Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence
Abstract
:1. Introduction
2. Strontium Aluminate Phosphors
2.1. Lanthanide Ion-Doped Strontium Aluminate Phosphors
2.2. Synthesis of Strontium Aluminate Phosphors
2.3. Codoping of Strontium Aluminate Phosphors with Various Trivalent Lanthanide Ions
2.4. Mechanisms of Long-Persistent Luminescence from Lanthanide-Doped Strontium Aluminate Phosphors
3. Calcium Aluminate Phosphors
3.1. Calcium Aluminate Phosphors with Diverse Colors
3.2. Codoping of Calcium Aluminate Phosphors with Various Trivalent Lanthanide Ions
3.3. Synthesis of Calcium Aluminate Phosphors
4. Barium Aluminates
4.1. Synthesis of Barium Aluminate Phosphors
4.2. Barium Aluminate Phosphors with Various Colors
4.3. Codoping of Barium Aluminate Phosphors with Various Trivalent Lanthanide Ions
5. Outlook
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brahme, A. Comprehensive Biomedical Physics; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ray, B. Phosphors and Luminescence. In Electronic Materials; Springer: Berlin/Heidelberg, Germany, 1991; pp. 211–223. [Google Scholar]
- Lehmann, W. Activators and co-activators in calcium sulfide phosphors. J. Lumin. 1972, 5, 87–107. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Maier, W.F. Combinatorial and High-Throughput Discovery and Optimizatiof Catalysts and Materials; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4: Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670. [Google Scholar] [CrossRef]
- Zúñiga-Rivera, N.; Salas-Castillo, P.; Chernov, V.; Díaz-Torres, L.; Meléndrez, R.; García-Gutierrez, R.; Carrillo-Torres, R.; Barboza-Flores, M. Thermally and optically stimulated luminescence in long persistent orthorhombic strontium aluminates doped with Eu, Dy and Eu, Nd. Opt. Mater. 2017, 67, 91–97. [Google Scholar] [CrossRef]
- Nazarov, M.; Brik, M.G.; Spassky, D.; Tsukerblat, B. Crystal field splitting of 5d states and luminescence mechanism in SrAl2O4: Eu2+ phosphor. J. Lumin. 2017, 182, 79–86. [Google Scholar] [CrossRef]
- Peng, M.; Hong, G. Reduction from Eu3+ to Eu2+ in BaAl2O4: Eu phosphor prepared in an oxidizing atmosphere and luminescent properties of BaAl2O4: Eu. J. Lumin. 2007, 127, 735–740. [Google Scholar] [CrossRef]
- Freeda, M.; Subash, T. Preparation and Characterization of Praseodymium doped Calcium Aluminate nanophosphor (CaAl2O4: Pr) by sol-gel method. Mater. Today Proc. 2017, 4, 4266–4273. [Google Scholar] [CrossRef]
- Blasse, G.; Wanamaker, W. Fluorescence of Eu+2 activated silicates. Philips Res. Rep. 1968, 23, 189. [Google Scholar]
- Anesh, M.; Gulrez, S.K.; Anis, A.; Shaikh, H.; Ali Mohsin, M.; Al-Zahrani, S. Developments in Eu+2-Doped Strontium Aluminate and Polymer/Strontium Aluminate Composite. Adv. Polym. Technol. 2014, 33. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.-E.; Kim, C.-H. Effect of composition and impurities on the phosphorescence of green-emitting alkaline earth aluminate phosphor. PLoS ONE 2016, 11, e0145434. [Google Scholar] [CrossRef]
- Saines, P.J.; Elcombe, M.M.; Kennedy, B.J. Lanthanide distribution in some doped alkaline earth aluminates and gallates. J. Solid State Chem. 2006, 179, 613–622. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.-E.; Kim, C.-H. Enhancement of Long-Persistent Phosphorescence by Solid-State Reaction and Mixing of Spectrally Different Phosphors. ACS Omega 2020. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Wang, J.; Dang, P.; Lian, H.; Lin, J. Two-Step Sol–Gel Synthetic Strategy for Highly Dispersed Eu2+ Luminescence Centers for Tuning Emission. Adv. Photonics Res. 2020, 1, 2000028. [Google Scholar] [CrossRef]
- Fritzen, D.L.; Giordano, L.; Rodrigues, L.C.; Monteiro, J.H. Opportunities for Persistent Luminescent Nanoparticles in Luminescence Imaging of Biological Systems and Photodynamic Therapy. Nanomaterials 2020, 10, 2015. [Google Scholar] [CrossRef]
- Francis, B.; Nolasco, M.M.; Brandão, P.; Ferreira, R.A.; Carvalho, R.S.; Cremona, M.; Carlos, L.D. Efficient Visible-Light-Excitable Eu3+ Complexes for Red Organic Light-Emitting Diodes. Eur. J. Inorg. Chem. 2020. [Google Scholar] [CrossRef]
- Tiwari, A.; Dhoble, S. Tunable lanthanide/transition metal ion-doped novel phosphors for possible application in w-LEDs: A review. Luminescence 2020, 35, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, M.; Yawalkar, M.; Mahajan, N.; Dhoble, S. Potential of Luminescent Materials In Phototherapy. Photodiagnosis Photodyn. Ther. 2020, 102082. [Google Scholar] [CrossRef]
- Kohale, R.; Dhoble, S. Development of Dy3+ activated K2MgP2O7 pyrophosphate phosphor for energy saving lamp. J. Lumin. 2013, 138, 153–156. [Google Scholar] [CrossRef]
- Huy, B.T.; Gerelkhuu, Z.; Chung, J.W.; Dao, V.-D.; Ajithkumar, G.; Lee, Y.-I. Enhanced light harvesting with chromium in NaLu0.70−xGd0.10F4:Yb0.18Er0.02Crx (0 ≤ x ≤ 0.25) upconversion system. Mater. Sci. Eng. B 2017, 223, 91–97. [Google Scholar] [CrossRef]
- Markose, K.K.; Anjana, R.; Antony, A.; Jayaraj, M. Synthesis of Yb3+/Er3+ co-doped Y2O3, YOF and YF3 UC phosphors and their application in solar cell for sub-bandgap photon harvesting. J. Lumin. 2018, 204, 448–456. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.; Huang, W.; Bettinelli, M.; Liu, X. Lanthanide-activated phosphors based on 4f-5d optical transitions: Theoretical and experimental aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef] [PubMed]
- Joos, J.J.; Smet, P.F.; Seijo, L.; Barandiarán, Z. Insights into the complexity of the excited states of Eu-doped luminescent materials. Inorg. Chem. Front. 2020, 7, 871–888. [Google Scholar] [CrossRef]
- Marin, R.; Jaque, D. Doping Lanthanide Ions in Colloidal Semiconductor Nanocrystals for Brighter Photoluminescence. Chem. Rev. 2021, 121, 1425–1462. [Google Scholar] [CrossRef]
- Dorenbos, P. Energy of the first 4f7→ 4f65d transition of Eu2+ in inorganic compounds. J. Lumin. 2003, 104, 239–260. [Google Scholar] [CrossRef]
- Sahu, I.P.; Bisen, D.; Brahme, N.; Tamrakar, R.K.; Shrivastava, R. Luminescence studies of dysprosium doped strontium aluminate white light emitting phosphor by combustion route. J. Mater. Sci. Mater. Electron. 2015, 26, 8824–8839. [Google Scholar] [CrossRef]
- Nazarov, M.; Brik, M.; Spassky, D.; Tsukerblat, B.; Nazida, A.N.; Ahmad-Fauzi, M. Structural and electronic properties of SrAl2O4: Eu2+ from density functional theory calculations. J. Alloys Compd. 2013, 573, 6–10. [Google Scholar] [CrossRef]
- Lindop, A.; Matthews, C.; Goodwin, D. The refined structure of SrO.6Al2O3. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 2940–2941. [Google Scholar] [CrossRef]
- Gedekar, K.; Wankhede, S.; Moharil, S.; Belekar, R. d–f luminescence of Ce3+ and Eu2+ ions in BaAl2O4, SrAl2O4 and CaAl2O4 phosphors. J. Adv. Ceram. 2017, 6, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Won, H.I.; Hayk, N.; Won, C.W.; Jeon, D.Y.; Kirakosyan, A.G. Preparation and characterization of Sr4Al2O7: Eu3+, Eu2+ phosphors. Mater. Sci. Eng. B 2011, 176, 1521–1525. [Google Scholar] [CrossRef]
- Fu, Z.; Ma, L.; Sahi, S.; Hall, R.; Chen, W. Influence of doping concentration on valence states of europium in SrAl2O4: Eu phosphors. J. Lumin. 2013, 143, 657–662. [Google Scholar] [CrossRef]
- Mindru, I.; Gingasu, D.; Patron, L.; Marinescu, G.; Calderon-Moreno, J.M.; Diamandescu, L.; Secu, M.; Oprea, O. Tb3+-doped alkaline-earth aluminates: Synthesis, characterization and optical properties. Mater. Res. Bull. 2017, 85, 240–248. [Google Scholar] [CrossRef]
- Katsumata, T.; Nabae, T.; Sasajima, K.; Matsuzawa, T. Growth and characteristics of long persistent SrAl2O4-and CaAl2O4-based phosphor crystals by a floating zone technique. J. Cryst. Growth 1998, 183, 361–365. [Google Scholar] [CrossRef]
- Jia, W.; Yuan, H.; Lu, L.; Liu, H.; Yen, W.M. Crystal growth and characterization of Eu2+, Dy3+: SrAl2O4 and Eu2+, Nd3+: CaAl2O4 by the LHPG method. J. Cryst. Growth 1999, 200, 179–184. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Z.; Tang, Z.; Zhang, J.; Zheng, Z.; Lu, X. The characterization and mechanism of long afterglow in alkaline earth aluminates phosphors co-doped by Eu2O3 and Dy2O3. Mater. Chem. Phys. 2001, 70, 156–159. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Xiong, Y.; Wang, D.; Yin, Q. SrAl2O4: Eu2+, Dy3+ phosphors derived from a new sol–gel route. Microelectron. J. 2004, 35, 379–382. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhou, Y.; Lü, M.; Zhang, A.; Ma, Q. Combustion synthesis of long-persistent luminescent MAl2O4: Eu2+, R3+ (M= Sr, Ba, Ca, R= Dy, Nd and La) nanoparticles and luminescence mechanism research. Acta Mater. 2007, 55, 2615–2620. [Google Scholar] [CrossRef]
- Mothudi, B.M.; Ntwaeaborwa, O.; Botha, J.; Swart, H. Photoluminescence and phosphorescence properties of MAl2O4: Eu2+, Dy3+ (M = Ca, Ba, Sr) phosphors prepared at an initiating combustion temperature of 500 °C. Phys. B Condens. Matter 2009, 404, 4440–4444. [Google Scholar] [CrossRef]
- Aroz, R.; Lennikov, V.; Cases, R.; Sanjuán, M.L.; Germán, F.; Muñoz, E. Laser synthesis and luminescence properties of SrAl2O4: Eu2+, Dy3+ phosphors. J. Eur. Ceram. Soc. 2012, 32, 4363–4369. [Google Scholar] [CrossRef] [Green Version]
- Gültekin, S.; Yıldırım, S.; Yılmaz, O.; Keskin, İ.Ç.; Katı, M.İ.; Çelik, E. Structural and optical properties of SrAl2O4: Eu2+/Dy3+ phosphors synthesized by flame spray pyrolysis technique. J. Lumin. 2019, 206, 59–69. [Google Scholar] [CrossRef]
- Halefoglu, Y.; Yüksel, M.; Derin, H.; Can, N.; Topaksu, M.; Ozturk, E.; Karacaoğlu, E. Preparation and photoluminescence properties of aluminate phosphors produced by combustion synthesis. Appl. Radiat. Isot. 2018, 142, 46–50. [Google Scholar] [CrossRef]
- Aitasalo, T.; Dereń, P.; Hölsä, J.; Jungner, H.; Krupa, J.-C.; Lastusaari, M.; Legendziewicz, J.; Niittykoski, J.; Stręk, W. Persistent luminescence phenomena in materials doped with rare earth ions. J. Solid State Chem. 2003, 171, 114–122. [Google Scholar] [CrossRef]
- Chernov, V.; Salas-Castillo, P.; Díaz-Torres, L.; Zúñiga-Rivera, N.; Ruiz-Torres, R.; Meléndrez, R.; Barboza-Flores, M. Thermoluminescence and infrared stimulated luminescence in long persistent monoclinic SrAl2O4: Eu2+, Dy3+ and SrAl2O4: Eu2+, Nd3+ phosphors. Opt. Mater. 2019, 92, 46–52. [Google Scholar] [CrossRef]
- Song, H.; Chen, D. Combustion synthesis and luminescence properties of SrAl2O4: Eu2+, Dy3+, Tb3+ phosphor. Lumin. J. Biol. Chem. Lumin. 2007, 22, 554–558. [Google Scholar] [CrossRef]
- Hölsä, J.; Jungner, H.; Lastusaari, M.; Niittykoski, J. Persistent luminescence of Eu2+ doped alkaline earth aluminates, MAl2O4: Eu2+. J. Alloys Compd. 2001, 323, 326–330. [Google Scholar] [CrossRef]
- Li, K.; Lian, H.; Han, Y.; Shang, M.; Van Deun, R.; Lin, J. BaLu6(Si2O7)2(Si3O10): Ce3+, Tb3+: A novel blue-green emission phosphor via energy transfer for UV LEDs. Dye. Pigment. 2017, 139, 701–707. [Google Scholar] [CrossRef]
- He, Q.; Fu, R.; Zhu, H.; He, H.; Song, X.; Liu, X. Synthesis and luminescence enhancement of CaySr4−x−yAl2O7:xEu2+ phosphors by a novel halide-assisted solid-state reaction method. J. Mater. Sci. Mater. Electron. 2018, 29, 10487–10493. [Google Scholar] [CrossRef]
- Singh, V.; Rao, T.G.; Zhu, J.-J. Preparation, luminescence and defect studies of Eu2+-activated strontium hexa-aluminate phosphor prepared via combustion method. J. Solid State Chem. 2006, 179, 2589–2594. [Google Scholar] [CrossRef]
- Havasi, V.; Tátrai, D.; Szabó, G.; Varga, E.; Erdőhelyi, A.; Sipos, G.; Kónya, Z.; Kukovecz, Á. On the effects of milling and thermal regeneration on the luminescence properties of Eu2+ and Dy3+ doped strontium aluminate phosphors. J. Lumin. 2020, 219, 116917. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Q.; Yan, W.; Chen, Y.; Shen, A.; Zhang, H. Relation between structure conversion and spectra-tuning properties of Eu 2+-doped strontium aluminate phosphor. J. Mater. Sci. 2017, 52, 8188–8199. [Google Scholar] [CrossRef]
- Misevicius, M.; Scit, O.; Grigoraviciute-Puroniene, I.; Degutis, G.; Bogdanoviciene, I.; Kareiva, A. Sol–gel synthesis and investigation of un-doped and Ce-doped strontium aluminates. Ceram. Int. 2012, 38, 5915–5924. [Google Scholar] [CrossRef]
- Dutczak, D.; Jüstel, T.; Ronda, C.; Meijerink, A. Eu2+ luminescence in strontium aluminates. Phys. Chem. Chem. Phys. 2015, 17, 15236–15249. [Google Scholar] [CrossRef] [Green Version]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.-H.; Garcia, A.; Le Mercier, T. Mechanism of phosphorescence appropriate for the long-lasting phosphors Eu2+-doped SrAl2O4 with codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Bierwagen, J.; Delgado, T.; Jiranek, G.; Yoon, S.; Gartmann, N.; Walfort, B.; Pollnau, M.; Hagemann, H. Probing traps in the persistent phosphor SrAl2O4: Eu2+, Dy3+, B3+-A wavelength, temperature and sample dependent thermoluminescence investigation. J. Lumin. 2020, 222, 117113. [Google Scholar] [CrossRef]
- Vitola, V.; Millers, D.; Bite, I.; Smits, K.; Spustaka, A. Recent progress in understanding the persistent luminescence in SrAl2O4: Eu, Dy. Mater. Sci. Technol. 2019, 35, 1661–1677. [Google Scholar] [CrossRef]
- Li, G.; Tian, Y.; Zhao, Y.; Lin, J. Recent progress in luminescence tuning of Ce3+ and Eu2+-activated phosphors for pc-WLEDs. Chem. Soc. Rev. 2015, 44, 8688–8713. [Google Scholar] [CrossRef]
- Menon, S.G.; Bedyal, A.; Pathak, T.; Kumar, V.; Swart, H.C. Sr4Al14O25: Eu2+, Dy3+@ZnO nanocomposites as highly efficient visible light photocatalysts for the degradation of aqueous methyl orange. J. Alloys Compd. 2020, 158370. [Google Scholar] [CrossRef]
- Chithambo, M. Thermal assistance in the optically stimulated luminescence of superluminous Sr4Al14O25: Eu2+, Dy3+. Phys. B Condens. Matter 2020, 412722. [Google Scholar] [CrossRef]
- Morrison, C.A. Host dependence of the rare-earth ion energy separation 4 fN–4fN−1 nl. J. Chem. Phys. 1980, 72, 1001–1002. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, A.; Luyt, A.; Revaprasadu, N.; Hillie, K.; vdM Steyn, W.; Coetsee, E.; Swart, H. Ethyl vinyl acetate copolymer—SrAl2O4:Eu,Dy and Sr4Al14O25:Eu,Dy phosphor-based composites: Preparation and material properties. J. Appl. Polym. Sci. 2010, 115, 579–587. [Google Scholar] [CrossRef]
- Vitola, V.; Bite, I.; Millers, D.; Zolotarjovs, A.; Laganovska, K.; Smits, K.; Spustaka, A. The boron effect on low temperature luminescence of SrAl2O4: Eu, Dy. Ceram. Int. 2020, 46, 26377–26381. [Google Scholar] [CrossRef]
- Zhang, S.; Nakai, Y.; Tsuboi, T.; Huang, Y.; Seo, H.J. Luminescence and microstructural features of Eu-activated LiBaPO4 phosphor. Chem. Mater. 2011, 23, 1216–1224. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Wang, J.; Wu, M.; Wang, C. Color-tunable phosphor of Eu2+ and Mn2+ codoped Ca2Sr(PO4)2 for UV light-emitting diodes. J. Phys. Chem. C 2014, 118, 12494–12499. [Google Scholar] [CrossRef]
- Kareiva, A. Aqueous sol-gel synthesis methods for the preparation of garnet crystal structure compounds. Mater. Sci. 2011, 17, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Suta, M.; Lavoie-Cardinal, F.; Wickleder, C. Underestimated color centers: Defects as useful reducing agents in lanthanide-activated luminescent materials. Angew. Chem. Int. Ed. 2020, 59, 10949–10954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Castaing, V.; Guo, D.; Viana, B. Rare-earths doped-nanoparticles prepared by pulsed laser ablation in liquids. Ceram. Int. 2020, 46, 26299–26308. [Google Scholar] [CrossRef]
- Delgado, T.; Bierwagen, J.; Gartmann, N.; Walfort, B.; Kinski, I.; Pollnau, M.; Hagemann, H. Characterization and afterglow of SrAl2O4: Eu, Dy for various phosphor applications. In Proceedings of the Fiber Lasers and Glass Photonics: Materials through Applications II, Online. 6–10 April 2020; p. 113571. [Google Scholar]
- Homayoni, H.; Sahi, S.; Ma, L.; Zhang, J.; Mohapatra, J.; Liu, P.; Sotelo, A.P.; Macaluso, R.T.; Davis, T.; Chen, W. X-ray excited luminescence and persistent luminescence of Sr2MgSi2O7: Eu2+, Dy3+ and their associations with synthesis conditions. J. Lumin. 2018, 198, 132–137. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, J.; Jiang, B.; Wei, X.; Yin, M.; Chen, Y. A new yellow long persistent luminescence phosphor Ca2Al2SiO7: Eu2+, Tm3+ found by co-doping Ln3+ (Ln = Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) with Eu2+ in Ca2Al2SiO7 host. J. Lumin. 2019, 206, 6–10. [Google Scholar] [CrossRef]
- Hai, O.; Ren, Q.; Wu, X.; Zhang, Q.; Zhang, Z.; Zhang, Z. Insights into the element gradient in the grain and luminescence mechanism of the long afterglow material Sr2MgSi2O7: Eu2+, Dy3+. J. Alloys Compd. 2019, 779, 892–899. [Google Scholar] [CrossRef]
- Dorenbos, P. Mechanism of persistent luminescence in Eu2+ and Dy3+ codoped aluminate and silicate compounds. J. Electrochem. Soc. 2005, 152, H107. [Google Scholar] [CrossRef] [Green Version]
- Rezende, M.d.S.; Montes, P.J.; Soares, F.d.S.; Santos, C.d.; Valerio, M.E. Influence of co-dopant in the europium reduction in SrAl2O4 host. J. SynchrotrRadiat. 2014, 21, 143–148. [Google Scholar] [CrossRef]
- Aitasalo, T.; Hölsä, J.; Jungner, H.; Krupa, J.-C.; Lastusaari, M.; Legendziewicz, J.; Niittykoski, J. Effect of temperature on the luminescence processes of SrAl2O4: Eu2+. Radiat. Meas. 2004, 38, 727–730. [Google Scholar] [CrossRef]
- Aitasalo, T.; Hölsä, J.; Jungner, H.; Lastusaari, M.; Niittykoski, J. Mechanisms of persistent luminescence in Eu2+, RE3+ doped alkaline earth aluminates. J. Lumin. 2001, 94, 59–63. [Google Scholar] [CrossRef]
- Swati, G.; Jaiswal, V.V.; Haranath, D. Rare-earth doping in afterglow oxide phosphors: Materials, persistence mechanisms, and dark vision display applications. In Spectroscopy of Lanthanide Doped Oxide Materials; Elsevier: Cambridge, UK, 2020; pp. 393–425. [Google Scholar]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Feng, W.-L.; Chen, Z. Effect of boric acid on structure, morphology and luminescent properties of divalent europium doped calcium aluminate phosphors. Optik 2014, 125, 1252–1254. [Google Scholar] [CrossRef]
- Freeda, M.; Subash, T. Comparision of Photoluminescence studies of Lanthanum, Terbium doped Calcium Aluminate nanophosphors (CaAl2O4: La, CaAl2O4: Tb) by sol-gel method. Mater. Today Proc. 2017, 4, 4302–4307. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, D. Synthesis of CaAl2O4: Eu, Nd long persistent phosphor by combustion processes and its optical properties. Mater. Lett. 2007, 61, 3673–3675. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.-E.; Kim, C.-H. Development of a blue emitting calcium-aluminate phosphor. PLoS ONE 2016, 11, e0162920. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Z.; Zhang, Z.; Nan, C. Influence of co-doping different rare earth ions on the luminescence of CaAl2O4-based phosphors. J. Eur. Ceram. Soc. 2003, 23, 175–178. [Google Scholar] [CrossRef]
- Dougill, M.W. Crystal structure of calcium monoaluminate. Nature 1957, 180, 292–293. [Google Scholar] [CrossRef]
- Guo, C.; Luan, L.; Chen, C.; Huang, D.; Su, Q. Preparation of Y2O2S: Eu3+ phosphors by a novel decomposition method. Mater. Lett. 2008, 62, 600–602. [Google Scholar] [CrossRef]
- Madhukumar, K.; Rajendra Babu, K.; Ajith Prasad, K.; James, J.; Elias, T.; Padmanabhan, V.; Nair, C. Luminescence studies of rare earth doped calcium aluminate phosphors. Int. J. Mod. Phys. B 2007, 21, 1971–1980. [Google Scholar] [CrossRef]
- Singh, V.; Natarajan, V.; Zhu, J.-J. Luminescence and EPR investigations of Mn activated calcium aluminate prepared via combustion method. Opt. Mater. 2007, 30, 468–472. [Google Scholar] [CrossRef]
- Stefani, R.; Rodrigues, L.C.V.; Carvalho, C.A.A.d.; Felinto, M.C.F.d.C.; Brito, H.F.d.; Lastusaari, M.; Hölsä, J. Persistent luminescence of Eu2+ and Dy3+ doped barium aluminate (BaAl2O4: Eu2+, Dy3+) materials. Opt. Mater. 2009, 31, 1815–1818. [Google Scholar] [CrossRef]
- Jia, D.; Wang, X.-j.; Van der Kolk, E.; Yen, W. Site dependent thermoluminescence of long persistent phosphorescence of BaAl2O4: Ce3+. Opt. Commun. 2002, 204, 247–251. [Google Scholar] [CrossRef]
- Singh, V.; Chakradhar, R.; Rao, J.; Zhu, J.-J. Studies on red-emitting Cr3+ doped barium aluminate phosphor obtained by combustion process. Mater. Chem. Phys. 2008, 111, 143–148. [Google Scholar] [CrossRef]
- Vrankić, M.; Gržeta, B.; Lützenkirchen-Hecht, D.; Bosnar, S.; Sǎrić, A. Chromium environment within Cr-doped BaAl2O4: Correlation of X-ray diffraction and X-ray absorption spectroscopy investigations. Inorg. Chem. 2015, 54, 11127–11135. [Google Scholar] [CrossRef]
- Feilong, S.; Junwu, Z. Blue-green BaAl2O4: Eu2+, Dy3+ phosphors synthesized via combustion synthesis method assisted by microwave irradiation. J. Rare Earths 2011, 29, 326–329. [Google Scholar]
- Roh, H.-S.; Cho, I.-S.; An, J.-S.; Cho, C.M.; Noh, T.H.; Yim, D.K.; Kim, D.-W.; Hong, K.S. Enhanced photoluminescence property of Dy3+ co-doped BaAl2O4: Eu2+ green phosphors. Ceram. Int. 2012, 38, 443–447. [Google Scholar] [CrossRef]
- Bem, D.B.; Dejene, F.; Luyt, A.; Swart, H. Luminescence studies of a combustion-synthesized blue–green BaAlxOy: Eu2+, Dy3+ nanoparticles. Phys. B Condens. Matter 2012, 407, 1561–1565. [Google Scholar] [CrossRef]
- Ryu, H.; Bartwal, K. Preparation of crystalline fibres of codoped BaAl2O4: Eu2+: Cr3+. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2009, 44, 69–73. [Google Scholar] [CrossRef]
- Lephoto, M.; Ntwaeaborwa, O.; Pitale, S.S.; Swart, H.; Botha, J.; Mothudi, B.M. Synthesis and characterization of BaAl2O4: Eu2+ co-doped with different rare earth ions. Phys. B Condens. Matter 2012, 407, 1603–1606. [Google Scholar] [CrossRef]
- Kaur, J.; Jaykumar, B.; Dubey, V.; Shrivastava, R.; Suryanarayana, N. RETRACTED ARTICLE: Optical properties of rare earth-doped barium aluminate synthesized by different methods-A Review. Res. Chem. Intermed. 2015, 41, 2317–2343. [Google Scholar] [CrossRef]
- Zhang, L.W.; Wang, L.; Zhu, Y.F. Synthesis and performance of BaAl2O4 with a wide spectral range of optical absorption. Adv. Funct. Mater. 2007, 17, 3781–3790. [Google Scholar] [CrossRef]
- Yin, X.; Lin, H.; Zhang, D.; Hong, R.; Tao, C.; Han, Z. Effect of alumina addition on the microstructure and luminescence properties of BaAl2O4: Eu2+-Al2O3 green fluorescent composite ceramics fabricated by spark plasma sintering. Ceram. Int. 2020, 46, 3801–3810. [Google Scholar] [CrossRef]
- Rodrigues, L.; Stefani, R.; Brito, H.d.; Felinto, M.; Hölsä, J.; Lastusaari, M.; Laamanen, T.; Malkamäki, M. Thermoluminescence and synchrotron radiation studies on the persistent luminescence of BaAl2O4: Eu2+, Dy3+. J. Solid State Chem. 2010, 183, 2365–2371. [Google Scholar] [CrossRef]
- Zhang, N.-B.; Bai, C.-G.; LI, Z.-y. Preparation of BaAl2O4 by microwave sintering. Trans. Nonferrous Met. Soc. China 2010, 20, 2020–2025. [Google Scholar] [CrossRef]
- Liu, Y.L.; Tang, X.M.; Chen, X.; Lei, B.F.; Feng, D.X. Preparation of the phosphors BaAl2O4: Eu, RE (RE = Dy, Ho) by microwave heating technique and observation of long phosphorescence. Chin. Chem. Lett. 1999, 10, 709–712. [Google Scholar]
- Huang, A.; Yang, Z.; Yu, C.; Chai, Z.; Qiu, J.; Song, Z. Tunable and white light emission of a single-phased Ba2Y (BO3) 2Cl: Bi3+, Eu3+ phosphor by energy transfer for ultraviolet converted white LEDs. J. Phys. Chem. C 2017, 121, 5267–5276. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Gong, M. Dual-emitting Ce3+, Tb3+ co-doped LaOBr phosphor: Luminescence, energy transfer and ratiometric temperature sensing. Chem. Eng. J. 2017, 307, 291–299. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Yuan, P.; Wang, T.W.; Yin, Z.Q.; Lu, F.C. Effect of Sr and Ca substitution of Ba on the photoluminescence properties of the Eu2+ activated Ba2MgSi2O7 phosphor. Ceram. Int. 2020, 46, 1374–1382. [Google Scholar] [CrossRef]
- Dhananjaya, N.; Nagabhushana, H.; Nagabhushana, B.; Rudraswamy, B.; Shivakumara, C.; Narahari, K.; Chakradhar, R. Enhanced photoluminescence of Gd2O3: Eu3+ nanophosphors with alkali (M = Li+, Na+, K+) metal ion co-doping. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 8–14. [Google Scholar] [CrossRef]
- Liu, D.; Xu, X.; Du, Y.; Qin, X.; Zhang, Y.; Ma, C.; Wen, S.; Ren, W.; Goldys, E.M.; Piper, J.A. Three-dimensional controlled growth of monodisperse sub-50 nm heterogeneous nanocrystals. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Tao, K.; Tian, Q.; Sun, K. Interaction between Y3+ and oleate ions for the cubic-to-hexagonal phase transformation of NaYF4 nanocrystals. J. Phys. Chem. C 2012, 116, 1732–1739. [Google Scholar] [CrossRef]


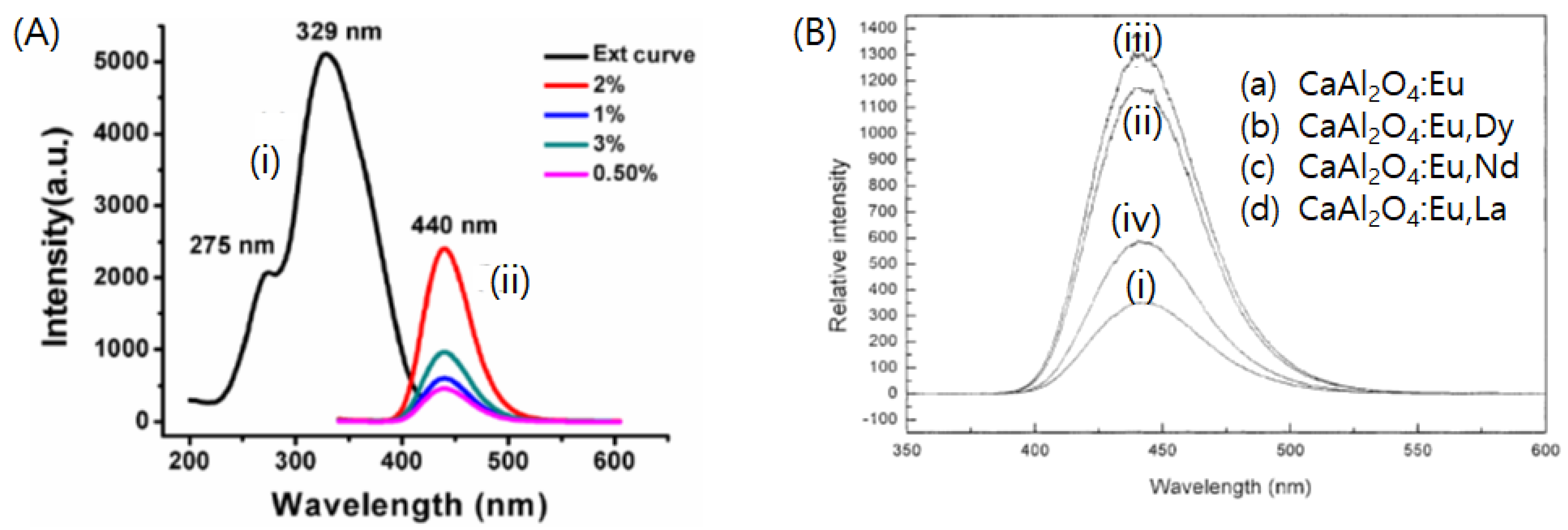
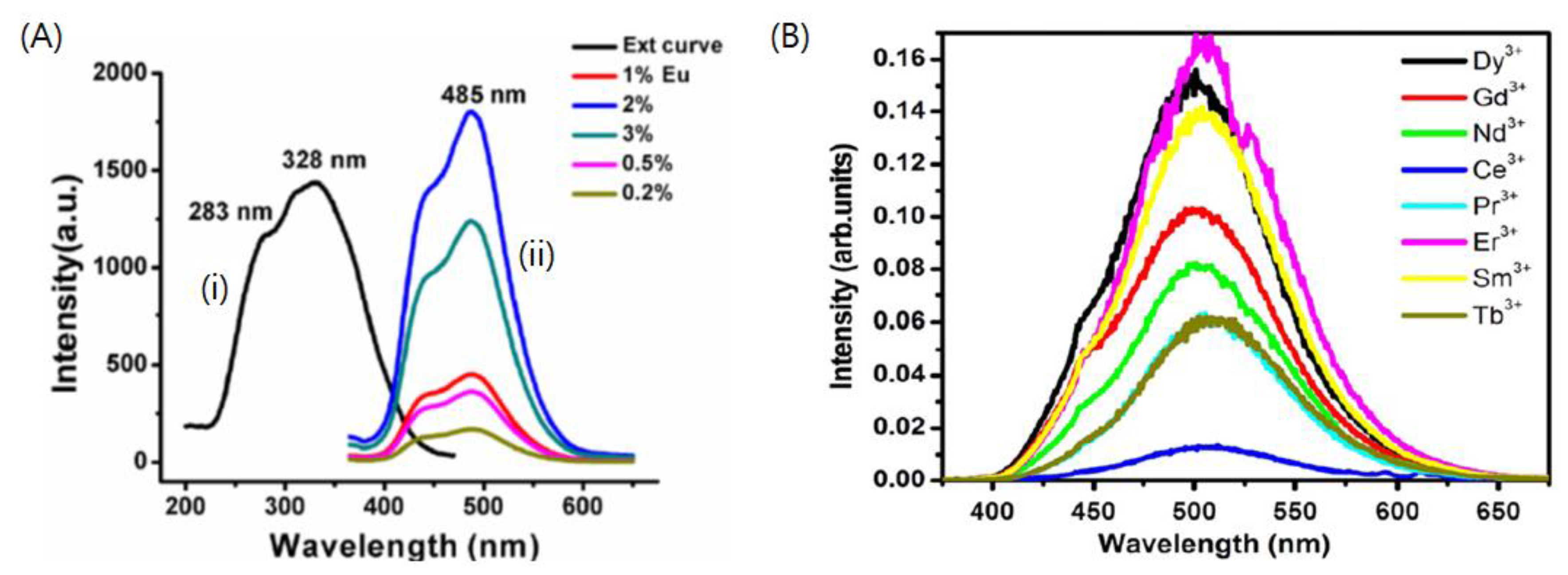

| Host Material | Activator | Co-Activator | Synthesis Method | Color or λ | Remarks | Ref |
|---|---|---|---|---|---|---|
| SrAl2O4 | Eu2+ | - | Solid-state reaction (1300 °C) | Green (λem = 515 nm(295 K) λem = 445 nm(20 K)) | It was found that the luminescent center is the same, but excitation processes are different at different temperatures. | [31] |
| Solid-state reaction (1250 °C) | Green (λem = 512 nm) | The position of the Eu 4f states showed the charge transfer transition. | [28] | |||
| Combustion method (600 °C) | Green (λex = 360 nm, λem = 513 nm) | The ratio of Eu2+ to Eu3+ is changed depending on the total concentration of Eu dopants, determining the luminescence color of the phosphors. | [32] | |||
| Combustion method using urea at 500 °C and calcinated at 1000 °C | Green (λem = 520 nm) | The luminescence mechanism and temperature dependence of bands intensities are discussed on the crystal field theory and the vibronic approach. | [7] | |||
| Dy3+ | - | Combustion method (600 °C) | λex = 356 nm, λem = 480 nm, 573 nm, 670 nm | The piezo-electricity was suggested to be responsible for producing mechanoluminescence in prepared phosphor. | [27] | |
| Tb3+ | Precursor route via the thermal decomposition of tartarate compounds | λem = 542 nm | They demonstrated that the precursor method via the thermal decomposition of multimetallic tartarate compounds is a quick, simple and inexpensive way for the preparation of alkaline-earth aluminate powder. | [33] | ||
| Eu2+ or Ce3+ | - | Combustion method (600 °C) | Eu2+:λex = 230, 350 nm, λem = 498 nm, Ce3+:λex = 266, 331 nm, λem = 371 nm | Experimental results matched with the predictions of Dorenbos’ model. | [30] | |
| Eu2+ or Nd3+ | - | Solid-state reaction (1000 °C) | - | The structures of the alkaline earth aluminates were systematically studied using a combination of synchrotron X-ray and neutron powder diffraction. | [13] | |
| Eu2+ | Dy3+ | Solid-state reaction (1300 °C) | Green (λex = 365 nm, λem = 520 nm) | They observed that Dy3+ ion creates the highly dense trapping level by acting as the hole-trap. | [5] | |
| Floating zone technique | Green (λem = 520 nm) | The intensities and the persistent times of the phosphorescences are found to depend on the growth atmosphere. | [34] | |||
| Laser-heated pedestal growth method | Green (λem = 520 nm) | It was found that multiple trapping centers are involved in the phosphorescence dynamic processes, which are responsible for the long persistence. | [35] | |||
| Solid-state reaction (900–1350 °C) | λem = 518 nm | The depth of Dy3+ trap levels is in the order of BaAl2O4 host > CaAl2O4 host > SrAl2O4 host. | [36] | |||
| Sol–gel method (900–1250 °C) | Green-blue (λem = 511 nm) | It was found that the single-phase SrAl2O4 was formed at 900 °C, which is 300 °C lower than the required temperature for the conventional solid-state reaction. | [37] | |||
| Combustion method (600 °C) | Yellow-green (λem = 516 nm) | They proposed that phosphor samples obtain a persistent luminescence with the aid of the energy transfer at the trap level. | [38] | |||
| Combustion method (500 °C) | λem = 528 nm | They found that the monoclinic crystal structures of both CaAl2O4 and SrAl2O4 are more appropriate in creating the traps, which is directly related to the long afterglow phenomena. | [39] | |||
| Laser synthesis | Green (λem = 520 nm) | This laser melting method is a promising route for the synthesis of ceramic phosphors. | [40] | |||
| Solid-state reaction (~1300 °C) | Green (λem = 520 nm) | A systematic investigation of the composition of phosphors, such as the concentrations of Eu2+,Dy3+, alkali metal, alkaline earth metal, Si ions. | [12] | |||
| Flame spray pyrolysis technique | Green (λem = 525 nm) | The flame spray pyrolysis technique was demonstrated to manufacture the rounded and spherical particles of SrAl2O4:Eu2+/Dy3+ phosphor without any problem. | [41] | |||
| Nd3+ | Combustion method (550 °C) | Green-blue (λex = 280 nm, λem = 612 nm) | Nd3+ trap levels can be thought of as the lanthanide element that causes long phosphorescence at room temperature. | [42] | ||
| Na+ | Solid-state reaction followed by ball-milling | Green (λem = 520 nm) | This report presents the factors affecting the luminescence properties of the Eu2+-, R3+-doped SrAl2O4. | [43] | ||
| Dy3+ or Nd3+ | Combustion method followed by annealing at 1150 °C | Green (Dy3+: λem = 515 nm, Nd3+: λem = 480 nm) | Eu2+ photoluminescence is observed to be shifted in a monoclinic/orthorhombic structure. | [44] | ||
| Dy3+,Tb3+ | Combustion method (600 °C) | Green (λem = 513 nm) | Compared with SrAl2O4:Eu2+,Dy3+ phosphor, the initial luminescence brightness improved, and the long afterglow time was prolonged in SrAl2O4:Eu2+, Tb3+ phosphor. | [45] | ||
| La3+–Lu3+, Y3+; excluding Pm3+ and Eu3+ | Solid-state reaction (1250–1300 °C) | Green (λem = 520 nm) | The co-doping by Dy3+ intensifies the luminescence by an order of magnitude, whereas the easily reducible rare earths, such as Sm3+ and Yb3+, suppressed both the afterglow and the thermoluminescence. | [46] | ||
| Sr4Al14O25 | Eu2+ | Dy3+, Er3+ | Solid-state reaction (1300 °C) | Green-blue (λem = 481, 492 nm, and 529 nm) | Appropriate Er3+ doping significantly enhanced the afterglow performance of the phosphors, but excessive Er3+ doping caused concentration quenching. | [47] |
| Sr4Al2O7 | Eu3+, Eu2+ | - | Solid-state reaction (1500 °C) | Red (λex = 450 nm, λem = 607 nm) | Sr4Al2O7 has higher emission intensity than Sr3Al2O6 due to the higher optimum doping concentration of Sr4Al2O7 phosphor. | [31] |
| Eu2+ | Ca2+ | Halide-assisted solid-state reaction (1450 °C) | Red (λem = 610 nm) | Partial substitution of Sr2+ by Ca2+ in Sr4Al2O7:Eu phosphors is found to be an efficient way to increase the proportion of longer wavelength emission and luminescence intensity. | [48] | |
| SrAl12O19 | Eu2+ | - | Combustion method (500 °C) | Red (λex = 341 nm,λem = 397 nm) | Europium ions were found to be present both in divalent as well as trivalent oxidation states in the sample, and Eu2+ was observed as the dominant luminescent site. | [49] |
| SrAl2O4, Sr4Al14O25 | Eu2+ | Dy3+ | Solid-state reaction followed by ball-milling | Green (SrAl2O4), Blue (Sr4Al14O25) | The significant loss of luminescence was observed below 2 μm average crystallite size, and performance could be partially restored by reductive annealing above 1000 °C. | [50] |
| Sr3Al2O6, SrAl2O4, Sr4Al14O25 | Eu2+ | - | Solid-state reaction (1350 °C) | Sr3Al2O6:Eu2+: λem = 510 nm Sr4Al14O25:Eu2+: λem = 483 nm | The influences of Al/Sr ratio, sintering temperature, the doping concentration of europium ions on structural transformation and luminescent properties of the phosphors were studied. | [51] |
| SrAl2O4, Sr4Al4O10, Sr3Al2O6 | Ce3+ | - | Sol–gel synthesis (700–1200 °C) | SrAl2O4:Ce: λex = 575~700 nm Sr3Al2O6:Ce: λex = 585~675 nm Sr4Al4O10:Ce: λex = 615 nm | The optical reflectance spectra clearly showed the influence of the strontium aluminate matrix on the optical properties of the synthesized phosphors. | [52] |
| SrAl2O4, SrAl4O7, SrAl12O19, Sr4Al14O25 | Eu2+ | - | Solid-state reaction (SrAl12O19:1300 °C, Sr4Al14O25:1400 °C, SrAl2O4:1350 °C) Citric acid method (SrAl4O7: 1050 °C) | SrAl12O19: λem = 397 nm SrAl4O7: λem = 470 nm Sr4Al14O25: λem = 490 nm | The Eu2+ emission spectra in the other aluminates showed the trend that the Eu2+ emission shifts to longer wavelengths with an increasing Sr/Al ratio. | [53] |
| Host Material | Activator | Co-Activator | Synthesis Method | Color or λ | Remarks | Ref |
|---|---|---|---|---|---|---|
| CaAl2O4 | Eu2+ | - | Solid-state reaction (1250–1300 °C) | Blue (λem = 440 nm) | The new mechanism was proposed, which involves the excited state absorption of two 530 nm photons via deep traps followed by trapping of electrons in shallow traps. | [75] |
| - | Solid-state reaction (1300 °C) | Blue (λem = 442 nm) | Good morphology and the best luminous intensity could be gained when H3BO3 mass ratio was 0.5 wt%. | [78] | ||
| Tb3+ | Precursor route via the thermal decomposition of tartarate compounds | λem = 542 nm | They demonstrated that the precursor method via the thermal decomposition of multimetallic tartarate compounds is a quick, simple and inexpensive way for the preparation of alkaline-earth aluminate powder. | [33] | ||
| Pr2+ | - | Sol–gel method | λem = 390 nm, 520 nm, 790 nm | The interlinked small granular structured particles finally formed bigger particles. | [9] | |
| Eu2+ or Nd3+ | - | Solid-state reaction (1000 °C) | - | A systematic study of the structures of the alkaline earth aluminates using a combination of synchrotron X-ray and neutron powder diffraction. | [13] | |
| La3+ or Tb3+ | - | Sol–gel method | Blue-green λem = 395 nm, 535 nm | Emission peak position is not altered by doping with La3+, Tb3+, but variation in the intensity is observed. | [79] | |
| Eu2+ or Ce3+ | - | Combustion method (600 °C) | Eu2+:λex = 275, 329 nm, λem = 440 nm Ce3+:λex = 247, 300 nm, λem = 370 nm | Experimental results matched with the predictions of Dorenbos’ model. | [30] | |
| Eu2+ | Nd3+ | Floating zone technique | Blue (λem = 450 nm) | The intensities and the persistent times of the phosphorescences are found to depend on the growth atmosphere. | [34] | |
| Laser-heated pedestal growth method | Blue (λem = 445 nm) | It was found that multiple trapping centers are involved in the phosphorescence dynamic processes, which is responsible for the long persistence. | [35] | |||
| Combustion method | Blue (λem = 440 nm) | Eu2+, Nd3+ co-doped calcium aluminate showed bright phosphorescence with a long duration. | [80] | |||
| Solid-state reaction (1300 °C) | Blue (λem = 442 nm) | The composition of the activator Eu2+ and the co-activator Nd3+, the doping conditions with alkaline earth metals, alkali metals, and Si were optimized. | [81] | |||
| Combustion method (550 °C) | Blue (λex = 355 nm, λem = 492 nm) | Nd3+ trap levels can be thought of as the lanthanide element that causes long phosphorescence at room temperature. | [42] | |||
| Dy3+ | Solid-state reaction (900–1350 °C) | λem = 445 nm | The depth of Dy3+ trap levels is in the order of BaAl2O4 host > CaAl2O4 host > SrAl2O4 host. | [36] | ||
| Combustion method (500 °C) | λem = 449 nm | They found that the monoclinic crystal structures of both CaAl2O4 and SrAl2O4 are more appropriate in creating the traps, which is directly related to the long afterglow phenomena. | [39] | |||
| Na+ | Solid-state reaction followed by ball-milling | λem = 440 nm, | This report presents the factors affecting the luminescence properties of the Eu2+-, R3+-doped SrAl2O4. | [43] | ||
| La3+ | Combustion method (600 °C) | blue-purple (λem = 440 nm) | They proposed that phosphor samples obtain a persistent luminescence with the aid of the energy transfer at the trap level. | [38] | ||
| Dy3+, Nd3+, La3+ | Solid-state reaction (1380 °C) | Blue (λem = 440 nm) | Both initial brightness and persistent afterglow time of CaAl2O4: Eu2+, Nd3+ is better than those of CaAl2O4: Eu2+,Dy3+, and CaAl2O4: Eu2+, La3+. | [82] | ||
| La3+–Lu3+, Y3+; except Pm3+, Eu3+ | Solid-state reaction (1250–1300 °C) | Green (λem = 440 nm) | The co-doping by Dy3+ intensifies the luminescence by an order of magnitude, whereas the easily reducible rare earths, such as Sm3+ and Yb3+, suppressed both the afterglow and the thermoluminescence. | [46] |
| Host Material | Activator | Co-Activator | Synthesis Method | Color or λ | Remarks | Ref |
|---|---|---|---|---|---|---|
| BaAl2O4 | Eu2+ | - | Solid-state reaction (1400 °C) | λex = 340 nm, λem = 498 nm | The Eu3+ reduction in BaAl2O4:Eu2+ prepared in the air could be explained with the charge compensation model. | [8] |
| Ce3+ | - | Solid-state reaction (900–1350 °C) | λex = 357 nm, 335 nm λem = 450 nm, 402 nm | Site-selective thermoluminescence spectra showed that traps were close to the corresponding Ce3+ ion. | [88] | |
| Cr3+ | - | Combustion method (500 °C) | Red (λem = 705 nm) | The site symmetry of Cr3+ ion in this phosphor is responsible for a distorted octahedron. | [89] | |
| Hydrothermal route followed by a thermal treatment | - | The dopant Cr3+ cations increased lattice strain and disturbed the crystallites to grow by acting as defects in the barium aluminate structure. | [90] | |||
| Eu2+ or Nd3+ | - | Solid-state reaction (1000 °C) | - | A systematic study of the structures of the alkaline earth aluminates using a combination of synchrotron X-ray and neutron powder diffraction. | [13] | |
| Eu2+ or Ce3+ | - | Combustion method (600 °C) | Eu2+:λex = 270, 328, 397 nm, λem = 485 nm, Ce3+:λex = 246, 292, 308 nm, λem = 386 nm | Experimental results matched well with the predictions of Dorenbos’ model. | [30] | |
| Eu2+ | Dy3+ | Solid-state reaction (900–1350 °C) | λem = 496 nm | The depth of Dy3+ trap levels is in the order of BaAl2O4 host > CaAl2O4 host > SrAl2O4 host. | [36] | |
| Solid-state reaction (700–1500 °C) | Green-blue (λem = 500 nm) | The dopant (Eu2+) and co-dopant (Dy3+) concentrations affect the crystallinity and luminescence properties of the materials. | [87] | |||
| Combustion method (500 °C) | λem = 450 nm | The hexagonal structure of BaAl2O4 can only produce shallow traps, resulting in a short afterglow. | [39] | |||
| Combustion method (400–600 °C) or Solid-state reaction (1500 °C) | λem = 505 nm | They found that the method of preparation has a significant effect on the defect structure of the materials. | [87] | |||
| Combustion synthesis method assisted by microwave irradiation | Blue-green (λem = 496 nm) | The surface of the BaAl2O4:Eu2+,Dy3+ powder samples showed lots of voids and pores. | [91] | |||
| Solid-state reaction (1300 °C) | Green (λex = 355 nm,λem = 499 nm) | The photoluminescence efficiency increased with increasing Eu2+ concentration until 3 mol% then decreased at higher concentrations due to the concentration quenching effect. | [92] | |||
| Combustion method (500 °C) | Blue–green (λex = 340 nm, λem = 505 nm) | The powders exhibited high initial brightness luminescence with subdued long afterglow characteristics. | [93] | |||
| Nd3+ | Combustion method (600 °C) | Green-blue (λem = 500 nm) | They proposed that phosphor samples obtain a lifetime of persistent luminescence with the aid of the energy transfer at the trap level. | [38] | ||
| Combustion method (550 °C) | Blue (λex = 355 nm, λem = 495 nm) | Nd3+ trap levels can be thought of as the lanthanide element that causes long phosphorescence at room temperature. | [42] | |||
| Cr3+ | Solid-state reaction (1300 °C) | - | Fibre shaped morphology of the grown material was formed with sharp surface morphology like single crystals. | [94] | ||
| Dy3+, Nd3+, Gd3+, Sm3+, Ce3+, Er3+, Pr3+ and Tb3+ | Combustion method (600 °C) | Blue-green (λem = 500 nm) | The highest intensity was observed from Er3+ co-doping, whereas the longest afterglow was observed from Nd3+ followed by Dy3+ co-doping. | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D. Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence. Nanomaterials 2021, 11, 723. https://doi.org/10.3390/nano11030723
Kim D. Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence. Nanomaterials. 2021; 11(3):723. https://doi.org/10.3390/nano11030723
Chicago/Turabian StyleKim, Doory. 2021. "Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence" Nanomaterials 11, no. 3: 723. https://doi.org/10.3390/nano11030723





