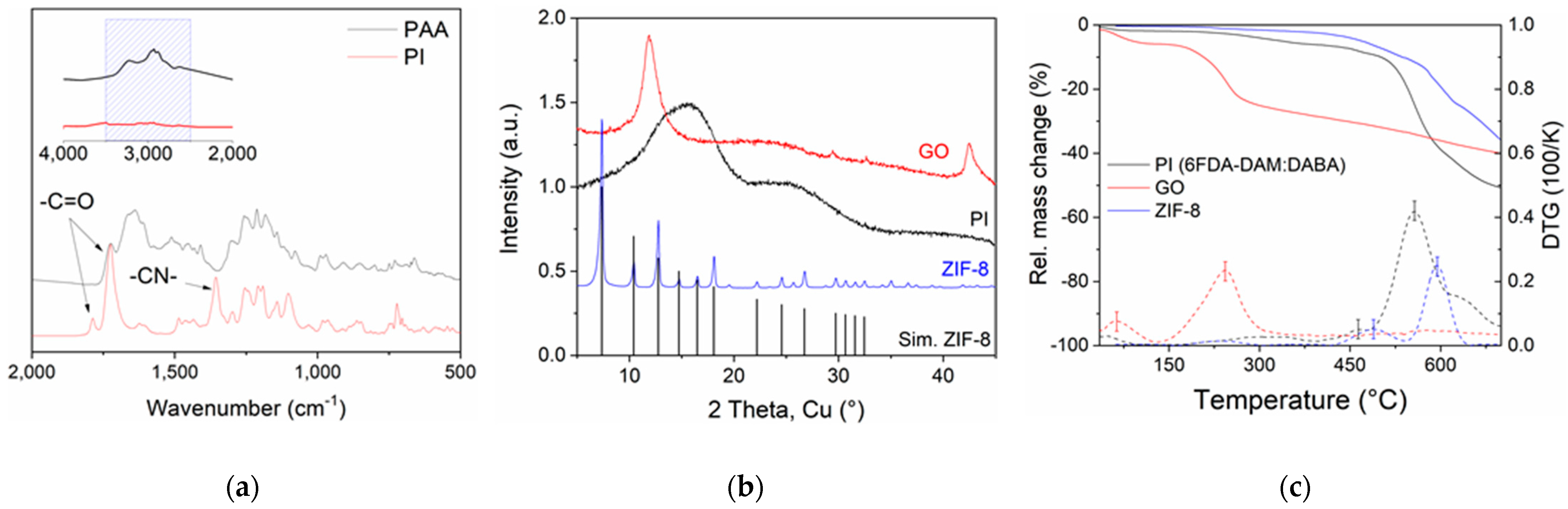
3.1. Materials Characterizations
3.1.1. 6FDA-Copolyimide and Nanoparticle Characterizations
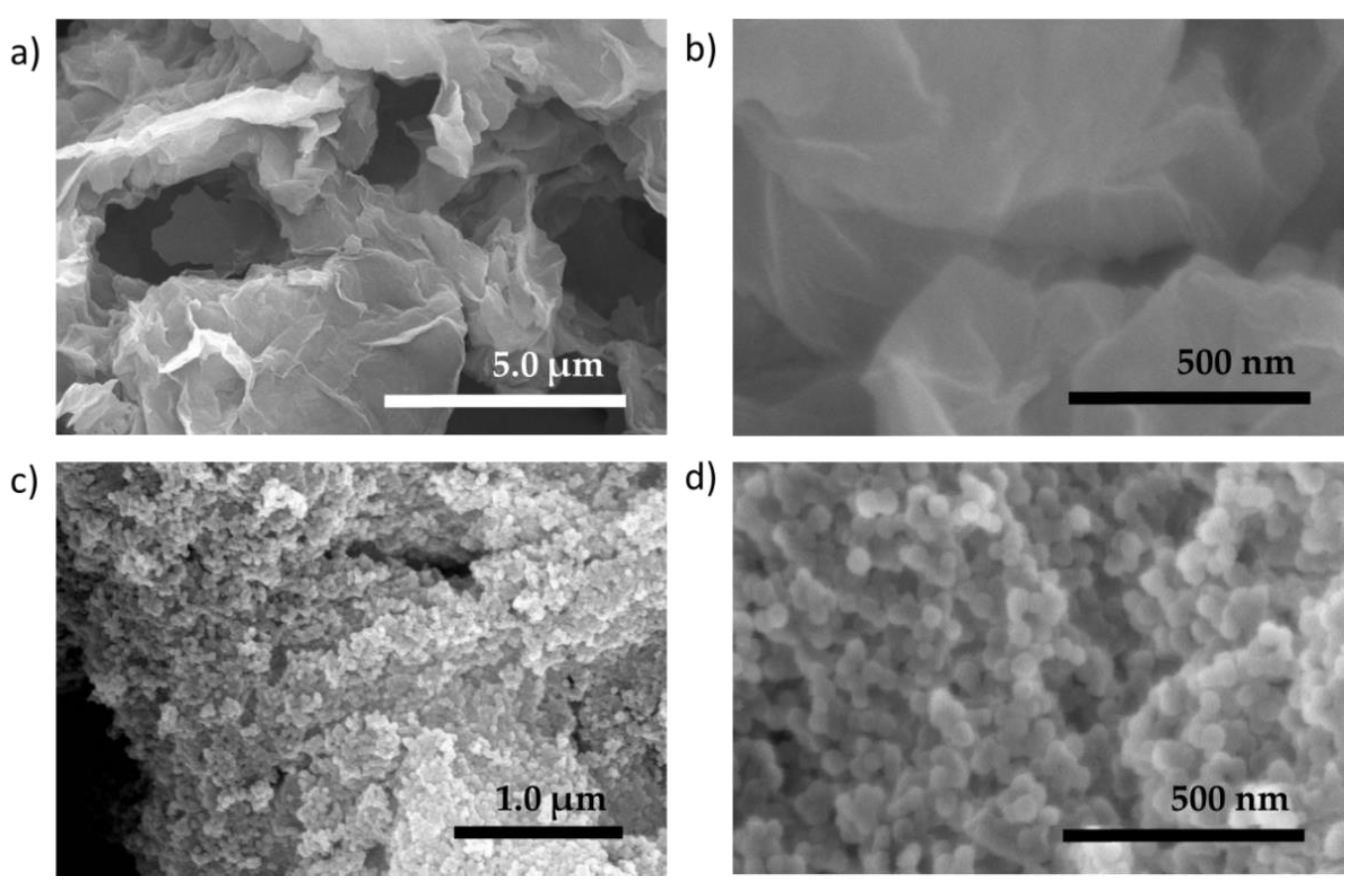
3.1.2. Membrane Characterizations
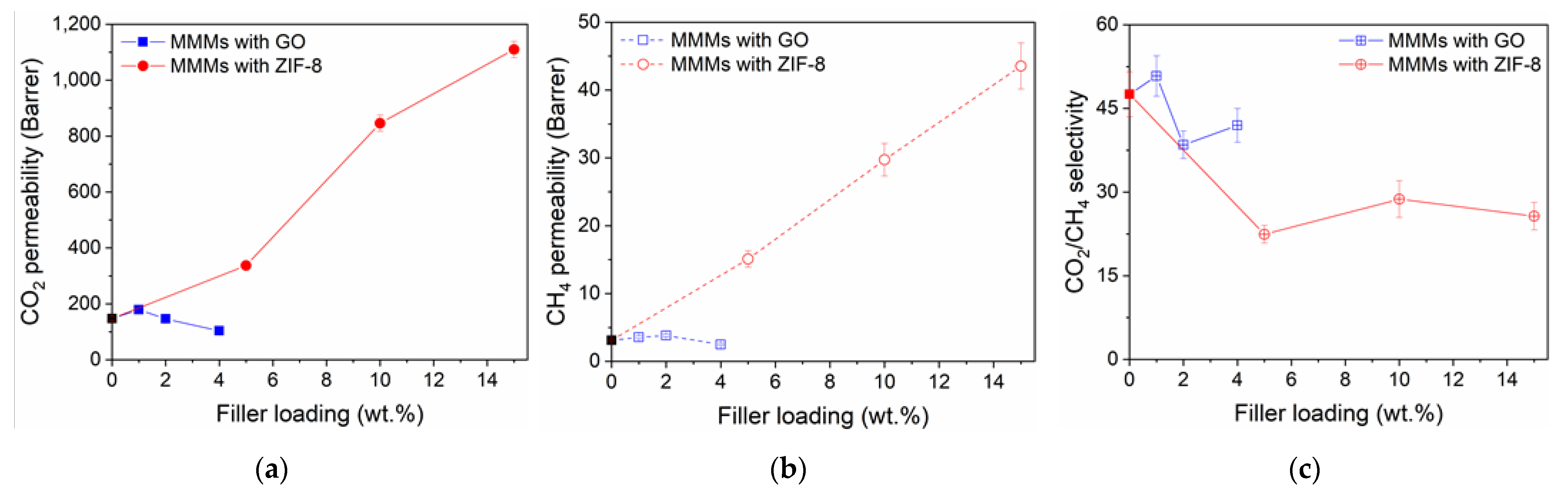
3.2. Gas Transport Properties
3.2.1. Gas Permeability and CO2/CH4 Selectivity
| Membrane | Gas Permeability (Barrer) | CO2/CH4 Selectivity | Feed Pressure (bar) | Ref. | |
|---|---|---|---|---|---|
| CO2 | CH4 | ||||
| 6FDA-DAM:DABA (4:1) * | 320.0 | - | 19.7 | 6.9 | [50] |
| 6FDA-DAM:DABA (3:1) | 199 ± 18 | 5.6 ± 0.3 | 35.9 ± 1.5 | 2 | [31] |
| 6FDA-DAM:DABA (3:1) | 147 ± 6.1 | 3.1 ± 0.1 | 47.5 ± 4.0 | 2 | This study |
| 6FDA-DAM:DABA (2:1) | 140.0 | 4.7 | 30.0 | 20 | [51] |
| 6FDA-DAM:DABA (3:2) | 158.9 | 4.2 | 37.8 | 6.9 | [52] |
3.2.2. Comparison with Upper Bounds
3.2.3. Performance at Various CO2 Partial Pressure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Plattner, G.-K., Xia, Y., Bex, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p.
- The Keeling Curve: Latest CO2 Reading; Scripps Institution of Oceanography: San Diego, CA, USA, 2020.
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Castro-Muñoz, R.; Budd, P.M. Boosting gas separation performance and suppressing the physical aging of polymers of intrinsic microporosity (PIM-1) by nanomaterial blending. Nanoscale 2020, 12, 23333–23370. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Etxeberria-Benavides, M.; David, O.; Johnson, T.; Łozińska, M.M.; Orsi, A.; Wright, P.A.; Mastel, S.; Hillenbrand, R.; Kapteijn, F.; Gascon, J. High performance mixed matrix membranes (MMMs) composed of ZIF-94 filler and 6FDA-DAM polymer. J. Membr. Sci. 2018, 550, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Martin-Gil, V.; Ahmad, M.; Castro-Muñoz, R.; Fila, V. Economic framework of membrane technologies for natural gas applications. Sep. Purif. Rev. 2018, 48, 298–324. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic–inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Melgar, V.M.A.; Kim, J.; Othman, M.R. Zeolitic imidazolate framework membranes for gas separation: A review of synthesis methods and gas separation performance. J. Ind. Eng. Chem. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Cravillon, J.; Schröder, C.A.; Bux, H.; Rothkirch, A.; Caro, J.; Wiebcke, M. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 2011, 14, 492–498. [Google Scholar] [CrossRef] [Green Version]
- McEwen, J.; Hayman, J.-D.; Yazaydin, A.O. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujita, K.; Miyake, Y.; Miyamoto, M.; Hasegawa, Y.; Makino, T.; Van Der Perre, S.; Remi, J.C.S.; Van Assche, T.; Baron, G.V.; et al. Adsorption and diffusion phenomena in crystal size engineered ZIF-8 MOF. J. Phys. Chem. C 2015, 119, 28430–28439. [Google Scholar] [CrossRef] [Green Version]
- Venna, S.R.; Zhu, M.; Li, S.; Carreon, M.A. Knudsen diffusion through ZIF-8 membranes synthesized by secondary seeded growth. J. Porous Mater. 2013, 21, 235–240. [Google Scholar] [CrossRef]
- Ibrahim, A.; Lin, Y. Gas permeation and separation properties of large-sheet stacked graphene oxide membranes. J. Membr. Sci. 2018, 550, 238–245. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Super gas barrier and selectivity of graphene oxide-polymer multilayer thin films. Adv. Mater. 2012, 25, 503–508. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; He, G.; Xin, Q.; Zhang, J.; Yang, L.; Li, Y.; Wu, H.; Zhang, Y.; Guiver, M.D.; et al. Graphene oxide membranes with heterogeneous nanodomains for efficient CO2 separations. Angew. Chem. Int. Ed. 2017, 56, 14246–14251. [Google Scholar] [CrossRef]
- Yang, K.; Dai, Y.; Ruan, X.; Zheng, W.; Yang, X.; Ding, R.; He, G. Stretched ZIF-8@GO flake-like fillers via pre-Zn(II)-doping strategy to enhance CO2 permeation in mixed matrix membranes. J. Membr. Sci. 2020, 601. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Kang, X.; Chen, M.; Zhang, C.; Bai, Y.; Dong, L. Enhanced CO2 separation of mixed matrix membranes with ZIF-8@GO composites as fillers: Effect of reaction time of ZIF-8@GO. Sep. Purif. Technol. 2019, 223, 113–122. [Google Scholar] [CrossRef]
- Anastasiou, S.; Bhoria, N.; Pokhrel, J.; Reddy, K.S.K.; Srinivasakannan, C.; Wang, K.; Karanikolos, G.N. Metal-organic framework/graphene oxide composite fillers in mixed-matrix membranes for CO2 separation. Mater. Chem. Phys. 2018, 212, 513–522. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Synergistic effect of adding graphene oxide and ZIF-301 to polysulfone to develop high performance mixed matrix membranes for selective carbon dioxide separation from post combustion flue gas. J. Membr. Sci. 2016, 514, 35–43. [Google Scholar] [CrossRef]
- Kratochvil, A.M.; Koros, W.J. Decarboxylation-induced cross-linking of a polyimide for enhanced CO2 plasticization resistance. Macromolecules 2008, 41, 7920–7927. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Pelletier, H.; Martin-Gil, V.; Castro-Muñoz, R.; Fila, V. Chemical crosslinking of 6FDA-ODA and 6FDA-ODA:DABA for improved CO2/CH4 separation. Membrane 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Chen, C.-C.; Kincer, M.R.; Koros, W.J. Thermal analysis and its application in evaluation of fluorinated polyimide membranes for gas separation. Polymers 2011, 52, 4073–4082. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; Fila, V.; Coronas, J. Enhancement of CO2/CH4 separation performances of 6FDA-based co-polyimides mixed matrix membranes embedded with UiO-66 nanoparticles. Sep. Purif. Technol. 2018, 192, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Ahmad, M.Z.; Fíla, V. Tuning of nano-based materials for embedding into low-permeability polyimides for a featured gas separation. Front. Chem. 2020, 7, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; Téllez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar] [CrossRef]
- Majumdar, S.; Tokay, B.; Martin-Gil, V.; Campbell, J.; Castro-Muñoz, R.; Ahmad, M.Z.; Fila, V. Mg-MOF-74/Polyvinyl acetate (PVAc) mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2020, 238, 116411. [Google Scholar] [CrossRef]
- Kawakami, H.; Mikawa, M.; Nagaoka, S. Gas transport properties in thermally cured aromatic polyimide membranes. J. Membr. Sci. 1996, 118, 223–230. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Martin-Gil, V.; Supinkova, T.; Lambert, P.; Castro-Muñoz, R.; Hrabanek, P.; Kocirik, M.; Fila, V. Novel MMM using CO2 selective SSZ-16 and high-performance 6FDA-polyimide for CO2/CH4 separation. Sep. Purif. Technol. 2021, 254, 117582. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Martin-Gil, V.; Muller, C. Enhanced CO2 permeability in Matrimid® 5218 mixed matrix membranes for separating binary CO2/CH4 mixtures. Sep. Purif. Technol. 2019, 210, 553–562. [Google Scholar] [CrossRef]
- Jankovský, O.; Marvan, P.; Nováček, M.; Luxa, J.; Mazánek, V.; Klímová, K.; Sedmidubský, D.; Sofer, Z. Synthesis procedure and type of graphite oxide strongly influence resulting graphene properties. Appl. Mater. Today 2016, 4, 45–53. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Martin-Gil, V.; Perfilov, V.; Sysel, P.; Fila, V. Investigation of a new co-polyimide, 6FDA-bisP and its ZIF-8 mixed matrix membranes for CO2/CH4 separation. Sep. Purif. Technol. 2018, 207, 523–534. [Google Scholar] [CrossRef]
- Martin-Gil, V.; Dujardin, W.; Sysel, P.; Koeckelberghs, G.; Vankelecom, I.; Fila, V. Effect of benzoic acid content on aging of 6FDA copolyimides based thin film composite (TFC) membranes in CO2/CH4 environment. Sep. Purif. Technol. 2019, 210, 616–626. [Google Scholar] [CrossRef]
- Hrabanek, P.; Zikanova, A.; Bernauer, B.; Fila, V.; Kočiřík, M. Butane isomer separation with composite zeolite MFI mebranes. Desalination 2009, 245, 437–443. [Google Scholar] [CrossRef]
- Pryde, C.A. IR studies of polyimides. I. Effects of chemical and physical changes during cure. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 711–724. [Google Scholar] [CrossRef]
- Qiu, W.; Xu, L.; Chen, C.-C.; Paul, D.R.; Koros, W.J. Gas separation performance of 6FDA-based polyimides with different chemical structures. Polymers 2013, 54, 6226–6235. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Kim, J.; Koros, W.; Paul, D. Effects of CO2 exposure and physical aging on the gas permeability of thin 6FDA-based polyimide membranesPart Without crosslinking. J. Membr. Sci. 2006, 282, 21–31. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Huang, F.; Rad, A.T.; Zheng, W.; Nieh, M.-P.; Cornelius, C. Hybrid organic-inorganic 6FDA-6pFDA and multi-block 6FDA-DABA polyimide SiO2–TiO2 nanocomposites: Synthesis, FFV, FTIR, swelling, stability, and X-ray scattering. Polymers 2017, 108, 105–120. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.E.; Lee, S.K.; Cho, Y.H.; Park, H.B. Effect of PEG-MEA and graphene oxide additives on the performance of Pebax®1657 mixed matrix membranes for CO2 separation. J. Membr. Sci. 2019, 572, 300–308. [Google Scholar] [CrossRef]
- Alberto, M.; Bhavsar, R.; Luque-Alled, J.M.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Impeded physical aging in PIM-1 membranes containing graphene-like fillers. J. Membr. Sci. 2018, 563, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Soyekwo, F.; Zhang, Q.; Hu, C.; Zhu, A.; Liu, Q. Graphene oxide nanosheets to improve permeability and selectivity of PIM-1 membrane for carbon dioxide separation. J. Ind. Eng. Chem. 2018, 63, 296–302. [Google Scholar] [CrossRef]
- Gonciaruk, A.; Althumayri, K.; Harrison, W.J.; Budd, P.M.; Siperstein, F.R. PIM-1/graphene composite: A combined experimental and molecular simulation study. Microporous Mesoporous Mater. 2015, 209, 126–134. [Google Scholar] [CrossRef]
- Lively, R.P.; Dose, M.E.; Xu, L.; Vaughn, J.T.; Johnson, J.; Thompson, J.A.; Zhang, K.; Lydon, M.E.; Lee, J.-S.; Liu, L.; et al. A high-flux polyimide hollow fiber membrane to minimize footprint and energy penalty for CO2 recovery from flue gas. J. Membr. Sci. 2012, 423–424, 302–313. [Google Scholar] [CrossRef]
- Wind, J.D.; Paul, D.R.; Koros, W.J. Natural gas permeation in polyimide membranes. J. Membr. Sci. 2004, 228, 227–236. [Google Scholar] [CrossRef]
- Eguchi, H.; Kim, D.J.; Koros, W.J. Chemically cross-linkable polyimide membranes for improved transport plasticization resistance for natural gas separation. Polymers 2015, 58, 121–129. [Google Scholar] [CrossRef]
- Song, Q.; Cao, S.; Pritchard, R.H.; Qiblawey, H.; Terentjev, E.M.; Cheetham, A.K.; Sivaniah, E. Nanofiller-tuned microporous polymer molecular sieves for energy and environmental processes. J. Mater. Chem. A 2015, 4, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Zhou, W.; Gallegos, J.M.; Liu, Y.; Chen, B. Exceptionally high acetylene uptake in a microporous metal−organic framework with open metal sites. J. Am. Chem. Soc. 2009, 131, 12415–12419. [Google Scholar] [CrossRef] [PubMed]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Téllez, C.; Coronas, J. Combination of MOFs and zeolites for mixed-matrix membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef] [PubMed]
- Knebel, A.; Bavykina, A.; Datta, S.J.; Sundermann, L.; Garzon-Tovar, L.; Lebedev, Y.; Durini, S.; Ahmad, R.; Kozlov, S.M.; Shterk, G.; et al. Solution processable metal–organic frameworks for mixed matrix membranes using porous liquids. Nat. Mater. 2020, 19, 1346–1353. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Fuoco, A. Porous liquids—Future for CO2 capture and separation? Curr. Res. Green Sustain. Chem. 2021, 4, 100070. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Bang, J.; Choi, J.; Lee, S.H.; Lee, J.-H.; Lee, J.S. 3-Dimensionally disordered mesoporous silica (DMS)-containing mixed matrix membranes for CO2 and non-CO2 greenhouse gas separations. Sep. Purif. Technol. 2014, 136, 286–295. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Cheong, W.L.; Lau, K.K.; Shariff, A.M. Facile fabrication of mixed matrix membranes containing 6FDA-durene polyimide and ZIF-8 nanofillers for CO2 capture. J. Ind. Eng. Chem. 2016, 44, 164–173. [Google Scholar] [CrossRef]
- Wijenayake, S.N.; Panapitiya, N.P.; Versteeg, S.H.; Nguyen, C.N.; Goel, S.; Balkus, J.K.J.; Musselman, I.H.; Ferraris, J.P. Surface cross-linking of ZIF-8/polyimide Mixed Matrix Membranes (MMMs) for gas separation. Ind. Eng. Chem. Res. 2013, 52, 6991–7001. [Google Scholar] [CrossRef]
- Visser, T.; Masetto, N.; Wessling, M. Materials dependence of mixed gas plasticization behavior in asymmetric membranes. J. Membr. Sci. 2007, 306, 16–28. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for Mixed-Matrix Membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef] [PubMed]
- Ogieglo, W.; Wessling, M.; Benes, N.E. Polymer relaxations in thin films in the vicinity of a penetrant- or temperature-induced glass transition. Macromolecules 2014, 47, 3654–3660. [Google Scholar] [CrossRef]

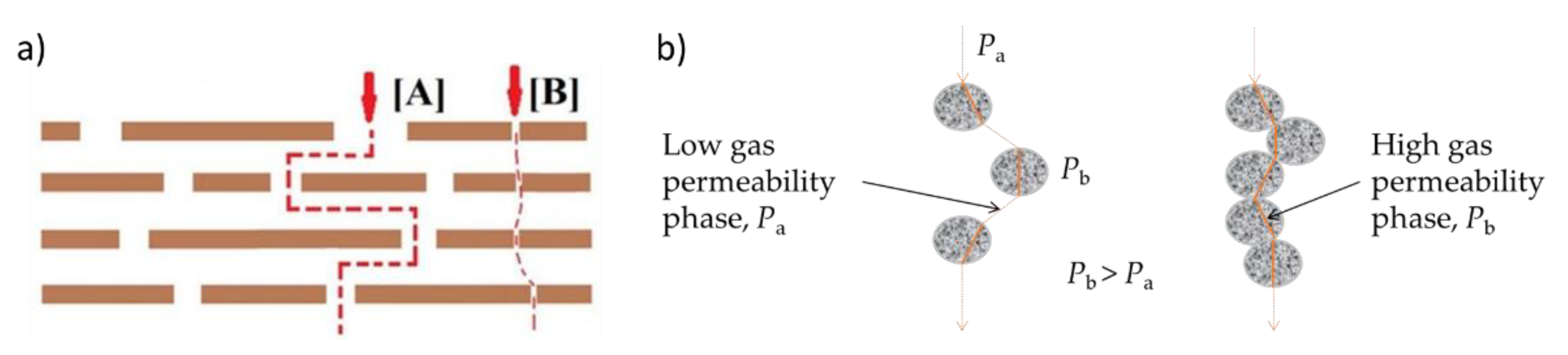
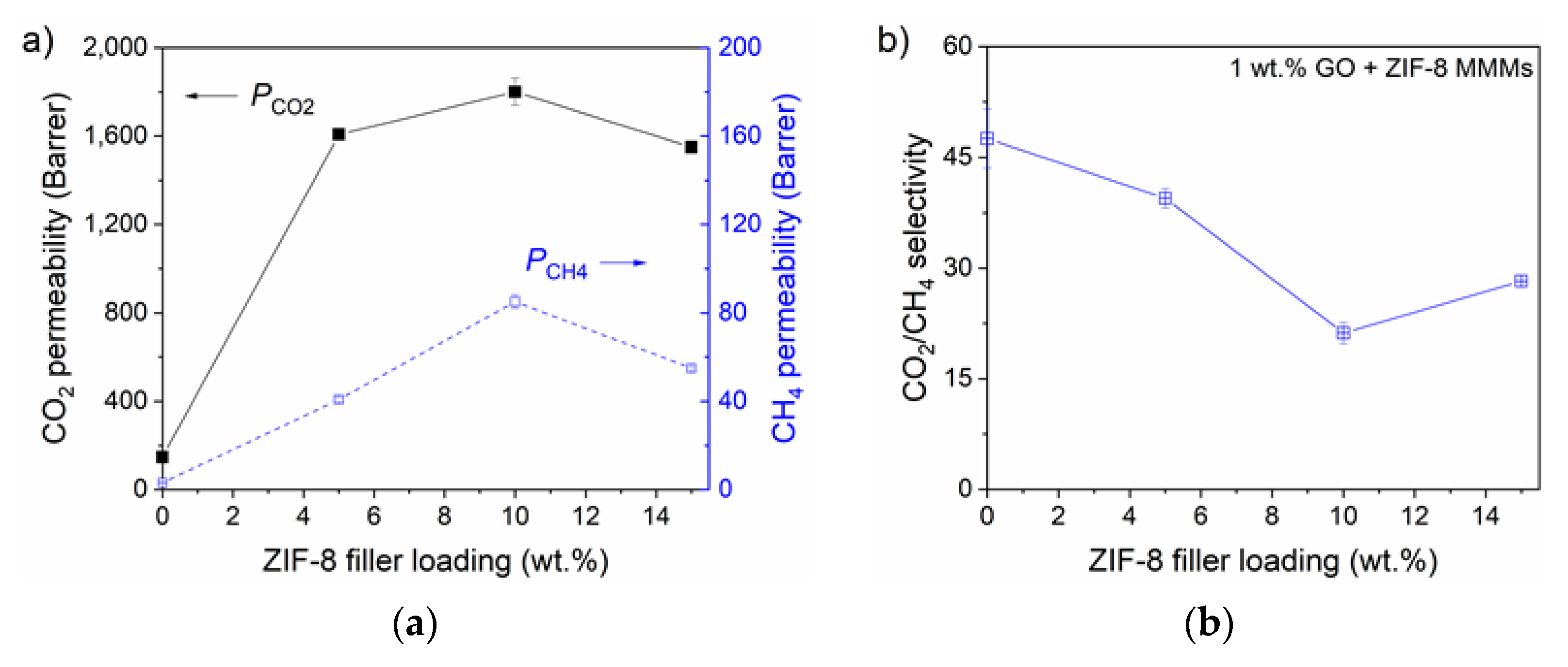
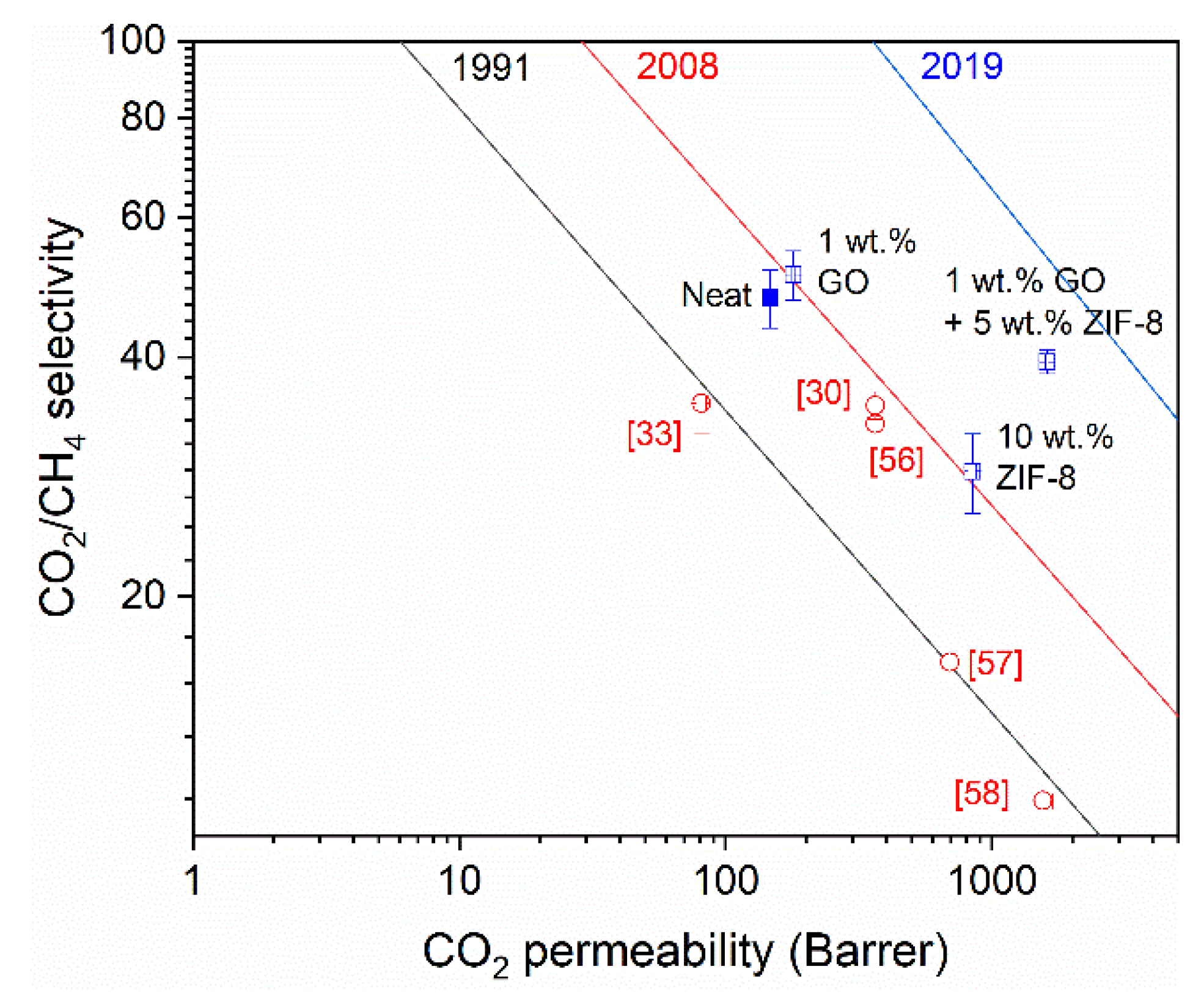
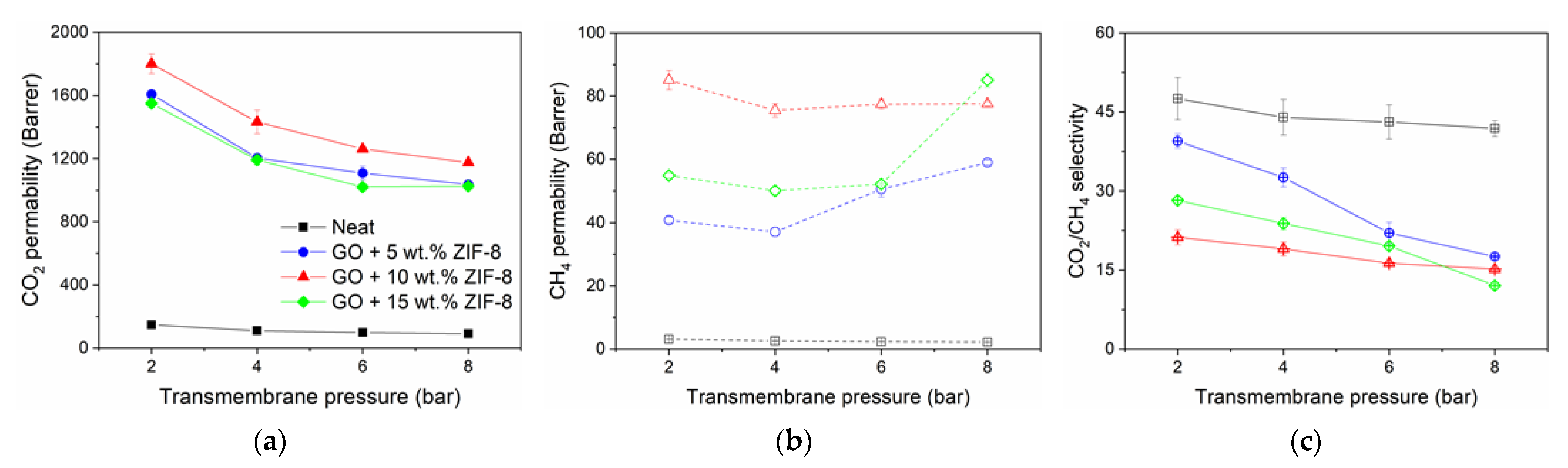
| Membrane | Decomposition Percentage (%) | Td (°C) | ||
|---|---|---|---|---|
| 100-350 °C | 351–490 °C | 491–700 °C | ||
| 6FDA-DAM:DABA (3:1) | 5.3 | 4.0 | 41.5 | 557 |
| MMM GO 1 wt.% | 5.6 | 3.0 | 31.7 | 555 |
| GO 2 wt.% | 8.1 | 3.7 | 34.4 | 557 |
| GO 4 wt.% | 9.3 | 3.9 | 45.3 | 557 |
| MMM ZIF-8 5 wt.% | 9.6 | 11.3 | 34.4 | 556 |
| ZIF-8 10 wt.% | 15.9 | 6.6 | 31.7 | 555 |
| ZIF-8 15 wt.% | 20.0 | 7.7 | 36.8 | 553 |
| MMM GO (1 wt.%)/ZIF-8 5 wt.% | 11.2 | 11.0 | 31.9 | 555 |
| GO (1 wt.%)/ZIF-8 10 wt.% | 15.8 | 6.6 | 31.7 | 554 |
| GO (1 wt.%)/ZIF-8 15 wt.% | 13.8 | 8.2 | 36.4 | 553 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, A.; Ahmad, M.Z.; Linkès, A.; Martin-Gil, V.; Castro-Muñoz, R.; Izak, P.; Sofer, Z.; Hintz, W.; Fila, V. 6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation. Nanomaterials 2021, 11, 668. https://doi.org/10.3390/nano11030668
Jain A, Ahmad MZ, Linkès A, Martin-Gil V, Castro-Muñoz R, Izak P, Sofer Z, Hintz W, Fila V. 6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation. Nanomaterials. 2021; 11(3):668. https://doi.org/10.3390/nano11030668
Chicago/Turabian StyleJain, Anand, Mohd Zamidi Ahmad, Audrey Linkès, Violeta Martin-Gil, Roberto Castro-Muñoz, Pavel Izak, Zdeněk Sofer, Werner Hintz, and Vlastimil Fila. 2021. "6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation" Nanomaterials 11, no. 3: 668. https://doi.org/10.3390/nano11030668
APA StyleJain, A., Ahmad, M. Z., Linkès, A., Martin-Gil, V., Castro-Muñoz, R., Izak, P., Sofer, Z., Hintz, W., & Fila, V. (2021). 6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation. Nanomaterials, 11(3), 668. https://doi.org/10.3390/nano11030668









