Two–Dimensional Disposable Graphene Sensor to Detect Na+ Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and the Fabrication of G–ISFET with ISM
2.2. Detection of Na+ Ions Using G–ISFET–ISM
3. Results and Discussion
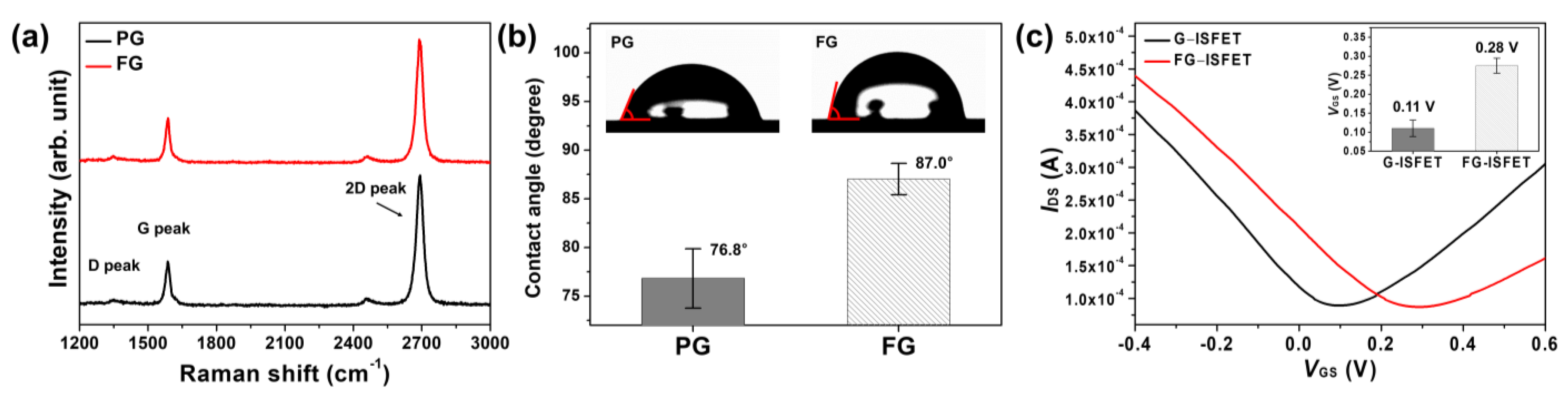
3.1. Fluorinated Graphene
3.2. Characteristics of G–ISFET and FG–ISFET
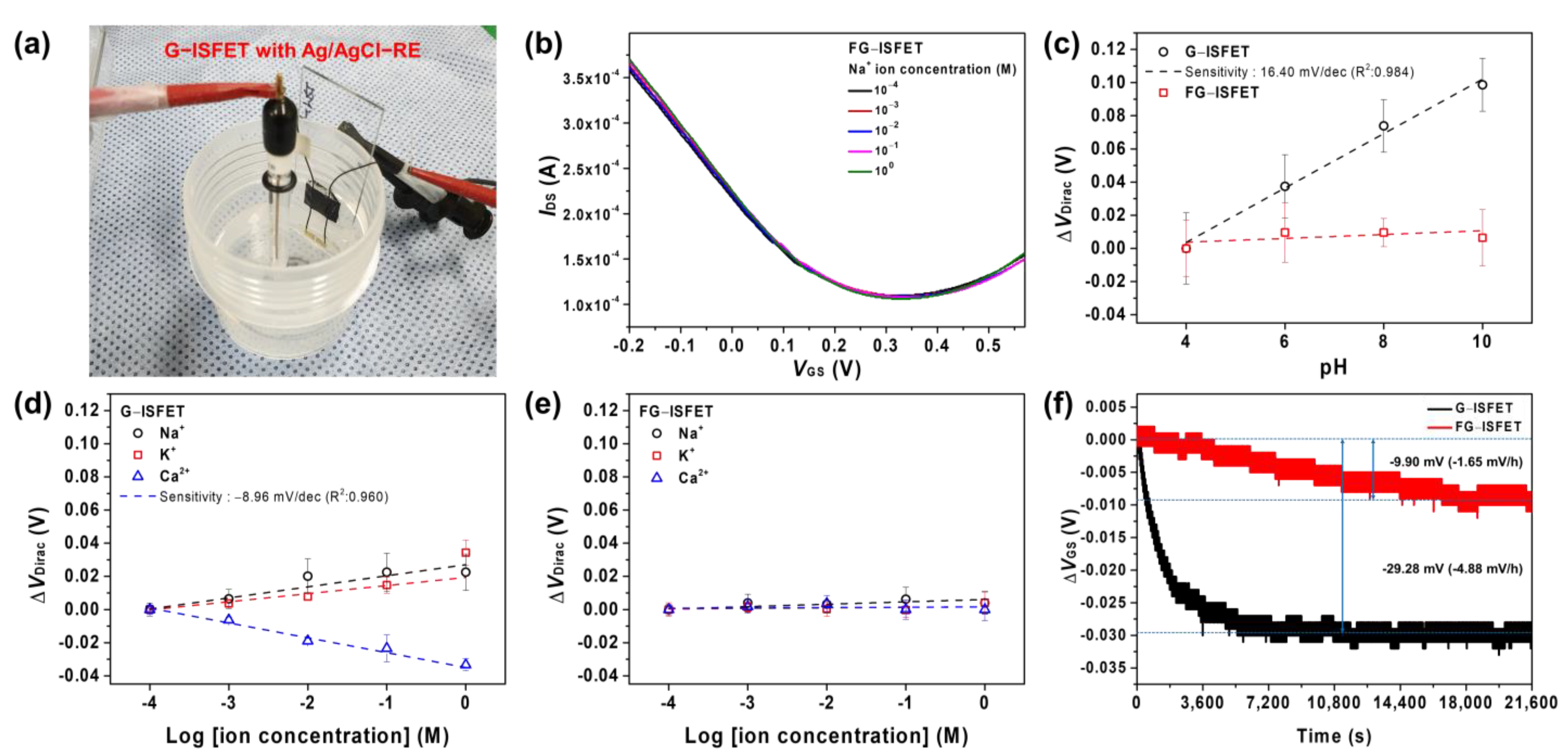
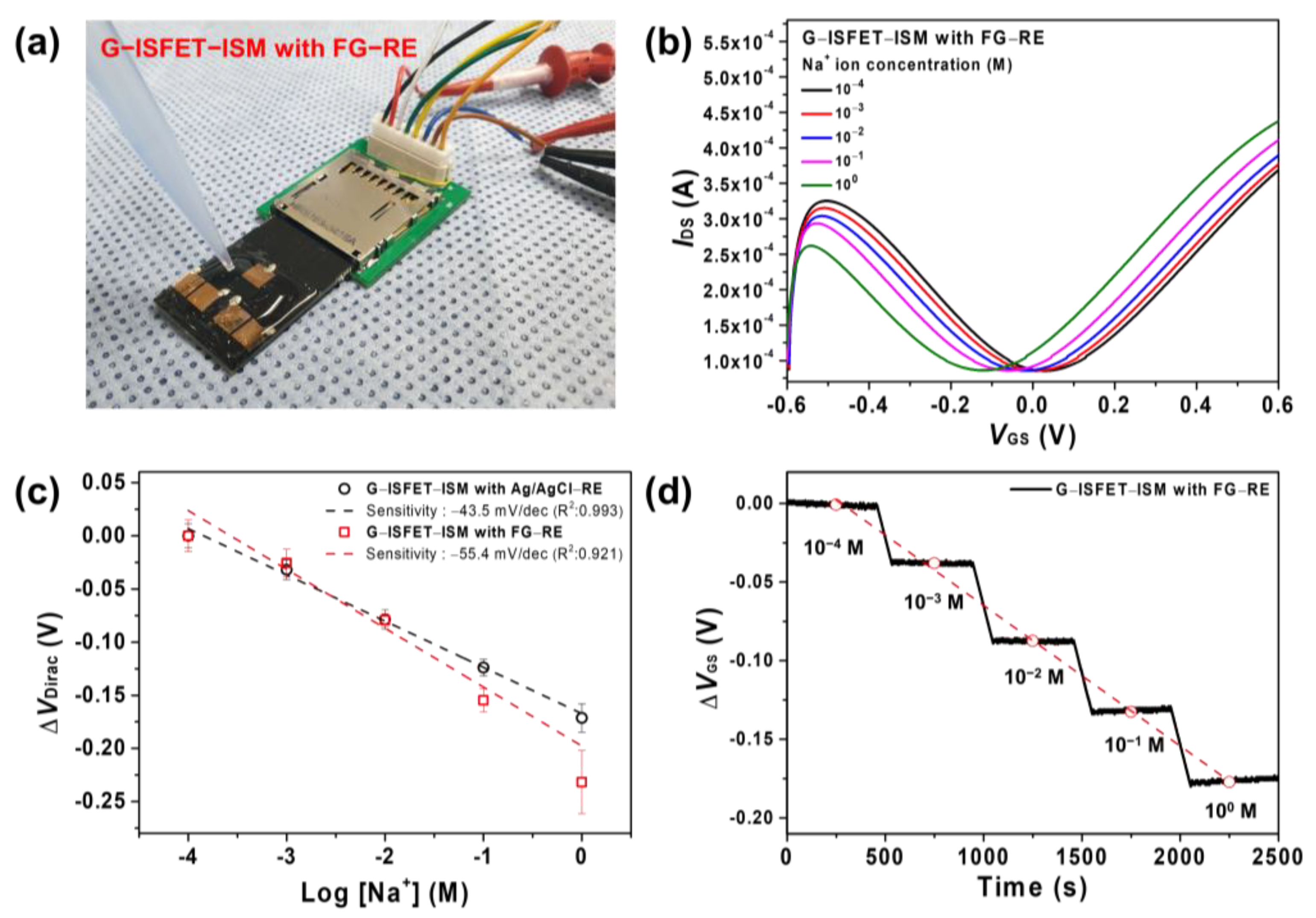
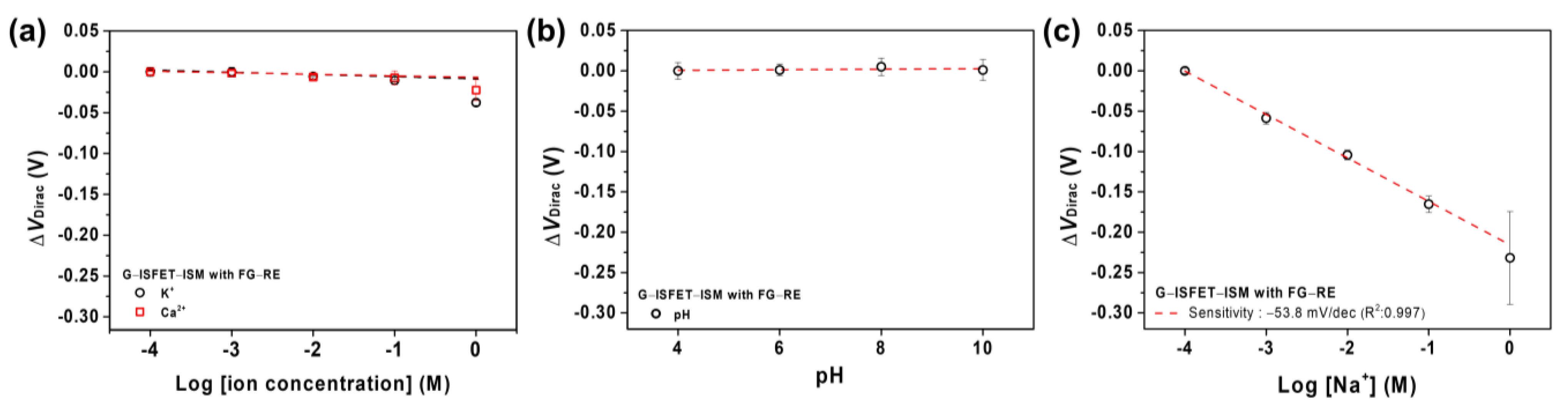
3.3. G–ISFET with ISM to Detect Na+ Ions Using Ag/AgCl–RE or FG–RE
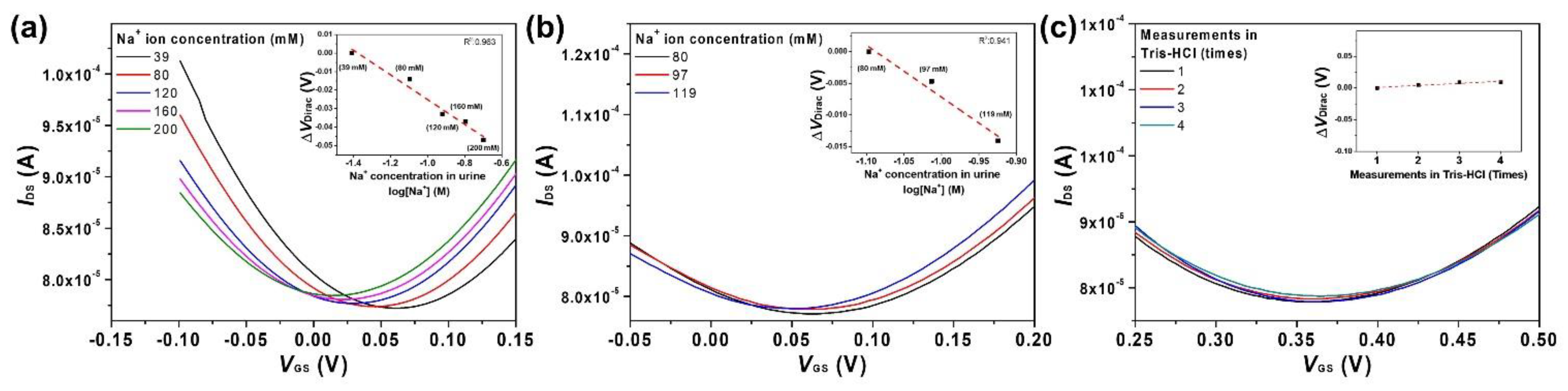
3.4. Urine Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hsu, H.-Y.; Wu, C.-Y.; Lee, H.-C.; Lin, J.-L.; Chin, Y.-L.; Sun, T.-P. Sodium and potassium sensors based on separated extended gate field effect transistor. Biomed. Eng. Appl. Basis Commun. 2009, 21, 441–444. [Google Scholar] [CrossRef]
- Sharp, R.L. Role of sodium in fluid homeostasis with exercise. J. Am. Coll. Nutr. 2006, 25, 231S–239S. [Google Scholar] [CrossRef]
- Gao, S.; Cui, X.; Wang, X.; Burg, M.B.; Dmitrieva, N.I. Cross-sectional positive association of serum lipids and blood pressure with serum sodium within the normal reference range of 135−145 mmol/L. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 598–606. [Google Scholar] [CrossRef]
- Muhsin, S.A.; Mount, D.B. Diagnosis and treatment of hypernatremia. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 189–203. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Padfield, P.L.; Seckl, J.R. Disorders of sodium balance. BMJ 2006, 332, 702–705. [Google Scholar] [CrossRef]
- Henry, D.A. Hyponatremia. Ann. Intern. Med. 2015, 163, ITC1–ITC16. [Google Scholar] [CrossRef]
- Nielsen, S.S. Sodium and potassium determinations by atomic absorption spectroscopy and inductively coupled plasma−optical emission spectroscopy. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010; pp. 87–93. [Google Scholar] [CrossRef]
- Coyne, M.D.; Lobene, A.; Neumann, C.; Lachcik, P.; Weaver, C.M.; Nie, L.H. Determination of bone sodium (Na) and Na exchange in pig leg using in vivo neutron activation analysis (IVNAA). Physiol. Meas. 2019, 40, 075009. [Google Scholar] [CrossRef]
- Banerjee, P.; Prasad, B. Determination of concentration of total sodium and potassium in surface and ground water using a flame photometer. Appl. Water Sci. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Bakker, E. A tunable detection range of ion−selective nano−optodes by controlling solvatochromic dye transducer lipophilicity. ChemComm 2019, 55, 12539–12542. [Google Scholar] [CrossRef]
- Kabaa, E.; Abdulateef, S.; Ahmed, N.M.; Hassan, Z.; Sabah, F.A. A novel porous silicon multi−ions selective electrode based extended gate field effect transistor for sodium, potassium, calcium, and magnesium sensor. Appl. Phys. A 2019, 125, 753. [Google Scholar] [CrossRef]
- Mlika, R.; Ouada, H.B.; Jaffrezic-Renault, N.; Dumazet, I.; Lamartine, R.; Gamoudi, M.; Guillaud, G. Study of ion−selective evaporated calixarene film used as a sensitive layer on ISFET sensors. Sens. Actuators B Chem. 1998, 47, 43–47. [Google Scholar] [CrossRef]
- Ito, K.; Satake, H.; Mori, Y.; Tseng, A.C.; Sakata, T. Biocompatible and Na+−sensitive thin−film transistor for biological fluid sensing. Sci. Technol. Adv. Mater. 2019, 20, 917–926. [Google Scholar] [CrossRef]
- Fakih, I.; Durnan, O.; Mahvash, F.; Napal, I.; Centeno, A.; Zurutuza, A.; Yargeau, V.; Szkopek, T. Selective ion sensing with high resolution large area graphene field effect transistor arrays. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Hernández, R.; Riu, J.; Rius, F.X. Determination of calcium ion in sap using carbon nanotube−based ion−selective electrodes. Analyst 2010, 135, 1979–1985. [Google Scholar] [CrossRef]
- Fu, W.; Jiang, L.; van Geest, E.P.; Lima, L.M.; Schneider, G.F. Sensing at the surface of graphene field−effect transistors. Adv. Mater. 2017, 29, 1603610. [Google Scholar] [CrossRef]
- Maruizumi, T.; Wegmann, D.; Suter, G.; Ammann, D.; Simon, W. Neutral carrier−based Na+−selective electrode for application in blood serum. Microchim. Acta 1986, 88, 331–336. [Google Scholar] [CrossRef]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon nanotube−based ion selective sensors for wearable applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef]
- Fu, W.; Nef, C.; Tarasov, A.; Wipf, M.; Stoop, R.; Knopfmacher, O.; Weiss, M.; Calame, M.; Schönenberger, C. High mobility graphene ion−sensitive field−effect transistors by noncovalent functionalization. Nanoscale 2013, 5, 12104–12110. [Google Scholar] [CrossRef]
- Kwak, J.; Chu, J.H.; Choi, J.-K.; Park, S.-D.; Go, H.; Kim, S.Y.; Park, K.; Kim, S.-D.; Kim, Y.-W.; Yoon, E.; et al. Near room−temperature synthesis of transfer−free graphene films. Nat. Commun. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Somanathan, T.; Prasad, K.; Ostrikov, K.K.; Saravanan, A.; Krishna, V.M. Graphene oxide synthesis from agro waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, W.H.; Oh, H.G.; Jeon, D.C.; Lim, J.M.; Song, K.S. Two-Channel Graphene pH Sensor Using Semi−Ionic Fluorinated Graphene Reference Electrode. Sensors 2020, 20, 4184. [Google Scholar] [CrossRef]
- Mazánek, V.; Jankovský, O.; Luxa, J.; Sedmidubský, D.; Janoušek, Z.; Šembera, F.; Mikulics, M.; Sofer, Z. Tuning of fluorine content in graphene: Towards large-scale production of stoichiometric fluorographene. Nanoscale 2015, 7, 13646–13655. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of grapheme. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Robinson, J.T.; Burgess, J.S.; Junkermeier, C.E.; Badescu, S.C.; Reinecke, T.L.; Perkins, F.K.; Zalautdniov, M.K.; Baldwin, J.W.; Culbertson, J.C.; Sheehan, P.E.; et al. Properties of fluorinated graphene films. Nano Lett. 2010, 10, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Xie, H.; Xia, K.; Zhang, Y. Challenge and Opportunities of Carbon Nanotubes. In Industrial Applications of Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 433–476. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated reference electrodes and their biosensing applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Nef, C.; Knopfmacher, O.; Tarasov, A.; Weiss, M.; Calame, M.; Schönenberger, C. Graphene transistors are insensitive to pH changes in solution. Nano Lett. 2011, 11, 3597–3600. [Google Scholar] [CrossRef]
- Sofue, Y.; Ohno, Y.; Maehashi, K.; Inoue, K.; Matsumoto, K. Highly sensitive electrical detection of sodium ions based on graphene field−effect transistors. Jpn. J. Appl. Phys. 2011, 50, 06GE07. [Google Scholar] [CrossRef]
- Villeneuve, P.-M.; Bagshaw, S.M. Assessment of urine biochemistry. In Critical Care Nephrology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–328.e1. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lei, K.F.; Tsai, S.-W.; Tsang, N.-M. Development of graphene−based sensors on paper substrate for the measurement of ph value of analyte. BioChip J. 2016, 10, 182–188. [Google Scholar] [CrossRef]
- Lei, N.; Li, P.; Xue, W.; Xu, J. Simple graphene chemiresistors as pH sensors: Fabrication and characterization. Meas. Sci. Technol. 2011, 22, 107002. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Q.; Li, Z.; Zhou, Q.; Fang, Y. Suspended graphene sensors with improved signal and reduced noise. Nano Lett. 2010, 10, 1864–1868. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Yamashiro, Y.; Matsumoto, K. Electrolyte−gated graphene field−effect transistors for detecting pH and protein adsorption. Nano Lett. 2009, 9, 3318–3322. [Google Scholar] [CrossRef] [PubMed]
- Maehashi, K.; Sofue, Y.; Okamoto, S.; Ohno, Y.; Inoue, K.; Matsumoto, K. Selective ion sensors based on ionophore−modified graphene field−effect transistors. Sens. Actuators B Chem. 2013, 187, 45–49. [Google Scholar] [CrossRef]
- Sohn, I.-Y.; Kim, D.-J.; Jung, J.-H.; Yoon, O.J.; Thanh, T.N.; Quang, T.T.; Lee, N.-E. pH sensing characteristics and biosensing application of solution−gated reduced graphene oxide field−effect transistors. Biosens. Bioelectron. 2013, 45, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Chang, S.-H.; Tsai, W.-L.; Chang, C.-T.; Wang, K.-Y.; Yang, P.-Y.; Cheng, H.-C. Highly sensitive pH sensors of extended−gate field−effect transistor with the oxygen−functionalized reduced Graphene oxide films on reverse pyramid substrates. IEEE Electron. Device Lett. 2015, 36, 1189–1191. [Google Scholar] [CrossRef]
- Ristein, J.; Zhang, W.; Speck, F.; Ostler, M.; Ley, L.; Seyller, T. Characteristics of solution gated field effect transistors on the basis of epitaxial graphene on silicon carbide. J. Phys. D Appl. Phys. 2010, 43, 345303. [Google Scholar] [CrossRef]
- Su, C.-Y.; Fu, D.; Lu, A.-Y.; Liu, K.-K.; Xu, Y.; Juang, Z.-Y.; Li, L.-J. Transfer printing of graphene strip from the graphene grown on copper wires. Nanotechnology 2011, 22, 185309. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Petrone, N.; Yu, J.; Nuckolls, C.; Hone, J.; Lin, Q. A solid-gated graphene FET sensor for PH measurements. In Proceedings of the 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 869–872. [Google Scholar] [CrossRef]
- Foley, K.F.; Boccuzzi, L. Urine calcium: Laboratory measurement and clinical utility. Lab. Med. 2010, 41, 683–686. [Google Scholar] [CrossRef]
- Pięk, M.; Wojciechowska, A.; Fendrych, K.; Piech, R.; Paczosa−Bator, B. A simple way to modify selectivity of sodium sensitive electrodes by using organic conductive crystals. Ionics 2019, 25, 2311–2321. [Google Scholar] [CrossRef]
- Salvo, P.; Melai, B.; Calisi, N.; Paoletti, C.; Bellagambi, F.; Kirchhain, A.; Trivella, M.G.; Fuoco, R.; Francesco, F.D. Graphene−based devices for measuring pH. Sens. Actuators B Chem. 2018, 256, 976–991. [Google Scholar] [CrossRef]
- Bridges, M.A.; Mattice, M.R. The significance of urinary pH: Critical observations. Ann. Intern. Med. 1941, 14, 1123–1136. [Google Scholar] [CrossRef]
- Rowe, D.; Bagga, H.; Betts, P. Normal variations in rate of albumin excretion and albumin to creatinine ratios in overnight and daytime urine collections in non−diabetic children. BMJ Clin. Res. Ed. 1985, 291, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Doumas, B.T.; Peters, T., Jr. Serum and urine albumin: A progress report on their measurement and clinical significance. Clin. Chim. Acta 1997, 258, 3–20. [Google Scholar] [CrossRef]
- Oh, H.-G.; Nam, H.-G.; Kim, D.-H.; Kim, M.-H.; Jhee, K.-H.; Song, K.S. Neuroblastoma cells grown on fluorine or oxygen treated graphene sheets. Mater. Lett. 2014, 131, 328–331. [Google Scholar] [CrossRef]
- Son, H.-G.; Oh, H.-G.; Park, Y.-S.; Kim, D.-H.; Lee, D.-S.; Park, W.-H.; Kim, H.J.; Cho, S.-M.; Lim, K.M.; Song, K.S. Micro cell array on silicon substrate using graphene sheet. Mater. Lett. 2017, 196, 385–387. [Google Scholar] [CrossRef]

| Subject No. | u–Na+ (mM) | u–K+ (mM) | Na+/K+ Ratio | u–Cl− (mM) | u–Cr3+ (mM) |
|---|---|---|---|---|---|
| S037 | 97.0 | 25.0 | 3.88 | 39.0 | 56.6 |
| S039 | 39.0 | 21.2 | 1.84 | 39.0 | 56.6 |
| S047 | 80.0 | 29.1 | 2.75 | 38.0 | 105.1 |
| S054 | 119.0 | 28.8 | 4.13 | 134.0 | 79.5 |
| Graphene–ISFET Channel | Detecting Ion/Sensing Range | Reference Electrode | Sensitivity | Ref. |
|---|---|---|---|---|
| Mechanical exfoliation | H+/pH 1–10.5 | gate–free | 30.8 Ω/pH | [31] |
| H+/pH 4–10 | gate–free | 2.13 kΩ/pH | [32] | |
| H+/pH 6–9 | Ag/AgCl | 17 mV/pH | [33] | |
| H+/pH 4–8.2 | Ag/AgCl | 30 mV/pH | [34] | |
| Mechanical exfoliation (without ISM) | K+, Na+/0–10−3 M | Ag/AgCl | − | [29,35] |
| Chemical exfoliation (rGO)) | H+/pH 6–9 | Ag/AgCl | 29 mV/pH | [36] |
| Chemical exfoliation (rGO + oxygen plasma) | H+/pH 1–13 | Ag/AgCl | 57 mV/pH | [37] |
| Epitaxial growth | H+/pH 3–12 | Ag/AgCl | 19.1 mV/pH | [38] |
| Chemical vapor deposition (CVD) growth | H+/pH 1.2–9 | Ag wire | 22 mV/pH | [39] |
| CVD growth + oxygenation (plasma) | H+/pH 5.3–9.3 | HfO2 | 57.5 mV/pH | [40] |
| H+/pH 4–10 | Ag/AgCl | 19.4 mV/pH | [22] | |
| H+/pH 4–10 | FG–RE (plasma) | 18.2 mV/pH | [22] | |
| CVD growth + ISM | K+, Na+, NH4+, NO3−, SO42−, HPO42−, and Cl−/10−6–10−1 M | Ag/AgCl | Sensitivity depends on ions (ΔIDS) | [14] |
| CVD growth + fluorination (fluorobenzene) | H+/pH 4–10 | Pt wire | <1 mV/pH | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.G.; Jeon, D.C.; Gianti, M.S.; Cho, H.S.; Jo, D.A.; Indriatmoko, M.N.; Jang, B.K.; Lim, J.M.; Cho, S.; Song, K.S. Two–Dimensional Disposable Graphene Sensor to Detect Na+ Ions. Nanomaterials 2021, 11, 787. https://doi.org/10.3390/nano11030787
Oh HG, Jeon DC, Gianti MS, Cho HS, Jo DA, Indriatmoko MN, Jang BK, Lim JM, Cho S, Song KS. Two–Dimensional Disposable Graphene Sensor to Detect Na+ Ions. Nanomaterials. 2021; 11(3):787. https://doi.org/10.3390/nano11030787
Chicago/Turabian StyleOh, Hong Gi, Dong Cheol Jeon, Mahmudah Salwa Gianti, Hae Shin Cho, Da Ae Jo, Muhammad Naufal Indriatmoko, Byoung Kuk Jang, Joon Mook Lim, Seungmin Cho, and Kwang Soup Song. 2021. "Two–Dimensional Disposable Graphene Sensor to Detect Na+ Ions" Nanomaterials 11, no. 3: 787. https://doi.org/10.3390/nano11030787
APA StyleOh, H. G., Jeon, D. C., Gianti, M. S., Cho, H. S., Jo, D. A., Indriatmoko, M. N., Jang, B. K., Lim, J. M., Cho, S., & Song, K. S. (2021). Two–Dimensional Disposable Graphene Sensor to Detect Na+ Ions. Nanomaterials, 11(3), 787. https://doi.org/10.3390/nano11030787







