An Overview of Recent Advances in the Synthesis and Applications of the Transition Metal Carbide Nanomaterials
Abstract
:1. Introduction
2. Synthesis Methods
2.1. Synthesis of 2D Carbides
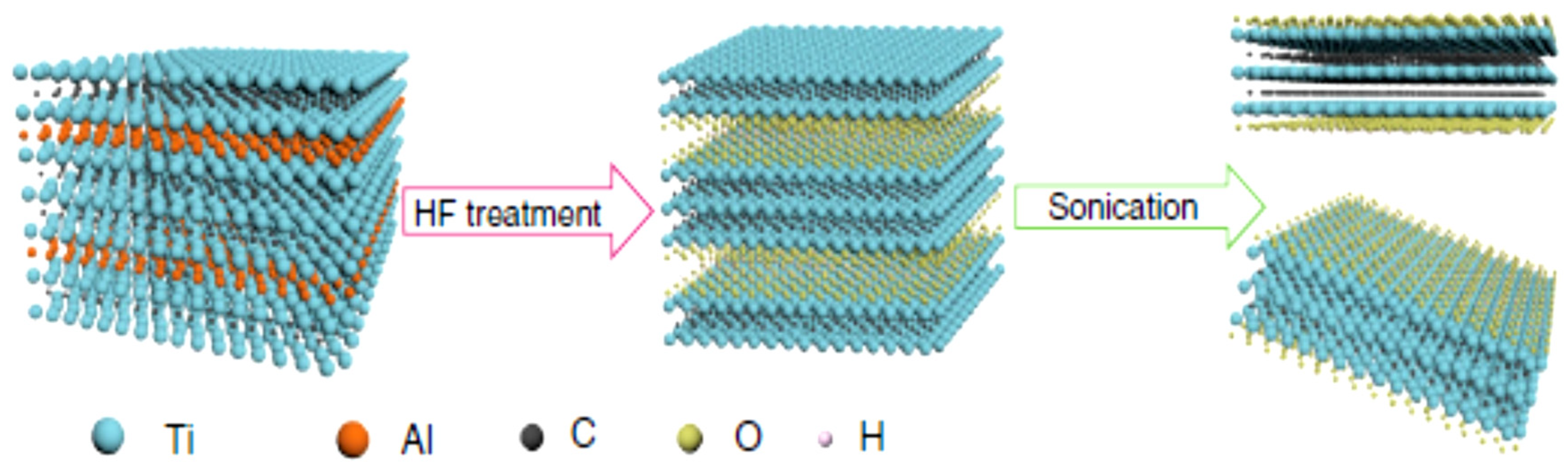
2.1.1. Acid Etching Method
2.1.2. Etching with Molten Salts
2.1.3. Acid Etching and Intercalation
2.1.4. Electrochemical Etching
2.2. Synthesis of TMCs Nano-Composites
2.2.1. One-Pot Solvothermal Method
2.2.2. Electrospinning Technique
2.2.3. Simple Stirring to Obtain Ag@Ti3C2Tx
2.3. Synthesis of TMCs Nano-Particles
2.3.1. Carbothermal Method
Direct Carburization of Metal
Carburization of Metal Plates
Carburization of Metal Wires
2.3.2. Solid-State Reaction Method
2.3.3. Liquid-Phase Synthesis Method
2.3.4. Spin Coating of Tungsten Nanoparticles
2.3.5. Sol-Gel Method
2.3.5.1. Urea Glass Route
2.3.5.2. Biopolymer Route
2.3.5.3. Inorganic Precursors
2.3.5.4. Organic Precursors
2.3.6. Solvothermal Synthesis of TMCs
2.3.7. TMCs by Thermal Reduction
2.3.8. TMCs by Direct Element Combination
2.3.9. TMCs by the Laser Ablation Method
2.4. Bimetallic Carbides
2.4.1. Thermal Decomposition Method
2.4.2. Carbothermal Hydrogen Reduction Method
2.5. Synthesis of Carbide Films
Epitaxial Growth at Low Temperature
- Co-evaporation of metal and C60; and
- Evaporation of C60 and magnetron sputtering of metal under Ar pressure.
2.6. Synthesis of Carbide Nano-Powders
2.6.1. Aqueous Synthesis
2.6.2. In-Situ Synthesis
2.6.3. Sol-Gel Using Inorganic Precursors
2.7. Synthesis of Carbide Nano-Fibers
2.7.1. Thermal Decomposition Method
3. Properties
3.1. Hydrolysis in Colloidal Solutions
3.2. Volumetric Changes in Ionic Liquids
3.3. Tunable Electronic Properties
3.4. Diffusion between Binary Carbides
3.5. Mechanical Properties
3.6. Magnetic Properties
3.7. Superconductivity
3.8. Electromagnetic Interference Shielding Property
4. Applications
4.1. Water Splitting
4.1.1. Hydrogen Evolution Reaction
4.1.2. Oxygen Evolution Reaction
4.2. Energy Storage
4.2.1. Batteries
4.2.2. Supercapacitors
4.3. As a Catalyst
4.3.1. Photocatalyst for Hydrogen Production
4.3.2. Carbon Monoxide Hydrogenation
4.3.3. Carbon Nano-Coils Synthesis
4.3.4. Catalytic Enhanced Hydro-Deoxygenation Selectivity
4.4. Environmental/Medicinal Application
4.4.1. CO2 Capture
4.4.2. Biosensors for Pesticides
4.4.3. Antipollution
4.4.4. Ecotoxicity
4.4.5. Phytotoxicity
4.4.6. Anti-Bacterial Activity
4.4.7. Chemiluminescence Biosensor
4.4.8. Cancer Therapeutics and Diagnostics
4.5. MXene in Memory Devices
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coville, N.J.; Mhlanga, S.D.; Nxumalo, E.N.; Shaikjee, A. A review of shaped carbon nanomaterials. S. Afr. J Sci. 2011, 107, 1–15. [Google Scholar] [CrossRef]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Stottlemyer, A.L.; Kelly, T.G.; Meng, Q.; Chen, J.G. Reactions of oxygen-containing molecules on transition metal carbides: Surface science insight into potential applications in catalysis and electrocatalysis. Surf. Sci. Rep. 2012, 67, 201–232. [Google Scholar] [CrossRef]
- Silveri, F.; Quesne, M.G.; Roldan, A.; De Leeuw, N.H.; Catlow, C.R.A. Hydrogen adsorption on transition metal carbides: A DFT study. Phys. Chem. Chem. Phys. 2019, 21, 5335–5343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Jiao, T.; Xing, R.; Zou, G.; Zhou, J.; Zhang, L.; Peng, Q. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene–2D layered electrode materials for energy storage. Prog. Nat. Sci. 2018, 28, 133–147. [Google Scholar] [CrossRef]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Pinto, D.; Pivak, Y.; Van Omme, J.T.; May, S.J.; Gogotsi, Y.; Taheri, M.L. Control of MXenes’ electronic properties through termination and intercalation. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: A review. RSC Adv. 2018, 8, 19895–19905. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Halim, J.; Persson, I.; Moon, E.J.; Kühne, P.; Darakchieva, V.; Persson, P.O.Å.; Eklund, P.; Rosen, J.; Barsoum, M.W. Electronic and optical characterization of 2D Ti2C and Nb2C (MXene) thin films. J. Phys. Condens. Matter 2019, 31, 165301. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Niu, T. Old materials with new properties II: The metal carbides. Nano Today 2018, 18, 12–14. [Google Scholar] [CrossRef]
- Lin, H.; Chen, L.; Lu, X.; Yao, H.; Chen, Y.; Shi, J. Two-dimensional titanium carbide MXenes as efficient non-noble metal electrocatalysts for oxygen reduction reaction. Sci. China Mater. 2018, 62, 662–670. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Liao, C.; Hafez, A.M.; Zhu, H.; Wu, S. Two-dimensional MXenes for energy storage. Chem. Eng. J. 2018, 338, 27–45. [Google Scholar] [CrossRef]
- Patterson, P.M.; Das, T.K.; Davis, B.H. Carbon monoxide hydrogenation over molybdenum and tungsten carbides. Appl. Catal. A General 2003, 251, 449–455. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Ma, L.; Gao, J.; Jiang, Y. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem. Eng. J. 2017, 128, 243–249. [Google Scholar] [CrossRef]
- Kim, S.K.; Qiu, Y.; Zhang, Y.-J.; Hurt, R.; Peterson, A. Nanocomposites of transition-metal carbides on reduced graphite oxide as catalysts for the hydrogen evolution reaction. Appl. Catal. B Environ. 2018, 235, 36–44. [Google Scholar] [CrossRef]
- Chouzier, S.; Afanasiev, P.; Vrinat, M.; Cseri, T.; Roy-Auberger, M. One-step synthesis of dispersed bimetallic carbides and nitrides from transition metals hexamethylenetetramine complexes. J. Solid State Chem. 2006, 179, 3314–3323. [Google Scholar] [CrossRef]
- Mahajan, M.; Rajpoot, S.; Pandey, O. In-situ synthesis of chromium carbide (Cr3C2) nanopowders by chemical-reduction route. Int. J. Refract. Met. Hard Mater. 2015, 50, 113–119. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, H.; Liu, S.; Shen, J.; Song, W.; Chen, J. Low temperature synthesis of chromium carbide (Cr3C2) nanopowders by a novel precursor method. Int. J. Refract. Met. Hard Mater. 2015, 48, 46–50. [Google Scholar] [CrossRef]
- Lei, M.; Zhao, H.; Yang, H.; Song, B.; Cao, L.; Li, P.; Tang, W. Syntheses of metal nitrides, metal carbides and rare-earth metal dioxymonocarbodiimides from metal oxides and dicyandiamide. J. Alloys Compd. 2008, 460, 130–137. [Google Scholar] [CrossRef]
- Han, N.; Yang, K.R.; Lu, Z.; Li, Y.; Xu, W.; Gao, T.; Cai, Z.; Zhang, Y.; Batista, V.S.; Liu, W.; et al. Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.-Y.; Duan, Y.; Gao, M.-R.; Lang, C.-C.; Zheng, Y.-R.; Yu, S.-H. A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 2017, 8, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Ling, C.; Shi, L.; Ouyang, Y.; Chen, Q.; Wang, J. Transition Metal-Promoted V2CO2 (MXenes): A New and Highly Active Catalyst for Hydrogen Evolution Reaction. Adv. Sci. 2016, 3, 1600180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Wang, Z.; Yang, Q.; Tan, L.; Dong, L.; Dong, M. Ultrathin Ti3C2Tx (MXene) Nanosheet-Wrapped NiSe2 Octahedral Crystal for Enhanced Supercapacitor Performance and Synergetic Electrocatalytic Water Splitting. Nano-Micro Lett. 2019, 11, 31. [Google Scholar] [CrossRef] [Green Version]
- Tomás-García, A.L.; Jensen, J.O.; Bjerrum, N.J.; Li, Q. Hydrogen evolution activity and electrochemical stability of selected transition metal carbides in concentrated phosphoric acid. Electrochim. Acta 2014, 137, 639–646. [Google Scholar] [CrossRef]
- Meyer, S.; Nikiforov, A.V.; Petrushina, I.M.; Köhler, K.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Transition metal carbides (WC, Mo2C, TaC, NbC) as potential electrocatalysts for the hydrogen evolution reaction (HER) at medium temperatures. Int. J. Hydrogen Energy 2015, 40, 2905–2911. [Google Scholar] [CrossRef]
- Peng, X.; Hu, L.; Wang, L.; Zhang, X.; Fu, J.; Huo, K.; Lee, L.Y.S.; Wong, K.-Y.; Chu, P.K. Vanadium carbide nanoparticles encapsulated in graphitic carbon network nanosheets: A high-efficiency electrocatalyst for hydrogen evolution reaction. Nano Energy 2016, 26, 603–609. [Google Scholar] [CrossRef]
- Kelly, T.G.; Hunt, S.T.; Esposito, D.V.; Chen, J.G. Monolayer palladium supported on molybdenum and tungsten carbide substrates as low-cost hydrogen evolution reaction (HER) electrocatalysts. Int. J. Hydrogen Energy 2013, 38, 5638–5644. [Google Scholar] [CrossRef]
- Wang, C.-H.; Kurra, N.; Alhabeb, M.; Chang, J.-K.; Alshareef, H.N.; Gogotsi, Y. Titanium Carbide (MXene) as a Current Collector for Lithium-Ion Batteries. ACS Omega 2018, 3, 12489–12494. [Google Scholar] [CrossRef] [PubMed]
- Pande, P.; Rasmussen, P.G.; Thompson, L.T. Charge storage on nanostructured early transition metal nitrides and carbides. J. Power Source 2012, 207, 212–215. [Google Scholar] [CrossRef]
- Madima, N.; Mishra, S.B.; Inamuddin, I.; Mishra, A.K. Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater. A review. Environ. Chem. Lett. 2020, 18, 1169–1191. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Karwowska, E.; Poźniak, S.; Wojciechowski, T.; Chlubny, L.; Olszyna, A.; Ziemkowska, W.; Jastrzębska, A.M. Influence of modification of Ti3C2 MXene with ceramic oxide and noble metal nanoparticles on its antimicrobial properties and ecotoxicity towards selected algae and higher plants. RSC Adv. 2019, 9, 4092–4105. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, A.; Ponraj, J.S.; Wang, C.; Peng, W.K.; Manavalan, R.K.; Dhanabalan, S.C.; Zhang, H.; Gaspar, J. Engineering of 2D transition metal carbides and nitrides MXenes for cancer therapeutics and diagnostics. J. Mater. Chem. B 2020, 8, 4990–5013. [Google Scholar] [CrossRef]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the synthesis of MXenes and other ultrathin 2D transition metal carbides and nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Milligan, C.; Ma, T.; Zhou, L.; Cui, Y.; Qi, Z.; Libretto, N.; Xu, B.; Luo, J.; et al. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Luo, S.; Wang, R.; Yin, J.; Jiao, T.; Chen, K.; Zou, G.; Zhang, L.; Zhou, J.; Zhang, L.; Peng, Q. Preparation and Dye Degradation Performances of Self-Assembled MXene-Co3O4 Nanocomposites Synthesized via Solvothermal Approach. ACS Omega 2019, 4, 3946–3953. [Google Scholar] [CrossRef] [Green Version]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Syamsai, R.; Grace, A.N. Synthesis, properties and performance evaluation of vanadium carbide MXene as supercapacitor electrodes. Ceram. Int. 2020, 46, 5323–5330. [Google Scholar] [CrossRef]
- Wei, C.; Tao, Y.; An, Y.; Tian, Y.; Zhang, Y.; Feng, J.; Qian, Y. Recent Advances of Emerging 2D MXene for Stable and Dendrite-Free Metal Anodes. Adv. Funct. Mater. 2020, 30, 2004613. [Google Scholar] [CrossRef]
- Aslam, M.K.; Xu, M. A Mini-Review: MXene composites for sodium/potassium-ion batteries. Nanoscale 2020, 12, 15993–16007. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Wang, K.; Li, D.; Ding, F.; Meng, D.; Wang, X.; Zhang, Z. One-pot synthesis of Ni/Ni3C/Ni3N nanocomposite: Structure, growth mechanism and magnetic properties. Mater. Res. Bull. 2017, 95, 79–85. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Moon, S.-H.; Kim, M.-C.; Kim, S.-J.; Choi, S.; Kim, E.-S.; Han, S.-B.; Park, K.-W. Molybdenum carbide embedded in carbon nanofiber as a 3D flexible anode with superior stability and high-rate performance for Li-ion batteries. Ceram. Int. 2018, 44, 7972–7977. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Pei, L.; Yue, S.; Ma, L.; Zhou, L.; Huang, Z.; He, Y.; Gao, J. Silver nanoparticles modified two-dimensional transition metal carbides as nanocarriers to fabricate acetycholinesterase-based electrochemical biosensor. Chem. Eng. J. 2018, 339, 547–556. [Google Scholar] [CrossRef]
- Dinh, K.N.; Liang, Q.; Du, C.-F.; Zhao, J.; Tok, A.I.Y.; Mao, H.; Yan, Q. Nanostructured metallic transition metal carbides, nitrides, phosphides, and borides for energy storage and conversion. Nano Today 2019, 25, 99–121. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Ticianelli, E.A. Analysis of the electrocatalytic activity of α-molybdenum carbide thin porous electrodes toward the hydrogen evolution reaction. Electrochim. Acta 2016, 220, 363–372. [Google Scholar] [CrossRef]
- Wang, X.-H.; Hao, H.-L.; Zhang, M.-H.; Li, W.; Tao, K.-Y. Synthesis and characterization of molybdenum carbides using propane as carbon source. J. Solid State Chem. 2006, 179, 538–543. [Google Scholar] [CrossRef]
- Lei, M.; Zhao, H.; Yang, H.; Song, B.; Tang, W. Synthesis of transition metal carbide nanoparticles through melamine and metal oxides. J. Eur. Ceram. Soc. 2008, 28, 1671–1677. [Google Scholar] [CrossRef]
- Singh, H.; Pandey, O. Direct synthesis of nanocrystalline tungsten carbide from scheelite ore by solid state reaction method. Ceram. Int. 2013, 39, 785–790. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Shi, M.-Q.; Ma, C.-A.; Chu, Y.-Q.; Zhu, A.-J. Simple, green self-construction approach for large-scale synthesis of hollow, needle-like tungsten carbide. Powder Technol. 2013, 235, 467–474. [Google Scholar] [CrossRef]
- Yeh, C.; Liu, E. Combustion synthesis of tantalum carbides TaC and Ta2C. J. Alloys Compd. 2006, 415, 66–72. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Zhu, Y.; Qian, Y. Magnesium-assisted formation of metal carbides and nitrides from metal oxides. Int. J. Refract. Met. Hard Mater. 2012, 31, 288–292. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, S.; Hu, W.; Zhang, Y.; Chen, C.; Wang, P.; He, J.; Yu, D.; Xu, B.; Lu, Y.; et al. Mechanochemically activated synthesis of zirconium carbide nanoparticles at room temperature: A simple route to prepare nanoparticles of transition metal carbides. J. Eur. Ceram. Soc. 2011, 31, 1491–1496. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Zhou, X.; Hong, Y.; Shao, J.; Zhang, Y.; Yan, Q.; Dong, X. Controlled synthesis of nickel carbide nanoparticles and their application in lithium storage. Chem. Eng. J. 2018, 352, 940–946. [Google Scholar] [CrossRef]
- Liu, M.-C.; Hu, Y.-M.; An, W.-Y.; Kong, L.-B.; Kang, L. Liquid phase synthesis of dendritic nickel carbide alloy with high conductivity for advanced energy storage. J. Energy Chem. 2017, 26, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Emin, S.; Altinkaya, C.; Semerci, A.; Okuyucu, H.; Yildiz, A.; Stefanov, P. Tungsten carbide electrocatalysts prepared from metallic tungsten nanoparticles for efficient hydrogen evolution. Appl. Catal. B Environ. 2018, 236, 147–153. [Google Scholar] [CrossRef]
- Giordano, C.; Antonietti, M. Synthesis of crystalline metal nitride and metal carbide nanostructures by sol–gel chemistry. Nano Today 2011, 6, 366–380. [Google Scholar] [CrossRef]
- Morishita, T.; Soneda, Y.; Hatori, H.; Inagaki, M. Carbon-coated tungsten and molybdenum carbides for electrode of electrochemical capacitor. Electrochim. Acta 2007, 52, 2478–2484. [Google Scholar] [CrossRef]
- Dollé, M.; Gosset, D.; Bogicevic, C.; Karolak, F.; Simeone, D.; Baldinozzi, G. Synthesis of nanosized zirconium carbide by a sol–gel route. J. Eur. Ceram. Soc. 2007, 27, 2061–2067. [Google Scholar] [CrossRef]
- Kelly, J.P.; Kanakala, R.; Graeve, O.A. A Solvothermal Approach for the Preparation of Nanostructured Carbide and Boride Ultra-High-Temperature Ceramics. J. Am. Ceram. Soc. 2010, 93, 3035–3038. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Mei, T.; Shi, L.; Zhu, Y.; Qian, Y. A thermal reduction route to nanocrystalline transition metal carbides from waste polytetrafluoroethylene and metal oxides. Mater. Chem. Phys. 2012, 137, 1–4. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Zhang, B.; Anbalgam, K.; Thomas, T.; Yang, M. Synthesis and application of nano-structured metal nitrides and carbides: A review. Prog. Solid State Chem. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Amendola, V.; Riello, P.; Meneghetti, M. Magnetic Nanoparticles of Iron Carbide, Iron Oxide, Iron@Iron Oxide, and Metal Iron Synthesized by Laser Ablation in Organic Solvents. J. Phys. Chem. C 2010, 115, 5140–5146. [Google Scholar] [CrossRef]
- Shi, W.; Zheng, Y.; Peng, H.; Wang, N.; Lee, C.S.; Lee, S.-T. Laser Ablation Synthesis and Optical Characterization of Silicon Carbide Nanowires. J. Am. Ceram. Soc. 2000, 83, 3228–3230. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, C.; Liu, J.; Tian, Z.; Shao, G. The formation of onion-like carbon-encapsulated cobalt carbide core/shell nanoparticles by the laser ablation of metallic cobalt in acetone. Carbon 2013, 55, 108–115. [Google Scholar] [CrossRef]
- Liang, C.; Ma, W.; Feng, Z.; Li, C. Activated carbon supported bimetallic CoMo carbides synthesized by carbothermal hydrogen reduction. Carbon 2003, 41, 1833–1839. [Google Scholar] [CrossRef]
- Jansson, U.; Högberg, H.; Palmqvist, J.-P.; Norin, L.; Malm, J.; Hultman, L.; Birch, J. Low temperature epitaxial growth of metal carbides using fullerenes. Surf. Coat. Technol. 2001, 142–144, 817–822. [Google Scholar] [CrossRef]
- Kadas, K.; Andersson, M.; Holmström, E.; Wende, H.; Karis, O.; Urbonaite, S.; Butorin, S.M.; Nikitenko, S.; Kvashnina, K.O.; Jansson, U.; et al. Structural properties of amorphous metal carbides: Theory and experiment. Acta Mater. 2012, 60, 4720–4728. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Huang, Z.; Liu, X.; Jiang, D. Carbothermal synthesis of ultra-fine zirconium carbide powders using inorganic precursors via sol–gel method. J. Sol-Gel Sci. Technol. 2007, 44, 81–85. [Google Scholar] [CrossRef]
- Kundu, D.; Black, R.; Adams, B.; Harrison, K.; Zavadil, K.; Nazar, L.F. Nanostructured Metal Carbides for Aprotic Li–O2Batteries: New Insights into Interfacial Reactions and Cathode Stability. J. Phys. Chem. Lett. 2015, 6, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Mochalin, V.N. Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal Solutions. Inorg. Chem. 2019, 58, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, N.; Krüner, B.; Van Aken, K.L.; Alhabeb, M.; Anasori, B.; Kaasik, F.; Gogotsi, Y.; Presser, V. Electrochemical in Situ Tracking of Volumetric Changes in Two-Dimensional Metal Carbides (MXenes) in Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 32089–32093. [Google Scholar] [CrossRef]
- Anasori, B.; Shi, C.; Moon, E.J.; Xie, Y.; Voigt, C.A.; Kent, P.R.C.; May, S.J.; Billinge, S.J.L.; Barsoum, M.W.; Gogotsi, Y. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. 2016, 1, 227–234. [Google Scholar] [CrossRef]
- Ghaffari, S.; Faghihi-Sani, M.; Golestani-Fard, F.; Nojabayy, M. Diffusion and solid solution formation between the binary carbides of TaC, HfC and ZrC. Int. J. Refract. Met. Hard Mater. 2013, 41, 180–184. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2019, 5, 235–258. [Google Scholar] [CrossRef]
- He, J.; Lyu, P.; Nachtigall, P. New two-dimensional Mn-based MXenes with room-temperature ferromagnetism and half-metallicity. J. Mater. Chem. C 2016, 4, 11143–11149. [Google Scholar] [CrossRef]
- Sun, W.; Xie, Y.; Kent, P.R.C. Double transition metal MXenes with wide band gaps and novel magnetic properties. Nanoscale 2018, 10, 11962–11968. [Google Scholar] [CrossRef]
- Bekaert, J.; Sevik, C.; Milošević, M.V. First-principles exploration of superconductivity in MXenes. Nanoscale 2020, 12, 17354–17361. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Tranca, D.; Zhang, J.; Hernández, F.R.; Su, Y.; Zhuang, X.; Zhang, F.; Seifert, G.; Feng, X. Molybdenum Carbide-Embedded Nitrogen-Doped Porous Carbon Nanosheets as Electrocatalysts for Water Splitting in Alkaline Media. ACS Nano 2017, 11, 3933–3942. [Google Scholar] [CrossRef]
- Chen, C.; Wu, A.; Yan, H.; Xiao, Y.; Tian, C.; Fu, H. Trapping [PMo12O40]3− clusters into pre-synthesized ZIF-67 toward MoxCoxC particles confined in uniform carbon polyhedrons for efficient overall water splitting. Chem. Sci. 2018, 9, 4746–4755. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Li, Y.; Guo, S.; Jin, T.; Li, H.; Wang, Y.; Jiao, L. Molybdenum carbide in-situ embedded into carbon nanosheets as efficient bifunctional electrocatalysts for overall water splitting. Electrochim. Acta 2019, 298, 305–312. [Google Scholar] [CrossRef]
- Yu, X.; Wang, T.; Yin, W.; Zhang, Y. Ti3C2 MXene nanoparticles modified metal oxide composites for enhanced photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 2704–2710. [Google Scholar] [CrossRef]
- Handoko, A.D.; Fredrickson, K.D.; Anasori, B.; Convey, K.W.; Johnson, L.R.; Gogotsi, Y.; Vojvodic, A.; Seh, Z.W. Tuning the Basal Plane Functionalization of Two-Dimensional Metal Carbides (MXenes) To Control Hydrogen Evolution Activity. ACS Appl. Energy Mater. 2018, 1, 173–180. [Google Scholar] [CrossRef]
- Ma, T.Y.; Cao, J.L.; Jaroniec, M.; Qiao, S.Z. Interacting Carbon Nitride and Titanium Carbide Nanosheets for High-Performance Oxygen Evolution. Angew. Chem. Int. Ed. 2016, 55, 1138–1142. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-doped Graphene-Supported Transition-metals Carbide Electrocatalysts for Oxygen Reduction Reaction. Sci. Rep. 2015, 5, srep10389. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Wang, D.; Du, F.; Chen, G.; Wang, C.; Wei, Y.; Gogotsi, Y.; Gao, Y.; Dall’Agnese, Y. Revealing the Pseudo-Intercalation Charge Storage Mechanism of MXenes in Acidic Electrolyte. Adv. Funct. Mater. 2019, 29, 1902953. [Google Scholar] [CrossRef]
- Bak, S.-M.; Qiao, R.; Yang, W.; Lee, S.; Yu, X.; Anasori, B.; Lee, H.; Gogotsi, Y.; Yang, X.-Q. Na-Ion Intercalation and Charge Storage Mechanism in 2D Vanadium Carbide. Adv. Energy Mater. 2017, 7, 1700959. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Tang, X.; Guo, X.; Wu, W.; Wang, G. 2D Metal Carbides and Nitrides (MXenes) as High-Performance Electrode Materials for Lithium-Based Batteries. Adv. Energy Mater. 2018, 8, 1801897. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Feng, T.; Gan, Y.; Fang, M.; Xia, Y.; Liang, C.; Tao, X.; Zhang, W. TiC/NiO Core/Shell Nanoarchitecture with Battery-Capacitive Synchronous Lithium Storage for High-Performance Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2015, 7, 11842–11848. [Google Scholar] [CrossRef]
- Ihsan, M.; Wang, H.; Majid, S.R.; Yang, J.; Kennedy, S.J.; Guo, Z.; Liu, H.K. MoO2/Mo2C/C spheres as anode materials for lithium ion batteries. Carbon 2016, 96, 1200–1207. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Qi, J.; Li, X.; Xiao, Y.; Yang, L.; Yu, X.; Wang, H.; Yuan, B.; Gao, Q. MoC/C nanowires as high-rate and long cyclic life anode for lithium ion batteries. Electrochim. Acta 2018, 277, 205–210. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Torelli, M.; Ren, C.E.; Ghidiu, M.; Ling, Z.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. 2D titanium carbide and transition metal oxides hybrid electrodes for Li-ion storage. Nano Energy 2016, 30, 603–613. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, H.; Wang, J.; Ding, B.; Xu, Y.; Chang, Z.; Hao, X. Three-dimensional porous MXene/layered double hydroxide composite for high performance supercapacitors. J. Power Source 2016, 327, 221–228. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Li, Z.; Zhang, Y.; Xing, B.; Zhang, C.; Zhou, A. Electrochemical performance of Ti3C2 supercapacitors in KOH electrolyte. J. Adv. Ceram. 2015, 4, 130–134. [Google Scholar] [CrossRef]
- Zhu, K.; Jin, Y.; Du, F.; Gao, S.; Gao, Z.; Meng, X.; Chen, G.; Wei, Y.; Gao, Y. Synthesis of Ti2CTx MXene as electrode materials for symmetric supercapacitor with capable volumetric capacitance. J. Energy Chem. 2019, 31, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Rakhi, R.B.; Ahmed, B.; Anjum, D.H.; Alshareef, H.N. Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2016, 8, 18806–18814. [Google Scholar] [CrossRef] [Green Version]
- Syamsai, R.; Kollu, P.; Jeong, S.K.; Grace, A.N. Synthesis and properties of 2D-titanium carbide MXene sheets towards electrochemical energy storage applications. Ceram. Int. 2017, 43, 13119–13126. [Google Scholar] [CrossRef]
- Syamsai, R.; Grace, A.N. Ta4C3 MXene as supercapacitor electrodes. J. Alloys Compd. 2019, 792, 1230–1238. [Google Scholar] [CrossRef]
- Shan, Q.; Mu, X.; Alhabeb, M.; Shuck, C.E.; Pang, D.; Zhao, X.; Chu, X.-F.; Wei, Y.; Du, F.; Chen, G.; et al. Two-dimensional vanadium carbide (V2C) MXene as electrode for supercapacitors with aqueous electrolytes. Electrochem. Commun. 2018, 96, 103–107. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Jiang, Q. Chemically functionalized two-dimensional titanium carbide MXene by in situ grafting-intercalating with diazonium ions to enhance supercapacitive performance. J. Phys. Chem. Solids 2018, 115, 172–179. [Google Scholar] [CrossRef]
- Tian, Y.; Que, W.; Luo, Y.; Yang, C.; Yin, X.; Kong, L.B. Surface nitrogen-modified 2D titanium carbide (MXene) with high energy density for aqueous supercapacitor applications. J. Mater. Chem. A 2019, 7, 5416–5425. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Hao, X.; Bi, J.; Wang, W.; Chen, Y.; Gao, X.; Sun, X.; Zhang, J. Bimetallic carbide Fe2MoC as electrode material for high-performance capacitive energy storage. Ceram. Int. 2018, 44, 21874–21881. [Google Scholar] [CrossRef]
- Djire, A.; Ajenifujah, O.T.; Sleightholme, A.E.; Rasmussen, P.; Thompson, L.T. Effects of surface oxygen on charge storage in high surface area early transition-metal carbides and nitrides. J. Power Source 2015, 275, 159–166. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Tian, Z.; Ye, Y.; Cai, Y.; Liang, C.; Terabe, K. A general strategy toward transition metal carbide/carbon core/shell nanospheres and their application for supercapacitor electrode. Carbon 2016, 100, 590–599. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Hood, Z.D.; Naguib, M.; Adhikari, S.P.; Wu, Z. Titania Composites with 2D Transition Metal Carbides as Photocatalysts for Hydrogen Production under Visible-Light Irradiation. ChemSusChem 2016, 9, 1490–1497. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Kikuchi, N.; Motojima, S. Catalytic effects of various metal carbides and Ti compounds for the growth of carbon nanocoils (CNCs). Mater. Lett. 2008, 62, 1462–1465. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, C.-J.; Luc, W.W.; Chen, J.G.; Bhan, A.; Jiao, F. Ordered Mesoporous Metal Carbides with Enhanced Anisole Hydrodeoxygenation Selectivity. ACS Catal. 2016, 6, 3506–3514. [Google Scholar] [CrossRef]
- Kunkel, C.; Viñes, F.; Illas, F. Transition metal carbides as novel materials for CO2 capture, storage, and activation. Energy Environ. Sci. 2016, 9, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Chen, X.; Ong, W.-J.; Macfarlane, D.R.; Zhao, X.; Cheetham, A.K.; Sun, C. Understanding of Electrochemical Mechanisms for CO2 Capture and Conversion into Hydrocarbon Fuels in Transition-Metal Carbides (MXenes). ACS Nano 2017, 11, 10825–10833. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. J. 2020, 388, 124340. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Cao, J.; Tang, Q.; Kang, P.; Zhu, C.; Ma, M. 2D a-Fe2O3 doped Ti3C2 MXene composite with enhanced visible light photocatalytic activity for degradation of Rhodamine B. Ceram. Int. 2018, 44, 19958–19962. [Google Scholar] [CrossRef]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Cai, C.; Wang, R.; Liu, S.; Yan, X.; Zhang, L.; Wang, M.; Tong, Q.; Jiao, T. Synthesis of self-assembled phytic acid-MXene nanocomposites via a facile hydrothermal approach with elevated dye adsorption capacities. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124468. [Google Scholar] [CrossRef]
- Jun, B.-M.; Heo, J.; Taheri-Qazvini, N.; Park, C.M.; Yoon, Y. Adsorption of selected dyes on Ti3C2Tx MXene and Al-based metal-organic framework. Ceram. Int. 2020, 46, 2960–2968. [Google Scholar] [CrossRef]
- Tariq, A.; Ali, S.I.; Akinwande, D.; Rizwan, S. Efficient Visible-Light Photocatalysis of 2D-MXene Nanohybrids with Gd3+- and Sn4+-Codoped Bismuth Ferrite. ACS Omega 2018, 3, 13828–13836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.A.; Ali, S.I.; Amin, F.; Tariq, A.; Rizwan, S. La- and Mn-Codoped Bismuth Ferrite/Ti3C2 MXene Composites for Efficient Photocatalytic Degradation of Congo Red Dye. ACS Omega 2019, 4, 8661–8668. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.A.; Tariq, A.; Zaheer, A.; Gul, S.; Ali, S.I.; Akinwande, D.; Rizwan, S. Ti3C2-MXene/Bismuth Ferrite Nanohybrids for Efficient Degradation of Organic Dyes and Colorless Pollutants. ACS Omega 2019, 4, 20530–20539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Zheng, K.; Li, S.; Jing, L.; Chen, P.; Xie, J. Synergistic Antimicrobial Titanium Carbide (MXene) Conjugated with Gold Nanoclusters. Adv. Healthc. Mater. 2020, 9, 2001007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Zhang, Q.; Wang, F.; Liu, Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens. Bioelectron. 2019, 124-125, 184–190. [Google Scholar] [CrossRef]
- Dong, L.M.; Ye, C.; Zheng, L.L.; Gao, Z.F.; Xia, F. Two-dimensional metal carbides and nitrides (MXenes): Preparation, property, and applications in cancer therapy. Nanophotonics 2020, 9, 2125–2145. [Google Scholar] [CrossRef] [Green Version]
- Lyu, B.; Choi, Y.; Jing, H.; Qian, C.; Kang, H.; Lee, S.; Cho, J.H. 2D MXene–TiO2 Core–Shell Nanosheets as a Data-Storage Medium in Memory Devices. Adv. Mater. 2020, 32, 1907633. [Google Scholar] [CrossRef]








| Catalyst | Media | Tafel Slope mV.dec−1 | Reference |
|---|---|---|---|
| Ni-PC * Mo2C-PC Ni/Mo2C-PC Pt/C | 1 M KOH | 145 140 101 52 | [25] |
| Com-Mo2C Mo2C@NPC Mo2C@2D-NPC Pt/C | 1 M KOH | 67 52 46 31 | [86] |
| PMo/ZIF-67-6-7N ZIF-67-7N RuO2 PMo/ZIF-67-6-6N ZIF-67-6N Pt/C | 1 M KOH | 35 39 66 50 88 30 | [87] |
| N-WC + nano-array WC nano-array N-WC powders WC powders Pt/C | 0.5 M H2SO4 | −75 −110 −149 −166 −29 | [24] |
| Pure Ni-foam Mo2C (0.000) ** Mo2C (0.005) Mo2C (0.01) Mo2C (0.02) Pt/C | 1 M KOH | 114.5 188.8 74.2 66.5 95.8 33.5 | [88] |
| Ti3C2Tx NiSe2 NiSe2/Ti3C2Tx Pt/C | 0.5 M H2SO4 | 222.2 46.9 37.7 30.5 | [27] |
| CATALYST | Media | Tafel Slope Value (mV.dec−1) | References |
|---|---|---|---|
| TMC electrodes | Phosphoric acid | [28] | |
| Cr | 76 | ||
| Mo | 67 | ||
| W | 56 | ||
| Nb | 135 | ||
| Ta | 93 | ||
| VC nano-sheets VC particles | 0.5 M H2SO4 | 56 81 | [30] |
| W1 at 150 °C (W, W2C, WC Phase Mix) | 0.5 M H2SO4 | 108 | [61] |
| W NPs * | 156 | ||
| W at 900 °C | 156 | ||
| W at 1000 °C | 145 | ||
| W at 1450 °C | 143 | ||
| MXene (LiF-HCl) MXene (10% HF) MXene (50% HF) Mo2CTx Ti3CTx Mo2Ti2C3Tx | 0.5 M H2SO4 | 128 138 190 75 88 99 | [90] |
| α-Mo2C at different loaded amounts | 0.1 M HClO4 | [51] | |
| 8.5 μg/cm−2 | 73.8 | ||
| 12.7μg/cm−2 | 79.6 | ||
| 16.9 μg/cm−2 | 75.5 | ||
| 25.4 μg/cm−2 | 75.5 | ||
| 50.8 μg/cm−2 | 77.8 | ||
| 100.8 μg/cm−2 | 79 |
| Energy Storage Devices | Type | Material |
|---|---|---|
| Batteries | LIBs | Anode: transition metal-based MXenes, |
| MoO2/Mo2C/C (nanosphere) | ||
| MoC/C nanowires | ||
| Multilayer MXene Ti3C2/carbon nanofiber | ||
| TiC/NiO core/shell nanoarchitecture | ||
| TiC nanowire | ||
| Mo2C nanofiber | ||
| Hybrid: Ti3C2Tx-Co2O4 Ti3C2Tx-NiCo2O4 | ||
| TiC, NbC, MoC, WC, and TaC | ||
| SIBs | Vanadium carbide-based MXenes | |
| MoS2/MXene-composite | ||
| Sb2O3 NPs disperse on MXene | ||
| Supercapacitors | MXene with rGO, CNT, PVA | |
| MXene Nanosheets wrapped in NiSe2 | ||
| MXene Nanosheets/Ni/Al layered double hydroxide | ||
| Functionalized MXene | ||
| Molybdenum iron carbide (bimetallic carbide) | ||
| V2C | ||
| V4C3 MXene | ||
| Carbon coated WC | ||
| W, Mo, and V carbide | ||
| Nitrogen modified MXene sheets | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Ashraf, I.; Mansoor, M.A.; Rizwan, S.; Iqbal, M. An Overview of Recent Advances in the Synthesis and Applications of the Transition Metal Carbide Nanomaterials. Nanomaterials 2021, 11, 776. https://doi.org/10.3390/nano11030776
Ahmad S, Ashraf I, Mansoor MA, Rizwan S, Iqbal M. An Overview of Recent Advances in the Synthesis and Applications of the Transition Metal Carbide Nanomaterials. Nanomaterials. 2021; 11(3):776. https://doi.org/10.3390/nano11030776
Chicago/Turabian StyleAhmad, Saba, Iffat Ashraf, Muhammad Adil Mansoor, Syed Rizwan, and Mudassir Iqbal. 2021. "An Overview of Recent Advances in the Synthesis and Applications of the Transition Metal Carbide Nanomaterials" Nanomaterials 11, no. 3: 776. https://doi.org/10.3390/nano11030776






