Characteristics and Photovoltaic Applications of Au-Doped ZnO–Sm Nanoparticle Films
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of Au-Doped ZnO–Sm Nanoparticles
2.2. Characterization
2.3. Preparation of Photo Anode and DSSC Fabrication
3. Results and Discussions
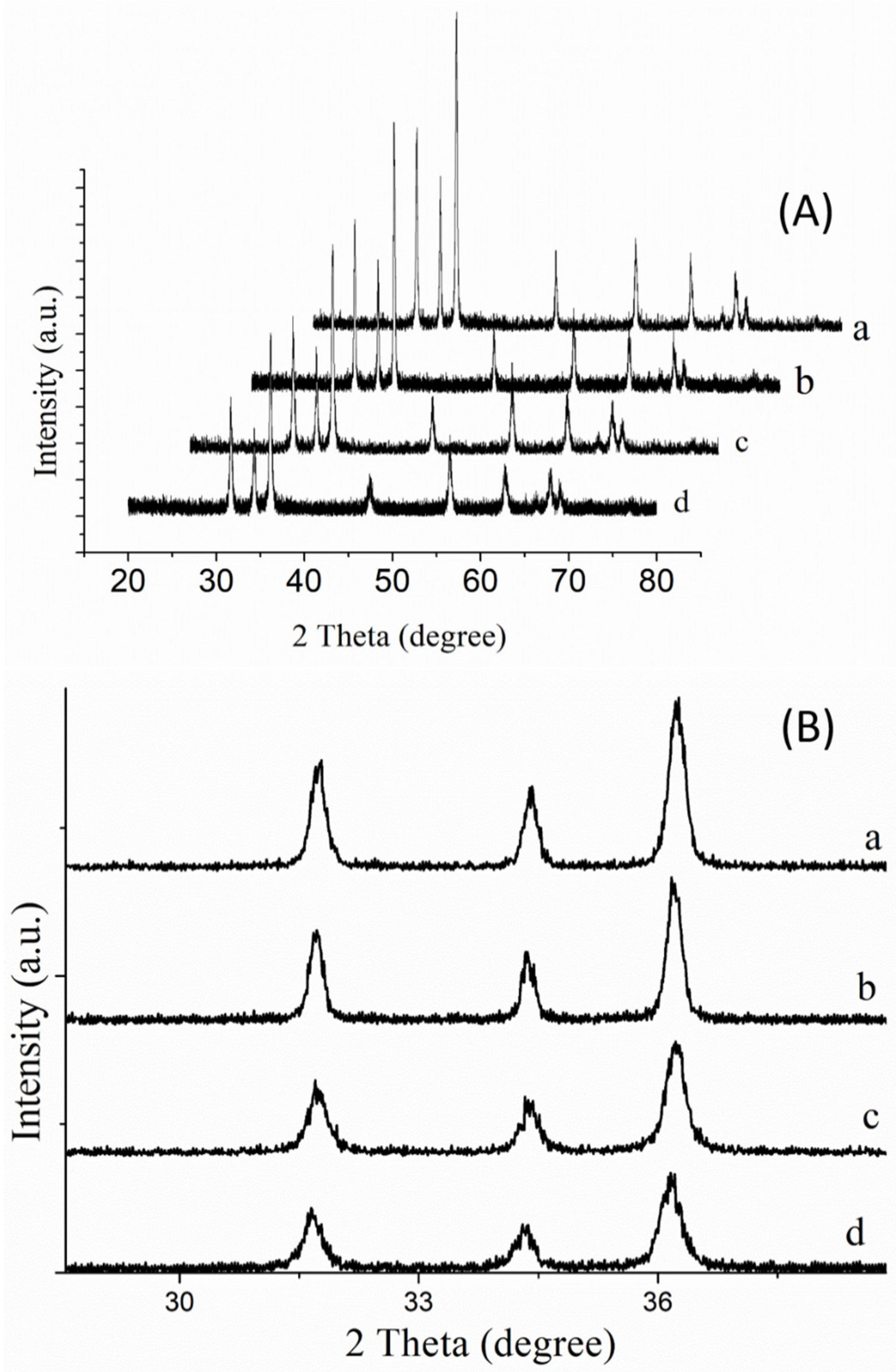
3.1. Structural Properties of Au-Doped ZnO–Sm Nanoparticles Films
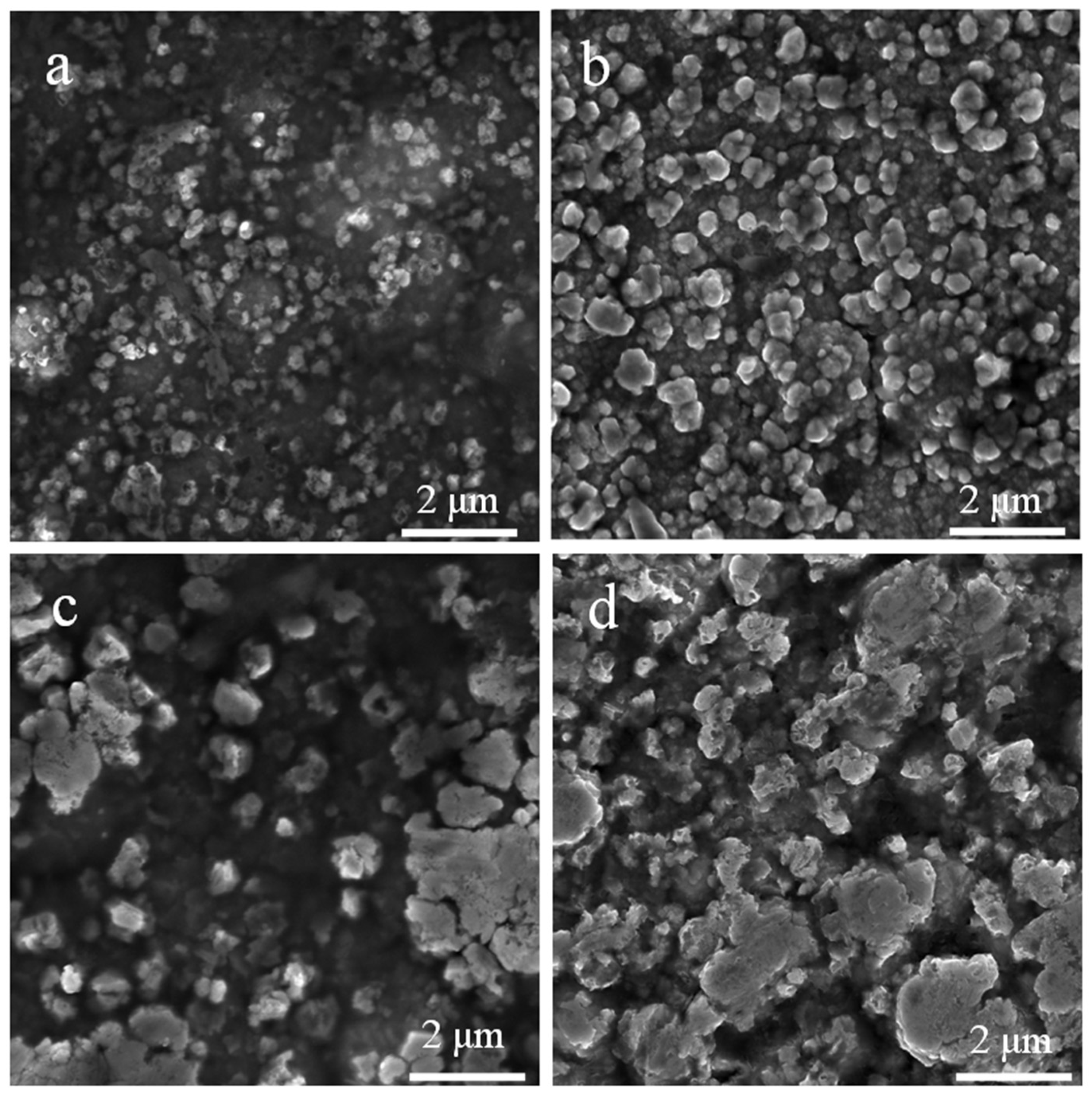
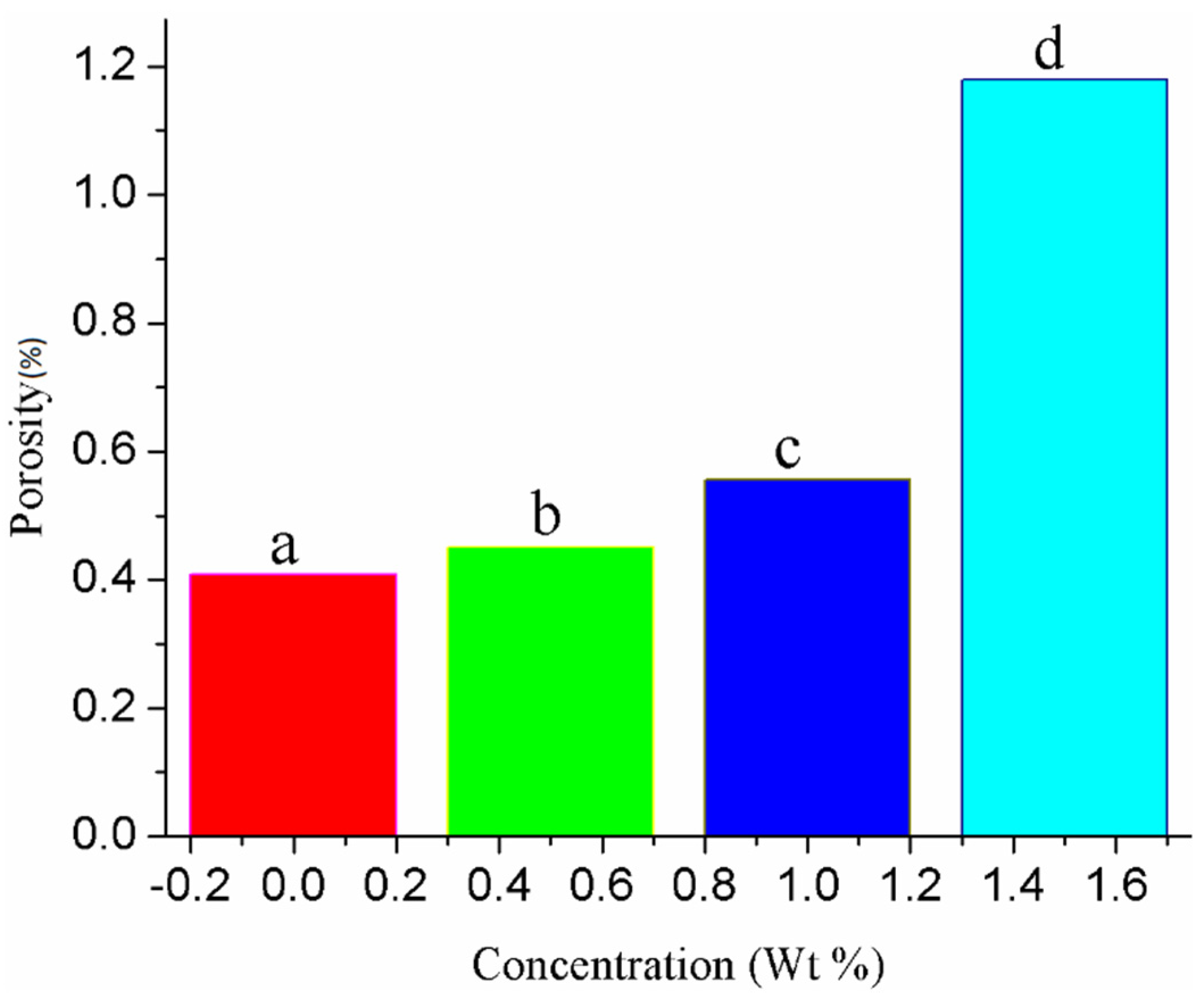
3.2. Morphological Properties of Au-Doped ZnO–Sm Nanoparticles Films
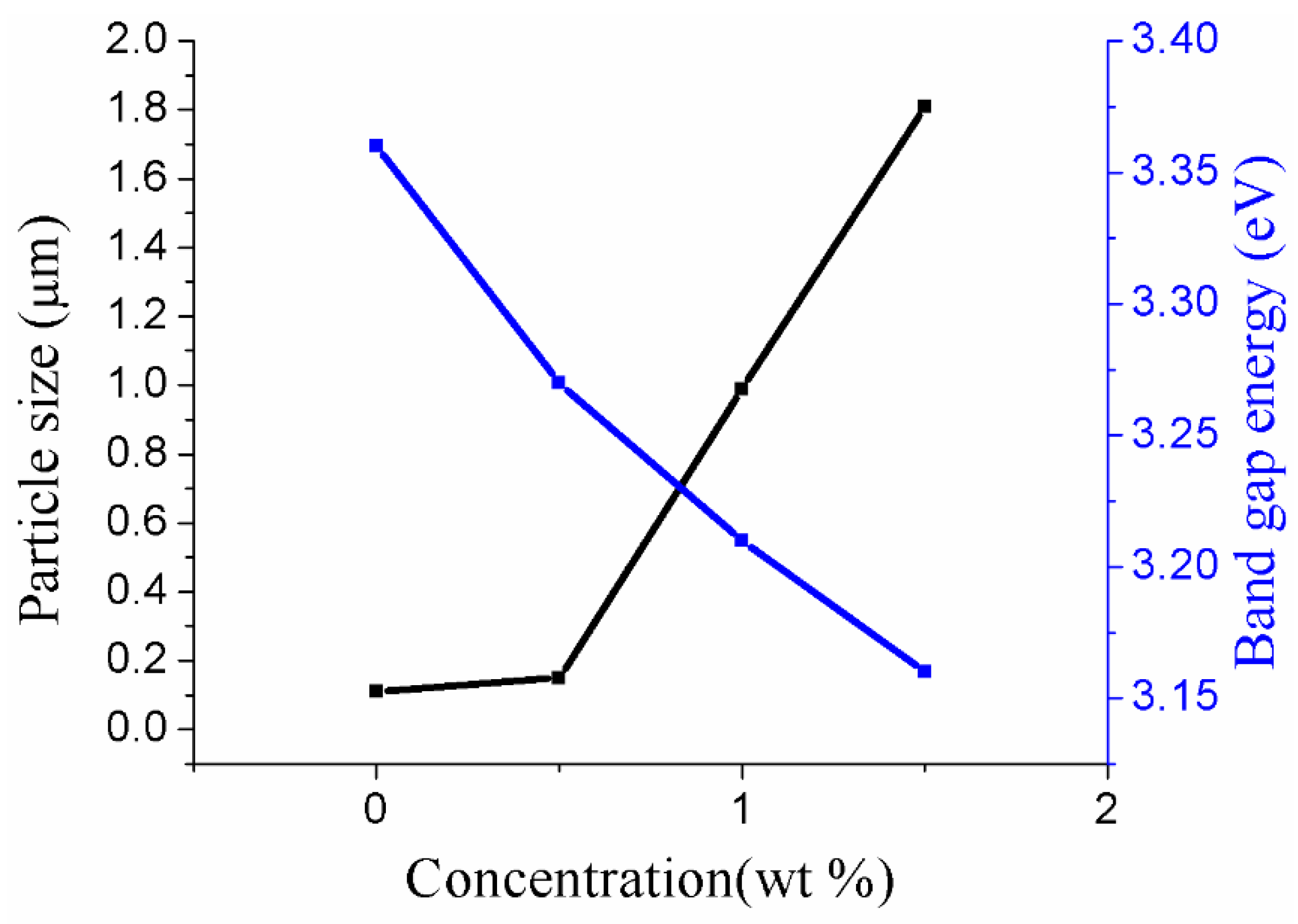
3.3. Optical Properties of Au-Doped ZnO–Sm Nanoparticle Films
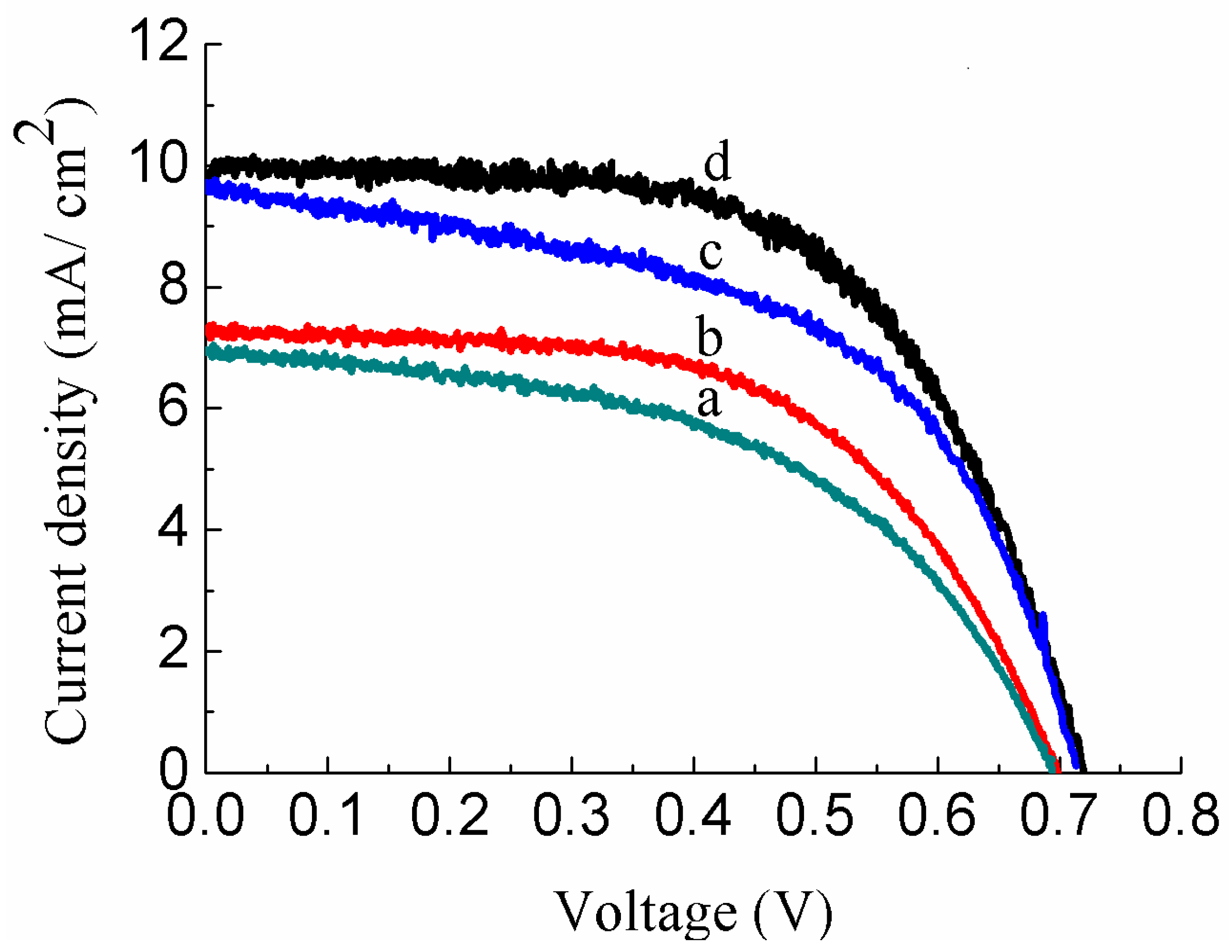
3.4. I–V Measurements of Au-Doped ZnO–Sm Films
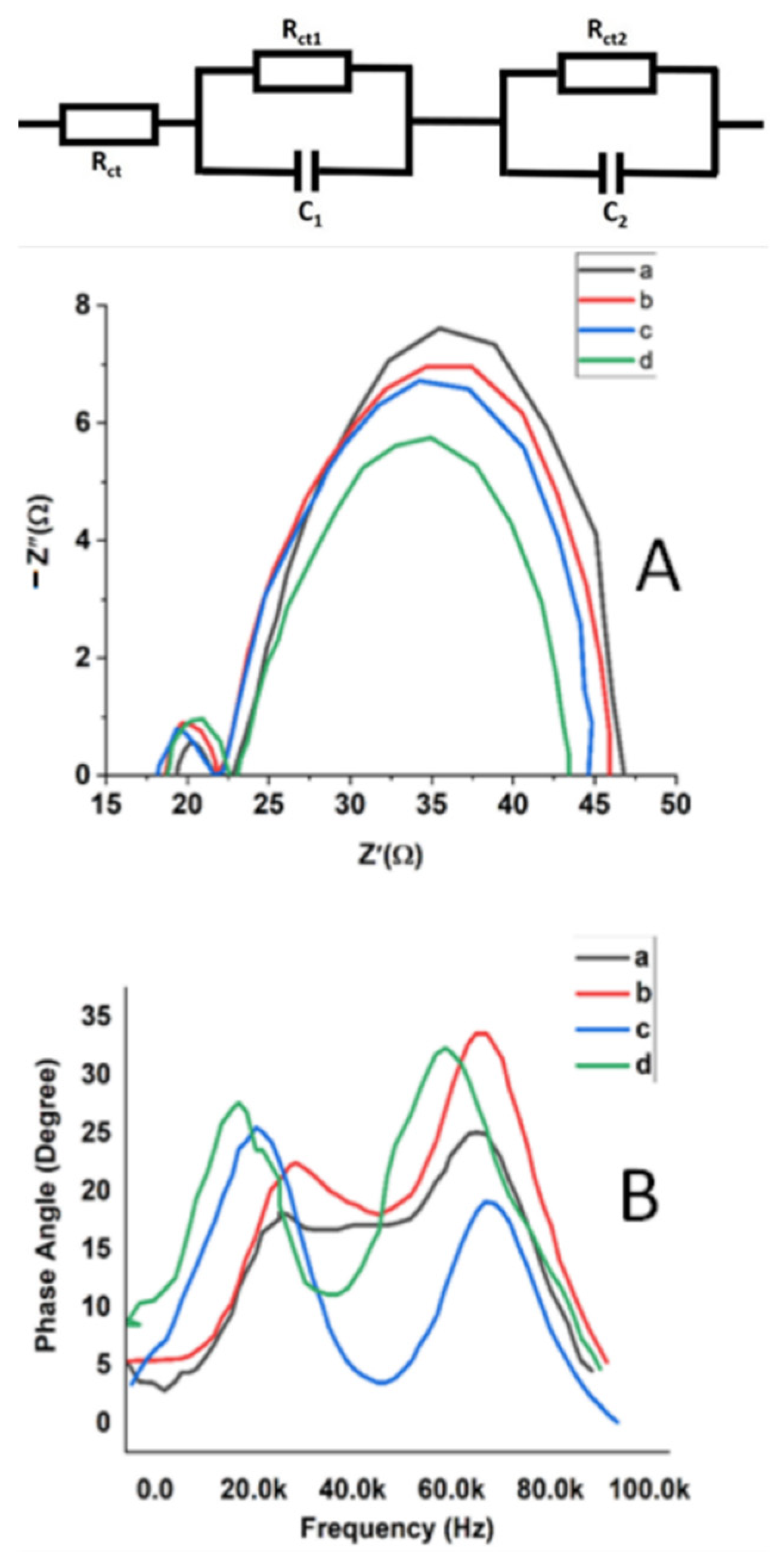
3.5. Impedance Spectroscopy Measurements of Au-Doped ZnO–Sm Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.; Lin, W.; Hon, M. Effects of post-annealing on the structure and properties of Al-doped zinc oxide films. Appl. Surf. Sci. 2001, 183, 18–25. [Google Scholar] [CrossRef]
- Du, Y.P.; Zhang, Y.W.; Sun, L.D.; Yan, C.H. Efficient energy transfer in monodisperse Eu-doped ZnO nanocrystals synthesized from metal acetylacetonates in high-boiling solvents. J. Phys. Chem. C 2008, 112, 12234–12241. [Google Scholar] [CrossRef]
- Gaponenko, S.V. Optical Properties of Semiconductor Nanocrystals; Cambridge University Press: Cambridge, UK, 1998; Volume 23. [Google Scholar]
- Guillén, E.; Peter, L.M.; Anta, J. Electron transport and recombination in ZnO-based dye-sensitized solar cells. J. Phys. Chem. C 2011, 115, 22622–22632. [Google Scholar] [CrossRef]
- Haase, M.; Riwotzki, K.; Meyssamy, H.; Kornowski, A. Synthesis and properties of colloidal lanthanide-doped nanocrystals. J. Alloy Compd. 2000, 303, 191–197. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S. Synthesis and characterization of samarium doped nano ZnOAg and its application to photocatalysis. Funct. Nanostruct. Proc. 2018, 7–9. [Google Scholar]
- Krivelevich, S.A.; Makoviichuk, M.I.; Selyukov, R.V. Excitation and De-excitation Cross Sections for Light-Emitting Nanoclusters in Rare Earth—Doped Silicon. Phys. Solid State 2005, 47, 9–12. [Google Scholar] [CrossRef]
- Kulyk, B.; Sahraoui, B.; Figà, V.; Turko, B.; Rudyk, V.; Kapustianyk, V. Influence of Ag, Cu dopants on the second and third harmonic response of ZnO films. J. Alloy Compd. 2009, 481, 819–825. [Google Scholar] [CrossRef] [Green Version]
- LeDoux, G.; Guillois, O.; Porterat, D.; Reynaud, C.; Huisken, F.; Kohn, B.; Paillard, V. Photoluminescence properties of silicon nanocrystals as a function of their size. Phys. Rev. B 2000, 62, 15942–15951. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.; Chen, H.; Ai, X.; Huang, X. Microstructures and optical properties of Cu-doped ZnO films prepared by radio frequency reactive magnetron sputtering. Appl. Surf. Sci. 2011, 257, 10036–10041. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, H.-Y.; Park, S.-J. Ag-ZnO photocatalyst anchored on carbon nanofibers: Synthesis, characterization, and photocatalytic activities. Synth. Met. 2016, 220, 533–537. [Google Scholar] [CrossRef]
- Peh, C.; Ke, L.; Ho, G. Modification of ZnO nanorods through Au nanoparticles surface coating for dye-sensitized solar cells applications. Mater. Lett. 2010, 64, 1372–1375. [Google Scholar] [CrossRef]
- Quintana, M.; Edvinsson, T.; Hagfeldt, A.A.; Boschloo, G. Comparison of Dye-Sensitized ZnO and TiO2Solar Cells: Studies of Charge Transport and Carrier Lifetime. J. Phys. Chem. C 2007, 111, 1035–1041. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, X.; Ren, S.; Tian, Z.; Fan, S.; Sun, S.; Liu, S.; Wang, Q. Improved visible solar absorber based on TiO2 nanotube film by surface-loading of plasmonic Au nanoparticles. J. Appl. Phys. 2013, 114, 63510. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Preparation and photocatalytic properties of visible light-driven samarium-doped ZnO nanorods. Ceram. Int. 2013, 39, 5833–5843. [Google Scholar] [CrossRef]
- Wang, X.; Kong, X.; Shan, G.; Yu, Y.I.; Sun, Y.; Feng, L.; Chao, K.; Lu, S.; Li, Y. Luminescence spectroscopy and visible upconversion properties of Er3+ in ZnO nanocrystals. J. Phys. Chem. B 2004, 108, 18408–18413. [Google Scholar] [CrossRef]
- Wei, S.; Chen, Y.; Ma, Y.; Shao, Z. Fabrication of CuO/ZnO composite films with cathodic co-electrodeposition and their photocatalytic performance. J. Mol. Catal. A Chem. 2010, 331, 112–116. [Google Scholar] [CrossRef]
- Shalev, G.; Schmitt, S.W.; Embrechts, H.; Brönstrup, G.; Christiansen, S. Enhanced photovoltaics inspired by the fovea centralis. Sci. Rep. 2015, 5, 8570. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, A.; Shalev, G. Geometry-driven carrier extraction enhancement in photovoltaic cells based on arrays of subwavelength light funnels. Nanoscale Adv. 2019, 1, 4755–4763. [Google Scholar] [CrossRef] [Green Version]
- Cheben, P.; Halir, R.; Schmid, J.H.; Atwater, H.A.; Smith, D.R. Subwavelength integrated photonics. Nature 2018, 560, 565–572. [Google Scholar] [CrossRef]
- Li, K.; Haque, S.; Martins, A.; Fortunato, E.; Martins, R.; Mendes, M.J.; Schuster, C.S. Light Trapping in Solar Cells: Simple Design Rules to Maximize Absorption. Optica 2020, 7, 1377–1384. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, C.; Liu, W.; Yang, S.; Wang, M.; Chen, T.; Zhu, C. Sequential deposition route to efficient Sb2S3 solar cells. J. Mater. Chem. A 2018, 6, 21320–21326. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Huang, J.; Fu, K.; Deng, X.; Ding, M.; Zhang, S.; Xu, X. Improving photovoltaic performance of dye sensitized solar cells based on a hierarchical structure with up/down converter. RSC Adv. 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Yao, N.; Huang, J.; Fu, K.; Deng, X.; Ding, M.; Shao, M.; Xu, X. Enhanced light harvesting of dye-sensitized solar cells with up/down conversion materials. Electrochim. Acta 2015, 154, 273–277. [Google Scholar] [CrossRef]
- Bora, T.; Kyaw, H.H.; Sarkar, S.; Pal, S.K.; Dutta, J. Highly efficient ZnO/Au Schottky barrier dye-sensitized solar cells: Role of gold nanoparticles on the charge-transfer process. Beilstein J. Nanotechnol. 2011, 2, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.H.; Tang, Y.B.; Liu, C.P.; Leung, Y.H.; Yuan, G.D.; Chen, L.M.; Wang, Y.Q.; Bello, I.; Zapien, J.A.; Zhang, W.J.; et al. Vertically aligned ZnO nanorod arrays sentisized with gold nanoparticles for Schottky barrier photovoltaic cells. J. Phys. Chem. C 2009, 113, 13433–13437. [Google Scholar] [CrossRef]
- Hafez, H.; Saif, M.; Abdel-Mottaleb, M.S.A. Down-converting lanthanide doped TiO2 photoelectrodes for efficiency enhancement of dye-sensitized solar cells. J. Power Sources 2011, 196, 5792. [Google Scholar] [CrossRef]
- Koao, L.; Dejene, F.; Kroon, R.; Swart, H. Effect of Eu3+ on the structure, morphology and optical properties of flower-like ZnO synthesized using chemical bath deposition. J. Lumin. 2014, 147, 85–89. [Google Scholar] [CrossRef]
- Panda, N.R.; Acharya, B.; Singh, T.B.; Gartia, R. Luminescence properties and decay kinetics of nano ZnO powder doped with cerium ions. J. Lumin. 2013, 136, 369–377. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y. Complete elimination of indoor formaldehyde over supported Pt catalysts with extremely low Pt content at ambient temperature. J. Catal. 2011, 280, 60–67. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Dakhel, A.A.; El-Hilo, M. Ferromagnetic nanocrystalline Gd-doped ZnO powder synthesized by coprecipitation. J. Appl. Phys. 2010, 107, 123905. [Google Scholar] [CrossRef]
- Singh, B.; Khan, Z.A.; Khan, I.; Ghosh, S. Highly conducting zinc oxide thin films achieved without post growth annealing. Appl. Phys. Lett. 2010, 97, 241903. [Google Scholar] [CrossRef]
- Hameed, A.S.H.; Karthikeyan, C.; Ahamed, A.P.; Thajuddin, N.; Alharbi, N.S.; Alharbi, S.A.; Ravi, G. In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci. Rep. 2016, 6, 24312. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.-W.; Shi, H.-Q.; Li, W.-N.; Xiao, H.-M.; Fu, S.-Y.; Cao, X.-Z.; Li, Z.-X. Lanthanum-doped ZnO quantum dots with greatly enhanced fluorescent quantum yield. J. Mater. Chem. 2012, 22, 8221–8227. [Google Scholar] [CrossRef]
- Pandey, P.; Kurchania, R.; Haque, F.Z. Rare earth ion (La, Ce, and Eu) doped ZnO nanoparticles synthesized via sol-gel method: Application in dye sensitized solar cells. Opt. Spectrosc. 2015, 119, 666–671. [Google Scholar] [CrossRef]
- Lu, J.G.; Ye, Z.Z.; Zeng, Y.J.; Zhu, L.P.; Wang, L.; Yuan, J.; Zhao, B.H.; Liang, Q.L. Structural, optical, and electrical properties of (Zn, Al) O films over a wide range of compositions. J. Appl. Phys. 2006, 100, 073714. [Google Scholar] [CrossRef]
- Houng, B.; Huang, C.J. Structure and properties of Ag embedded aluminum doped ZnO nanocomposite thin films prepared through a Sol—Gel process. Surf. Coat. Technol. 2006, 201, 3188–3192. [Google Scholar] [CrossRef]
- Divya, N.; Pradyumnan, P. Solid state synthesis of erbium doped ZnO with excellent photocatalytic activity and enhanced visible light emission. Mater. Sci. Semicond. Process. 2016, 41, 428–435. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kroon, R.; Swart, H. Photocatalytic and biological applications of Ag and Au doped ZnO nanomaterial synthesized by combustion. Vacuum 2018, 157, 508–513. [Google Scholar] [CrossRef]
- Latthe, S.S.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. Transparent, Adherent, and Photocatalytic SiO2-TiO2 Coatings on Polycarbonate for Self-Cleaning Applications. Coatings 2014, 4, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 6, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Kang, Y. Synthesis of NaBiO3/Bi2O3 heterojunction-structured photocatalyst and its photocatalytic mechanism. Mater. Lett. 2014, 117, 94–97. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, B.; Li, Y.F.; Ding, Z.H.; Li, J.C.; Wang, H.Z.; Zhang, Z.Z.; Zhang, L.G.; Zhao, H.F.; Shen, D.Z. Chemical states of gold doped in ZnO films and its effect on electrical and optical properties. J. Alloy Compd. 2014, 585, 479–484. [Google Scholar] [CrossRef]
- Yan, Y.; Al-Jassim, M.M.; Wei, S.-H. Doping of ZnO by group-IB elements. Appl. Phys. Lett. 2006, 89, 181912. [Google Scholar] [CrossRef]
- Wang, Q.; Moser, J.E.; Grätzel, M. Electrochemical Impedance Spectroscopic Analysis of Dye-Sensitized Solar Cells. J. Phys. Chem. B 2005, 109, 14945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshikawa, T.; Ikebe, T.; Kikuchi, R.; Eguchi, K. Effects of electrolyte in dye-sensitized solar cells and evaluation by impedance spectroscopy. Electrochim. Acta 2006, 51, 5286–5294. [Google Scholar] [CrossRef]
- Van de Lagemaat, J.; Park, N.G.; Frank, A.J. The role of electrical potential, charge transport, and recombination in determining the photopotential and photocurrent conversion efficiency (IPCE) of dye-sensitized nanocrystalline solar cells was studied. J. Phys. Chem. B 2000, 104, 2044–2052. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Maier, W.F.; Gratzel, M. An Iodine/Triiodide Reduction Electrocatalyst for Aqueous and Organic Media. J. Electrochem. Soc. 1997, 144, 876–884. [Google Scholar] [CrossRef]




| Samples | Content of ZnO (wt %) | Content of Sm (wt %) | Content of Au (wt %) |
|---|---|---|---|
| a | 100 | 1 | 0.0 |
| b | 100 | 1 | 0.5 |
| c | 100 | 1 | 1.0 |
| d | 100 | 1 | 1.5 |
| Samples | Content of ZnO (wt %) | Content of Sm (wt %) | Content of Au (wt %) | Particle Size (μm) | Band Gap Eg (eV) |
|---|---|---|---|---|---|
| a | 100 | 1 | 0.0 | 0.11 | 3.36 |
| b | 100 | 1 | 0.5 | 0.15 | 3.27 |
| c | 100 | 1 | 1.0 | 0.99 | 3.21 |
| d | 100 | 1 | 1.5 | 1.81 | 3.16 |
| Cell Parameters | Samples | |||
|---|---|---|---|---|
| a | b | c | d | |
| JSC (mA/cm2) | 6.96 ± 0.018 | 7.02 ± 0.056 | 9.63 ± 0.022 | 9.97 ± 0.019 |
| VOC (V) | 0.694 ± 0.003 | 0.699 ± 0.004 | 0.714 ± 0.002 | 0.719 ± 0.004 |
| FF | 0.52 ± 0.003 | 0.59 ± 0.005 | 0.54 ± 0.004 | 0.61 ± 0.003 |
| η (%) | 2.47 ± 0.002 | 3.26 ± 0.013 | 3.70 ± 0.003 | 4.35 ± 0.002 |
| Rct2 (Ω) | 130.62 ± 0.14 | 113.84 ± 0.10 | 96.47 ± 0.21 | 90.57 ± 0.16 |
| τ (ms) | 16 ± 1 | 21 ± 3 | 32 ± 1 | 39 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Irshad, K.; Ur Rehman, S.; Javed, M.S.; Hasan, M.A.; Ali, H.M.; Ali, A.; Malik, M.Z.; Islam, S. Characteristics and Photovoltaic Applications of Au-Doped ZnO–Sm Nanoparticle Films. Nanomaterials 2021, 11, 702. https://doi.org/10.3390/nano11030702
Saleem M, Irshad K, Ur Rehman S, Javed MS, Hasan MA, Ali HM, Ali A, Malik MZ, Islam S. Characteristics and Photovoltaic Applications of Au-Doped ZnO–Sm Nanoparticle Films. Nanomaterials. 2021; 11(3):702. https://doi.org/10.3390/nano11030702
Chicago/Turabian StyleSaleem, Muhammad, Kashif Irshad, Saif Ur Rehman, M. Sufyan Javed, Mohd Abul Hasan, Hafiz Muhammad Ali, Amjad Ali, Muhammad Zeeshan Malik, and Saiful Islam. 2021. "Characteristics and Photovoltaic Applications of Au-Doped ZnO–Sm Nanoparticle Films" Nanomaterials 11, no. 3: 702. https://doi.org/10.3390/nano11030702







