HKUST-1@IL-Li Solid-state Electrolyte with 3D Ionic Channels and Enhanced Fast Li+ Transport for Lithium Metal Batteries at High Temperature
Abstract
1. Introduction
2. Experimental Section
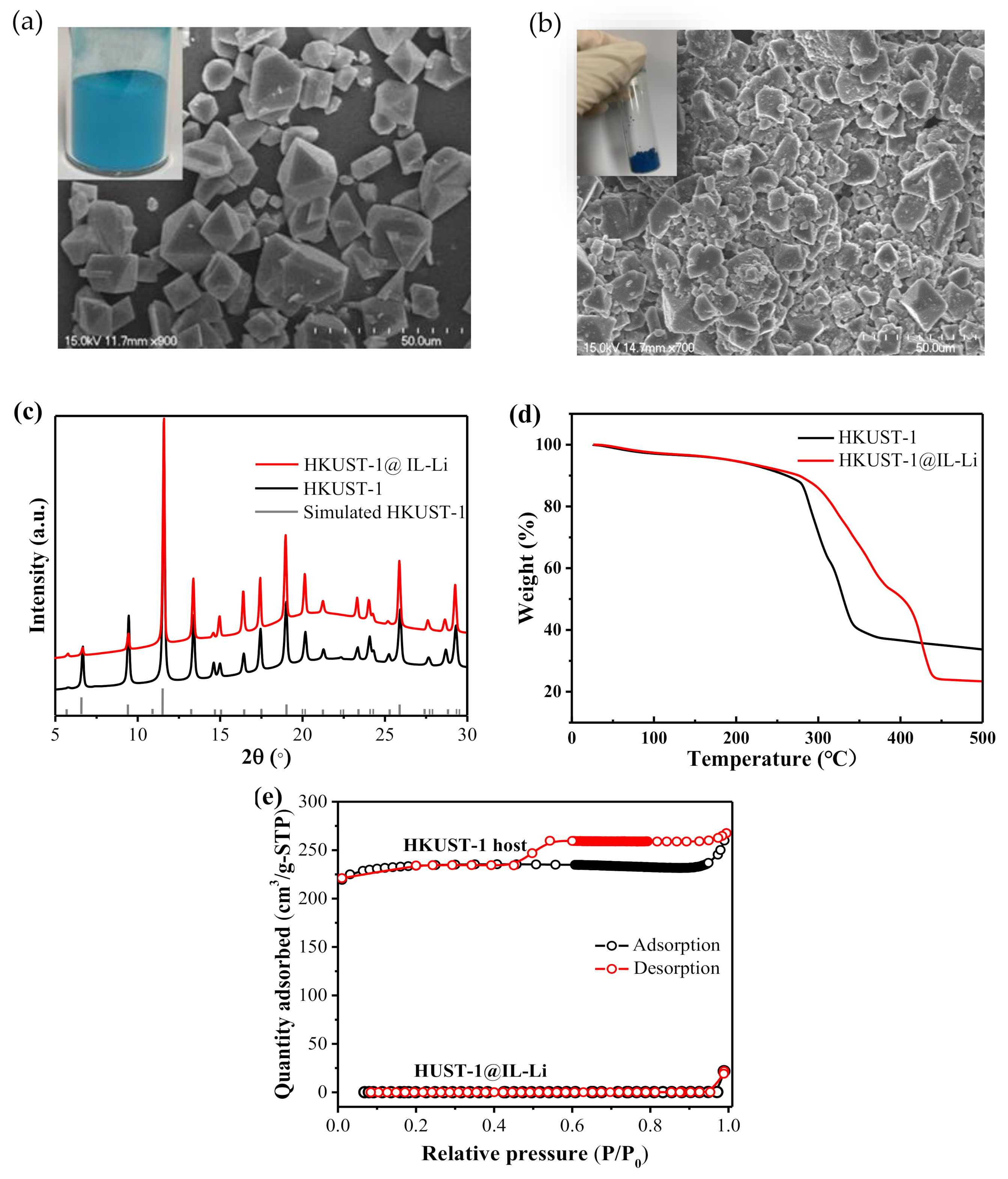
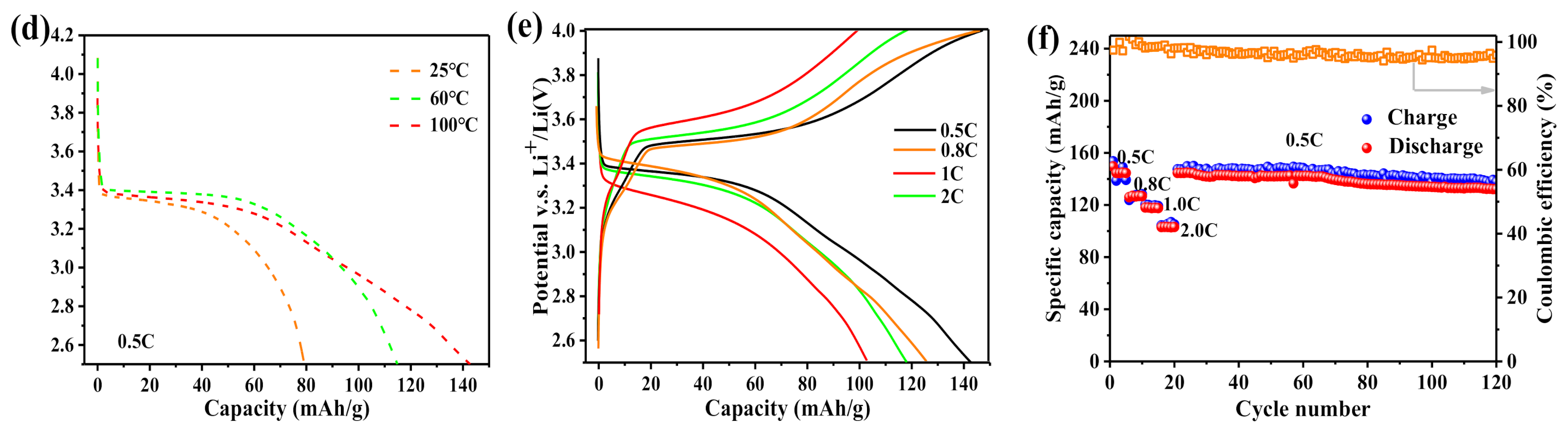
3. Results and Discussion

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Lin, X.; Salari, M.; Arava, L.M.; Ajayan, P.M.; Grinstaff, M.W. High temperature electrical energy storage: Advances, challenges, and frontiers. Chem. Soc. Rev. 2016, 45, 5848–5887. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Li, J.Y.; Zhang, C.H.; Guo, Y.G. Passivation of Lithium metal anode via hybrid Ionic liquid electrolyte toward stable Li plating/stripping. Adv. Sci. 2017, 4, 1600400. [Google Scholar] [CrossRef]
- Plylahan, N.; Kerner, M.; Lim, D.-H.; Matic, A.; Johansson, P. Ionic liquid and hybrid ionic liquid/organic electrolytes for high temperature lithium-ion battery application. Electrochim. Acta 2016, 216, 24–34. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Lee, H.; Toprakci, M.O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Liu, B.; Fu, K.; Gong, Y.; Yang, C.; Yao, Y.; Wang, Y.; Wang, C.; Kuang, Y.; Pastel, G.; Xie, H.; et al. Rapid thermal annealing of cathode-garnet interface toward high-temperature solid state batteries. Nano Lett. 2017, 17, 4917–4923. [Google Scholar] [CrossRef]
- Xing, H.; Liao, C.; Yang, Q.; Veith, G.; Guo, B.; Sun, X.; Ren, Q.; Hu, Y.; Dai, S. Ambient lithium–SO2 batteries with ionic liquids as electrolytes. Angew. Chem. 2014, 126, 2131–2135. [Google Scholar] [CrossRef]
- Del Sesto, R.E.; McCleskey, T.M.; Macomber, C.; Ott, K.C.; Koppisch, A.T.; Baker, G.A.; Burrell, A.K. Limited thermal stability of imidazolium and pyrrolidinium ionic liquids. Thermochim. Acta 2009, 491, 118–120. [Google Scholar] [CrossRef]
- Del Sestoa, R.E.; McCleskeya, T.M.; Macombera, C.; Ott, K.C.; Koppischb, A.T.; Bakerc, G.A.; Burrell, A.K. Z-scheme water splitting under visible light irradiation over powdered metal-complex/semiconductor hybrid photocatalysts mediated by reduced graphene oxide. J. Mater. Chem. A 2015, 3, 13283–13290. [Google Scholar]
- Miner, E.M.; Park, S.S.; Dinca, M. High Li+ and Mg2+ conductivity in a Cu-Azolate metal–organic framework. J. Am. Chem. Soc. 2019, 141, 4422–4427. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zheng, S.S.; Xue, H.G.; Pang, H. Metal-organic frameworks/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Xu, Q.X.; Zhang, X.Q.; Zeng, S.J.; Bai, L.; Zhang, S.J. Ionic liquid incorporated metal organic framework for high ionic conductivity over extended temperature range. ACS Sustain. Chem. Eng. 2019, 7, 7892–7899. [Google Scholar] [CrossRef]
- Shen, L.; Wu, H.B.; Liu, F.; Brosmer, J.L.; Shen, G.R.; Wang, X.F.; Zink, J.I.; Xiao, Q.F.; Cai, M.; Wang, G.; et al. Creating lithium-ion electrolytes with biomimetic ionic channels in metal–organic frameworks. Adv. Mater. 2018, 30, 1707476. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as proton conductors—challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, Z.Q.; Ying, W.; Chen, L.P.; Liu, Y.Z.; Wang, X.B.; Jiang, Z.J.; Chen, B.L.; Peng, X.S. A DNA-threaded ZIF-8 membrane with high proton conductivity and low methanol permeability. Adv. Mater. 2018, 30, 1705155. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Tulchinsky, Y.; Dinca, M. Single-Ion Li+, Na+, and Mg2+ solid electrolytes supported by a mesoporous anionic Cu–Azolate metal–organic framework. J. Am. Chem. Soc. 2017, 139, 13260–13263. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Wang, Z.N.; Wang, S.; Wang, A.L.; Liu, X.; Chen, J.; Zeng, Q.H.; Zhang, L.; Liu, W.; Zhang, L.Y. Covalently linked metal-organic framework (MOF)-polymer all-solid-state electrolyte membranes for room temperature high performance lithium batteries. J. Mater. Chem. A 2018, 6, 17227–17234. [Google Scholar] [CrossRef]
- Bai, S.Y.; Sun, Y.; Yi, J.; He, Y.B.; Qiao, Y.; Zhou, H.S. High-power Li-metal anode enabled by metal-organic framework modified electrolyte. Joule 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tu, Z.; Archer, L.A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 2014, 13, 961–969. [Google Scholar] [CrossRef]
- Fujie, K.; Yamada, T.; Ikeda, R.; Kitagawa, H. Introduction of an ionic liquid into the micropores of a metal–organic framework and its anomalous phase behavior. Angew. Chem. 2014, 126, 11484–11487. [Google Scholar] [CrossRef]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, W.; Payzant, E.A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef]
- Kimura, T.; Kato, A.; Hotehama, C.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Preparation and characterization of lithium ion conductive Li3SbS4 glass and glass-ceramic electrolytes. Solid State Ion. 2019, 333, 45–49. [Google Scholar] [CrossRef]
- Mo, S.; Lu, P.; Ding, F.; Xu, Z.; Liu, J.; Liu, X.; Xu, Q. High-temperature performance of all-solid-state battery assembled with 95(0.7Li2S-0.3P2S5)-5Li3PO4 glass electrolyte. Solid State Ion. 2016, 296, 37–41. [Google Scholar] [CrossRef]
- Wang, Y.; Richards, W.D.; Bo, S.-H.; Miara, L.J.; Ceder, G. Computational prediction and evaluation of solid-state sodium superionic conductors Na7P3X11 (X. = O., S, Se). Chem. Mater. 2017, 29, 7475–7482. [Google Scholar] [CrossRef]
- Matsuo, M.; Remhof, A.; Martelli, P.; Caputo, R.; Ernst, M.; Miura, Y.; Sato, T.; Oguchi, H.; Maekawa, H.; Takamura, H.; et al. Complex hydrides with (BH4)− and (NH2)− anions as new lithium fast-ion conductors. J. Am. Chem. Soc. 2009, 131, 16389–16391. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, V.; Weppner, W. Li6ALa2Nb2O12 (A=Ca, Sr, Ba): A new class of fast lithium ion conductors with garnet-like structure. J. Am. Ceram. Soc. 2005, 88, 411–418. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Lithium ion conductivity of Li5+xBaxLa3−xTa2O12 (x = 0–2) with garnet-related structure in dependence of the barium content. Ionics 2007, 13, 195–203. [Google Scholar] [CrossRef]
- Sheng, O.; Jin, C.; Luo, J.; Yuan, H.; Fang, C.; Huang, H.; Gan, Y.; Zhang, J.; Xia, Y.; Liang, C.; et al. Ionic conductivity promotion of polymer electrolyte with ionic liquid grafted oxides for all-solid-state lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 12934–12942. [Google Scholar] [CrossRef]
- Vasudevan, S.; Fullerton-Shirey, S.K. Effect of nanoparticle shape on the electrical and thermal properties of solid polymer electrolytes. J. Phys. Chem. C 2019, 123, 10720–10726. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.J.; Lee, J.C.; Braun, P.V.; Park, H.S. Extremely durable, flexible supercapacitors with greatly improved performance at high temperatures. ACS Nano 2015, 9, 8569–8577. [Google Scholar] [CrossRef]
- Qin, J.; Lan, Q.; Liu, N.; Zhao, Y.; Song, Z.; Zhan, H. A metal-free battery working at −80 °C. Energy Storage Mater. 2020, 26, 585–592. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.X.; Fan, L.Z.; Nan, C.W.; Zhang, Q. Intercalated electrolyte with high transference number for dendrite-free solid-state lithium batteries. Adv. Funct. Mater. 2019, 29, 1901047. [Google Scholar] [CrossRef]
- Georén, P.; Lindbergh, G. Characterization and modelling of the transport properties in lithium battery gel electrolytes: Part, I. the binary electrolyte PC/LiClO4. Electrochim. Acta 2004, 49, 3497–3505. [Google Scholar] [CrossRef]
- Van Humbeck, J.F.; Aubrey, M.L.; Alsbaiee, A.; Ameloot, R.; Coates, G.W.; Dichtel, W.R.; Long, J.R. Tetraarylborate polymer networks as single-ion conducting solid electrolytes. Chem. Sci. 2015, 6, 5499–5505. [Google Scholar] [CrossRef]
- Ge, B.C.; Wang, J.; Sun, Y.; Guo, J.X.; Fernandez, C.; Peng, Q.M. Heterojunction-composited architecture for Li−O2 batteries with low overpotential and long-term cyclability. ACS Appl. Energy Mater. 2020, 3, 3789–3797. [Google Scholar] [CrossRef]
- Zou, G.Z.; Guo, J.X.; Liu, X.Y.; Zhang, Q.R.; Huang, G.; Fernandez, C.; Peng, Q.M. Hydrogenated core-shell MAX@K2Ti8O17 pseudocapacitance with ultra-fast sodium storage and long-term cycling. Adv. Energy Mater. 2017, 7, 1700700. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Tan, R.; Wang, H.B.; Yang, L.Y.; Hu, J.T.; Chen, H.B.; Pan, F. A metal-organic-framework-based electrolyte with nanowetted interfaces for high-energy-density solid-state lithium battery. Adv. Mater. 2018, 30, 1704436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Wang, Z.J.; Yang, L.Y.; Wang, H.B.; Song, Y.L.; Han, L.; Yang, K.; Hu, J.T.; Chen, H.B.; Pan, F. Boosting interfacial Li+ transport with a MOF-based ionic conductor for solid-state batteries. Nano Energy 2018, 49, 580–587. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. A new approach toward improved low temperature performance of Li-ion battery. Electrochem. Commun. 2002, 4, 928–932. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Kalaga, K.; Gullapalli, H.; Babu, G.; Reddy, A.L.M.; Ajayan, P.M. Hexagonal boron nitride-based electrolyte composite for Li-Ion battery operation from room temperature to 150 °C. Adv. Energy Mater. 2016, 6, 1600218. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Han, X.; Gong, Y.; Fu, K.K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2017, 16, 572–579. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Chen, T.; Song, S.; Li, Y.; Bae, J. HKUST-1@IL-Li Solid-state Electrolyte with 3D Ionic Channels and Enhanced Fast Li+ Transport for Lithium Metal Batteries at High Temperature. Nanomaterials 2021, 11, 736. https://doi.org/10.3390/nano11030736
Li M, Chen T, Song S, Li Y, Bae J. HKUST-1@IL-Li Solid-state Electrolyte with 3D Ionic Channels and Enhanced Fast Li+ Transport for Lithium Metal Batteries at High Temperature. Nanomaterials. 2021; 11(3):736. https://doi.org/10.3390/nano11030736
Chicago/Turabian StyleLi, Man, Tao Chen, Seunghyun Song, Yang Li, and Joonho Bae. 2021. "HKUST-1@IL-Li Solid-state Electrolyte with 3D Ionic Channels and Enhanced Fast Li+ Transport for Lithium Metal Batteries at High Temperature" Nanomaterials 11, no. 3: 736. https://doi.org/10.3390/nano11030736
APA StyleLi, M., Chen, T., Song, S., Li, Y., & Bae, J. (2021). HKUST-1@IL-Li Solid-state Electrolyte with 3D Ionic Channels and Enhanced Fast Li+ Transport for Lithium Metal Batteries at High Temperature. Nanomaterials, 11(3), 736. https://doi.org/10.3390/nano11030736






