Single-Step Green Synthesis of Highly Concentrated and Stable Colloidal Dispersion of Core-Shell Silver Nanoparticles and Their Antimicrobial and Ultra-High Catalytic Properties
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Synthesis of Ag NPs-PTA and Bare Ag NPs
3. Characterization
3.1. Antimicrobial Tests
3.2. Catalytic Reduction of 4-NP
4. Results and Discussion
4.1. Antimicrobial Response Analysis
4.2. Catalytic Reduction of 4-NP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.; Roh, J.; Kim, S.; Choi, K.; Yi, J.; Kim, Y.; Yoon, J. Biofilm-inactivating activity of silver nanoparticles: A comparison with silver ions. J. Ind. Eng. Chem. 2013, 19, 614–619. [Google Scholar] [CrossRef]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Shaban, S.M.; Kim, J.; Kim, D.-H. One-pot synthesis of highly stable and concentrated silver nanoparticles with enhanced catalytic activity. Korean J. Chem. Eng. 2019, 36, 988–995. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The role of calix[n]arenes and pillar[n]arenes in the design of silver nanoparticles: Self-assembly and application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kheybari, S.; Samadi, N.; Hosseini, S.V.; Fazeli, A.; Fazeli, M.R. Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. DARU J. Pharm. Sci. 2010, 18, 168–172. [Google Scholar]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Ghaffari-Moghaddam, M.; Hadi-Dabanlou, R. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. J. Ind. Eng. Chem. 2014, 20, 739–744. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.J.; Moon, J.H.; Kim, J.H.; Heo, D.N.; Bang, J.B.; Lim, H.N.; Kwon, I.K. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J. Ind. Eng. Chem. 2018, 66, 196–202. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Núñez, N.V.; del Turrent, L.C.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Achilias, D.S.; Kosmidou, E.; Vakalopoulou, E.; Ioannidou, M.D. Green synthesis of silver nanoparticles and study of their antimicrobial properties. J. Polym. Environ. 2018, 26, 423–433. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H.; Park, J.E.; Osaka, T.; Park, S.G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahri, M. Metal-polymer nanocomposites for functional applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Ryu, B.H.; Choi, Y.; Park, H.S.; Byun, J.H.; Kong, K.; Lee, J.O.; Chang, H. Synthesis of highly concentrated silver nanosol and its application to inkjet printing. Colloids Surf. A Physicochem. Eng. Asp. 2005, 270–271, 345–351. [Google Scholar] [CrossRef]
- Balantrapu, K.; Goia, D.V. Silver nanoparticles for printable electronics and biological applications. J. Mater. Res. 2009, 24, 2828–2836. [Google Scholar] [CrossRef]
- Toisawa, K.; Hayashi, Y.; Takizawa, H. Synthesis of highly concentrated Ag nanoparticles in a heterogeneous solid-liquid system under ultrasonic irradiation. Mater. Trans. 2010, 51, 1764–1768. [Google Scholar] [CrossRef] [Green Version]
- Gakiya-Teruya, M.; Palomino-Marcelo, L.; Rodriguez-Reyes, J.C.F. Synthesis of highly concentrated suspensions of silver nanoparticles by two versions of the chemical reduction method. Methods Protoc. 2019, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, J.; Ma, G. Extremely concentrated silver nanoparticles stabilized in aqueous solution by Bovine Serum Albumin (BSA). Nano-Struct. Nano-Objects 2019, 19, 100349. [Google Scholar] [CrossRef]
- La Spina, R.; Mehn, D.; Fumagalli, F.; Rossi, F.; Gilliland, D.; Holland, M.; Reniero, F. Synthesis of citrate-stabilized silver nanoparticles modified by thermal and ph preconditioned tannic acid. Nanomaterials 2020, 10, 2031. [Google Scholar] [CrossRef]
- Yang, W.; Hu, W.; Zhang, J.; Wang, W.; Cai, R.; Pan, M.; Huang, C.; Chen, X.; Yan, B.; Zeng, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem. Eng. J. 2021, 405, 126629. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, Y.; Das, P.; Zhang, L.; Zhang, Y.; Fang, H.; Wang, L.; Cao, Y. Synthesis, dissolution, and regeneration of silver nanoparticles stabilized by tannic acid in aqueous solution. J. Nanoparticle Res. 2019, 21. [Google Scholar] [CrossRef]
- Fei, J.; Zhao, J.; Du, C.; Wang, A.; Zhang, H.; Dai, L.; Li, J. One-pot ultrafast self-assembly of autofluorescent polyphenol-based core@shell nanostructures and their selective antibacterial applications. ACS Nano 2014, 8, 8529–8536. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, X.; Guo, Y.; Niu, H.; Cai, Y. Enhanced catalytic application of Au@polyphenol-metal nanocomposites synthesized by a facile and green method. J. Mater. Chem. A 2014, 2, 14807–14811. [Google Scholar] [CrossRef]
- Ke, F.; Zhu, J.; Qiu, L.-G.; Jiang, X. Controlled synthesis of novel Au@MIL-100(Fe) core–shell nanoparticles with enhanced catalytic performance. Chem. Commun. 2013, 49, 1267–1269. [Google Scholar] [CrossRef]
- Ali, A.; Baheti, V.; Militky, J.; Khan, Z.; Tunakova, V.; Naeem, S. Copper coated multifunctional cotton fabrics. J. Ind. Text. 2018, 48, 448–464. [Google Scholar] [CrossRef]
- Ali, A.; Baheti, V.; Militky, J.; Khan, Z.; Gilani, S.Q.Z. Comparative performance of copper and silver coated stretchable fabrics. Fibers Polym. 2018, 19, 607–619. [Google Scholar] [CrossRef]
- Ali, A.; Baheti, V.; Militky, J.; Khan, Z. Utility of silver-coated fabrics as electrodes in electrotherapy applications. J. Appl. Polym. Sci. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Wang, X.; Cao, W.; Xiang, Q.; Jin, F.; Peng, X.; Li, Q.; Jiang, M.; Hu, B.; Xing, X. Silver nanoparticle and lysozyme/tannic acid layer-by-layer assembly antimicrobial multilayer on magnetic nanoparticle by an eco-friendly route. Mater. Sci. Eng. C 2017, 76, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Hussain, F.; Khurshid, M.F.; Masood, R.; Ibrahim, W. Developing antimicrobial calcium alginate fibres from neem and papaya leaves extract. J. Wound Care 2017, 26, 778–783. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1150–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Shen, W.; Xu, Q.; Tan, R.; Song, W. Properties of polyacrylic acid-coated silver nanoparticle ink for inkjet printing conductive tracks on paper with high conductivity. Mater. Chem. Phys. 2014, 147, 550–556. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Berezin, O.; Kamyshny, A. Triggering the sintering of silver nanoparticles at room temperature. ACS Nano 2010, 4, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Goia, D.V.; Matijević, E. Preparation of highly concentrated stable dispersions of uniform silver nanoparticles. J. Colloid Interface Sci. 2003, 260, 75–81. [Google Scholar] [CrossRef]
- Shon, Y.S.; Cutler, E. Aqueous synthesis of alkanethiolate-protected Ag nanoparticles using bunte salts. Langmuir 2004, 20, 6626–6630. [Google Scholar] [CrossRef]
- Yang, J.; Yin, H.; Jia, J.; Wei, Y. Facile synthesis of high-concentration, stable aqueous dispersions of uniform silver nanoparticles using aniline as a reductant. Langmuir 2011, 27, 5047–5053. [Google Scholar] [CrossRef]
- Tang, C.; Hu, D.; Cao, Q.; Yan, W.; Xing, B. Silver nanoparticles-loaded activated carbon fibers using chitosan as binding agent: Preparation, mechanism, and their antibacterial activity. Appl. Surf. Sci. 2017, 394, 457–465. [Google Scholar] [CrossRef]
- Qasim, M.; Udomluck, N.; Chang, J.; Park, H.; Kim, K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomed. 2018, 13, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Ryu, D.; Choi, S.; Lee, D. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, M. Silver nanoparticle supported on halloysite nanotubes catalyzed reduction of 4-nitrophenol (4-NP). Appl. Surf. Sci. 2009, 255, 3989–3993. [Google Scholar] [CrossRef]
- Kästner, C.; Thünemann, A.F. Catalytic reduction of 4-nitrophenol using silver nanoparticles with adjustable activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Meng, X. Carbon spheres with controllable silver nanoparticle doping. J. Phys. Chem. C 2010, 114, 977–982. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Q.; Guan, H.; Liu, S. In situ synthesis of silver nanoparticles dispersed or wrapped by a Cordyceps sinensis exopolysaccharide in water and their catalytic activity. RSC Adv. 2015, 5, 69790–69799. [Google Scholar] [CrossRef]
- Rashid, M.H.; Mandal, T.K. Synthesis and catalytic application of nanostructured silver dendrites. J. Phys. Chem. C 2007, 111, 16750–16760. [Google Scholar] [CrossRef]
- Shin, K.S.; Cho, Y.K.; Choi, J.Y.; Kim, K. Facile synthesis of silver-deposited silanized magnetite nanoparticles and their application for catalytic reduction of nitrophenols. Appl. Catal. A Gen. 2012, 413–414, 170–175. [Google Scholar] [CrossRef]
- An, Q.; Yu, M.; Zhang, Y.; Ma, W.; Guo, J.; Wang, C. Fe3O4@carbon microsphere supported Ag-Au bimetallic nanocrystals with the enhanced catalytic activity and selectivity for the reduction of nitroaromatic compounds. J. Phys. Chem. C 2012, 116, 22432–22440. [Google Scholar] [CrossRef]
- Baruah, B.; Gabriel, G.J.; Akbashev, M.J.; Booher, M.E. Facile synthesis of silver nanoparticles stabilized by cationic polynorbornenes and their catalytic activity in 4-nitrophenol reduction. Langmuir 2013, 29, 4225–4234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horecha, M.; Kaul, E.; Horechyy, A.; Stamm, M. Polymer microcapsules loaded with Ag nanocatalyst as active microreactors. J. Mater. Chem. A 2014, 2, 7431. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.C.; Ren, C.L.; Dong, Y.L.; Chang, Y.P.; Zhou, M.; Chen, X.G. Facile synthesis of multifunctional graphene oxide/AgNPs-Fe3O4 nanocomposite: A highly integrated catalysts. Chem. Eng. J. 2012, 211–212, 412–420. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Zhang, L. In situ synthesis of monodisperse silver nanoparticles on sulfhydryl-functionalized poly(glycidyl methacrylate) microspheres for catalytic reduction of 4-nitrophenol. Ind. Eng. Chem. Res. 2015, 54, 6480–6488. [Google Scholar] [CrossRef]


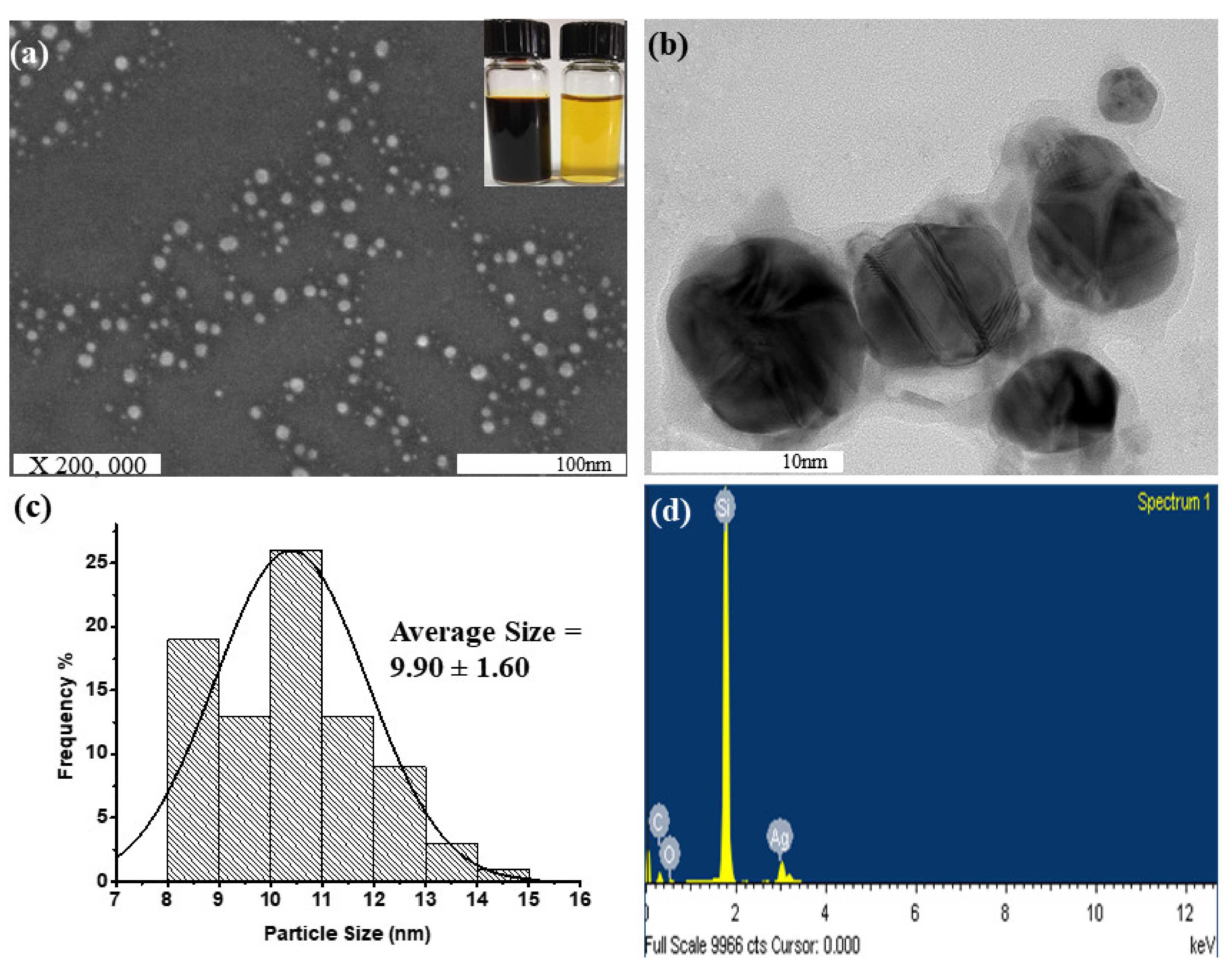
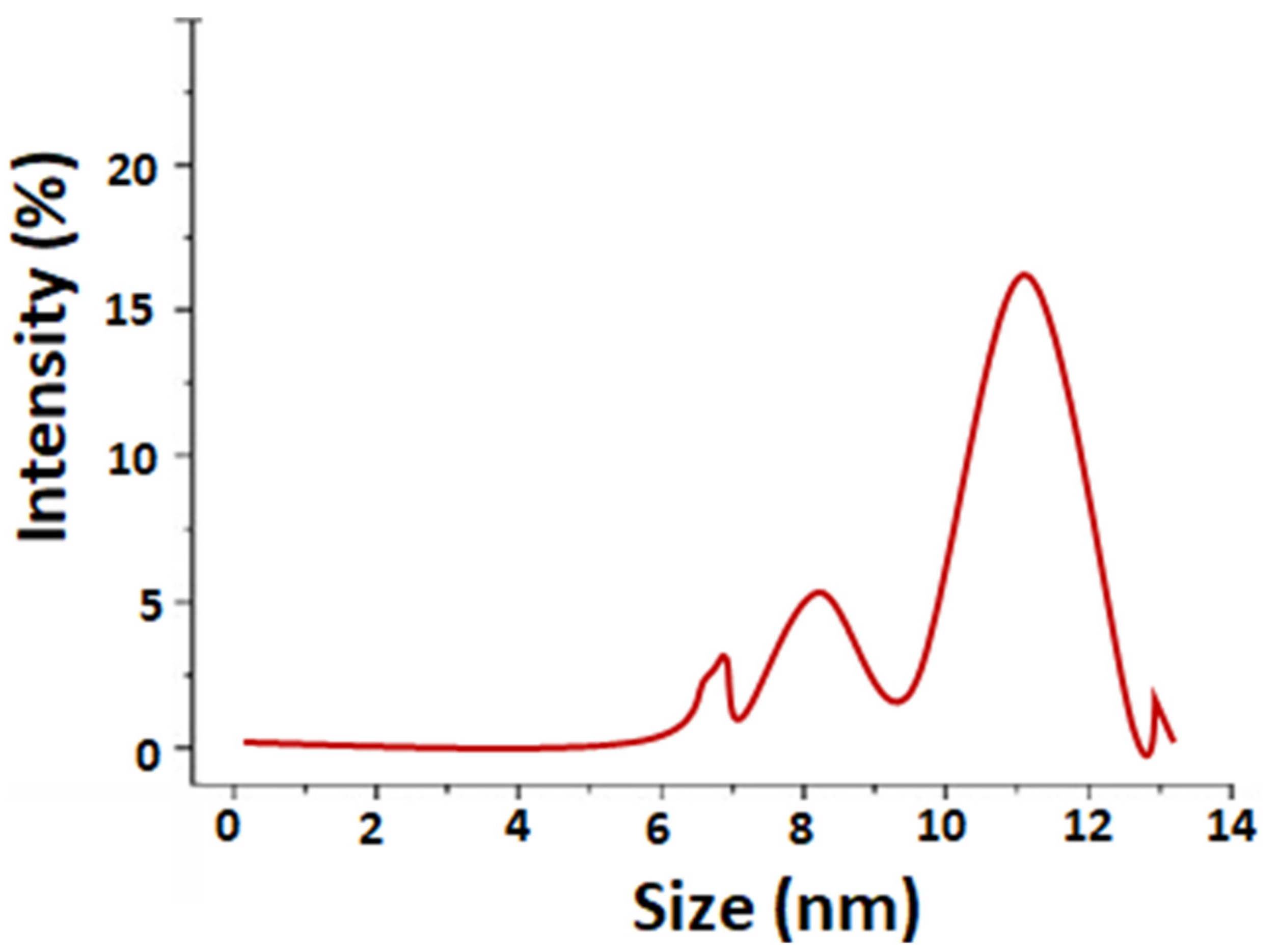


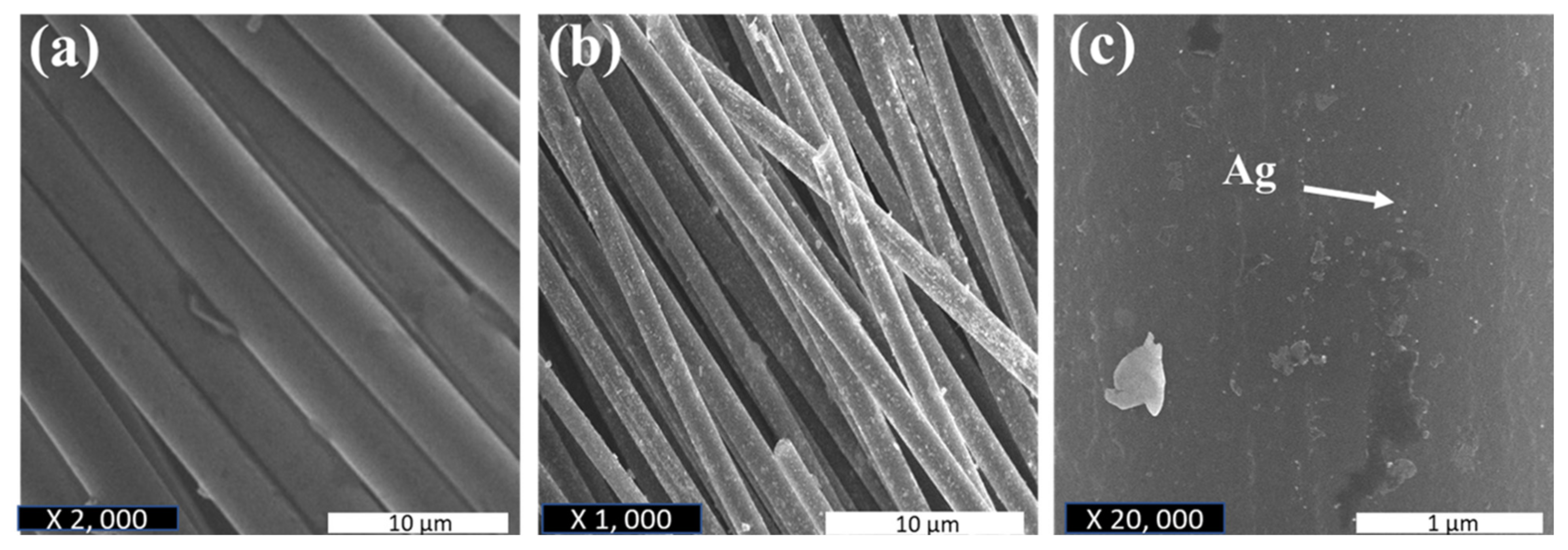


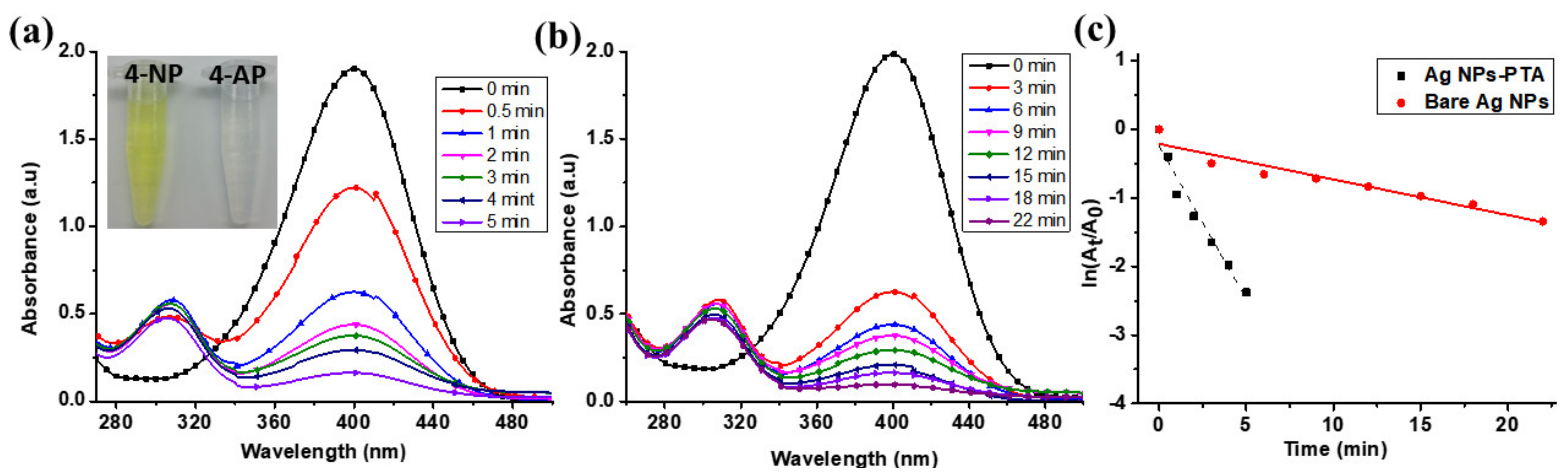
| Initial AgNO3 Concentration. (M) | Synthesis Time/Aqueous Stability | Limitation of the Synthesis Process | Particle Size (nm) | Synthesis Method | Ref. |
|---|---|---|---|---|---|
| 0.147 | 30 min/>15 months | Relatively lower conversion | 9 | Green chemistry | Current Study |
| 1.9 | 25 h/- | Long reaction time under extreme precautions | 30 | Chemical | [38] |
| 1.65 | 2 h/3 months | Long reaction time, no reproducibility, broad size distribution | 20–230 | Chemical | [39] |
| 0.94 | 0.75 h/ 6 months | A relatively high temperature is required. Particles are not stable at low/mild alkaline condition | 5–80 | Chemical | [40] |
| 0.83 | 0.75 h/14 months | Use of toxic chemicals | 14 | Chemical | [4] |
| 0.43 | 10 h/- | High energy input (200 W), long reaction time, and use of environmentally hazardous materials | 20–30 | Microwave | [21] |
| 0.27 | 7 min/- | Not stable at higher concentrations (>0.3M) | 26 | Chemical | [41] |
| 0.16 | 4.5 h/- | Two-phase, complicated process | 4 | Chemical | [42] |
| 0.02 | 2 min/- | Relatively low concentration and use of hazardous and toxic chemicals | 10 | Chemical | [43] |
| Nanocatalyst Structure | Catalyst (mg/mL) | Rate Constant K (s−1) | Knor. (mL·s−1·mg−1) | Reference |
|---|---|---|---|---|
| Halloysite nanotubes-Ag | 8.00 × 10−3 | 6.96 × 10−7 | 8.70 × 10−5 | [47] |
| Ag@PAA | 2.97 × 10−2 | 15.45 × 10−3 | 4.59 × 10−4 | [48] |
| Ag-NP/C composite | 1.00 × 10−0 | 1.69 × 10−3 | 1.69 × 10−3 | [49] |
| EPS–Ag nanocomposites | 2.60 × 10−2 | 1.26 × 10−3 | 4.80 × 10−2 | [50] |
| Ag NPs@PAA | 2.03 × 10−4 | 7.6 × 10−2 | 374.94 | [4] |
| TSC-Ag-1.4 | 1.33 × 10−3 | 3.64 × 10−4 | 2.7 × 10−1 | [51] |
| Fe3O4/SiO2-Ag | 2.00 × 10−2 | 5.50 × 10−3 | 2.8 × 10−1 | [52] |
| Fe3O4-@C@Ag | 1.00 × 10−2 | 3.72 × 10−3 | 3.7 × 10−1 | [53] |
| TAC-Ag-1.4 | 1.33 × 10−3 | 1.65 × 10−3 | 1.24 | [51] |
| Fe3O4-@C@Ag-Au | 1.00 × 10−2 | 15.80 × 10−3 | 1.58 | [53] |
| AgNP-PG-5K | 4.00 × 10−3 | 5.50 × 10−3 | 1.38 | [54] |
| Ag/SiO2 1.08 | 1.1 × 10−3 | 2.53 × 10−3 | 2.30 | [55] |
| Graphene oxide/Ag NPs−Fe3O4 | 8.1 × 10−3 | 2.67 × 10−2 | 3.30 | [56] |
| TAC-Ag-1.0 | 1.33 × 10−3 | 5.19 × 10−3 | 3.90 | [51] |
| Ag NPs@PGMA-SH composite | 9.00 × 10−4 | 3.94 × 10−3 | 4.38 | [57] |
| AgNPs-PTA | 1.33 × 10−4 | 8.18 × 10−2 | 615.04 | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Sattar, M.; Hussain, F.; Tareen, M.H.K.; Militky, J.; Noman, M.T. Single-Step Green Synthesis of Highly Concentrated and Stable Colloidal Dispersion of Core-Shell Silver Nanoparticles and Their Antimicrobial and Ultra-High Catalytic Properties. Nanomaterials 2021, 11, 1007. https://doi.org/10.3390/nano11041007
Ali A, Sattar M, Hussain F, Tareen MHK, Militky J, Noman MT. Single-Step Green Synthesis of Highly Concentrated and Stable Colloidal Dispersion of Core-Shell Silver Nanoparticles and Their Antimicrobial and Ultra-High Catalytic Properties. Nanomaterials. 2021; 11(4):1007. https://doi.org/10.3390/nano11041007
Chicago/Turabian StyleAli, Azam, Mariyam Sattar, Fiaz Hussain, Muhammad Humble Khalid Tareen, Jiri Militky, and Muhammad Tayyab Noman. 2021. "Single-Step Green Synthesis of Highly Concentrated and Stable Colloidal Dispersion of Core-Shell Silver Nanoparticles and Their Antimicrobial and Ultra-High Catalytic Properties" Nanomaterials 11, no. 4: 1007. https://doi.org/10.3390/nano11041007
APA StyleAli, A., Sattar, M., Hussain, F., Tareen, M. H. K., Militky, J., & Noman, M. T. (2021). Single-Step Green Synthesis of Highly Concentrated and Stable Colloidal Dispersion of Core-Shell Silver Nanoparticles and Their Antimicrobial and Ultra-High Catalytic Properties. Nanomaterials, 11(4), 1007. https://doi.org/10.3390/nano11041007








